Abstract
The origins and role of the Warburg effect have remained uncertain for many years. Two recent studies demonstrate that an embryonic- and cancer-cell-specific isoform of the enzyme pyruvate kinase M2 (PKM2) is regulated by binding to phospho-tyrosine motifs and promotes increased cell growth and tumor development. PKM2 enhances the use of glycolytic intermediates for macromolecular biosynthesis and tumor growth. These findings illustrate the distinct advantages of this metabolic phenotype in cancer cell growth.
The Warburg effect
The high proliferative rate of cancerous cells results in metabolic needs distinct from those of their normal counterparts. Most cells in the adult body are quiescent, with nutrients directed primarily toward energy production to maintain cellular machinery. By contrast, cancerous cells must divide their incoming nutrients between energy production and macromolecular biosynthesis to support cell growth and DNA replication. This difference in cell metabolism was initially observed nearly a century ago by Otto Warburg when he described that cancer cells take up much higher amounts of glucose than normal cells and exhibit increased rates of glycolysis and lactate production even in the presence of oxygen [1]. This metabolic phenotype is now termed aerobic glycolysis and is known as the Warburg effect. A wide variety of tumor types, arising from different kinds of cells, converge on this metabolic program to meet their energetic demands. Such convergence indicates that this phenotype provides tumor cells with a growth and/or survival advantage. However, it has remained unclear how and why the Warburg effect occurs.
Several contributing factors have been proposed to explain the Warburg effect, and two recent studies indicate a new mechanism. First, the presence of hypoxia within the tumor microenvironment can contribute to a glycolytic metabolism via hypoxia inducible factor-mediated upregulation of glycolytic genes [2]. Second, the activation of certain oncoproteins, such as Akt (also termed protein kinase B) [3,4], Bcr–Abl [Breakpoint cluster region–Abelson murine leukemia oncogene; the result of a t(9;22) translocation] [5,6] and Myc (Myelocytomatosis viral oncogene homolog) [7], can all directly promote increased glucose uptake by regulating the expression of the glucose transporter Glut1. In addition to these established influences on aerobic glycolysis, Christofk et al. [8,9] have recently demonstrated that the glycolytic enzyme pyruvate kinase (PK) might have a key role in the origins of the Warburg effect. Interestingly, they show that altered PK expression and activity in cancer cells, more specifically the expression of one specific alternative splice isoform, pyruvate kinase M2 (PKM2), and its association with phospho-tyrosine (pTyr) motifs, enables cells to use predominantly aerobic glycolysis instead of oxidative phosphorylation. Furthermore, they suggest that this change benefits cancer cells by enabling them to redirect glycolytic metabolites away from oxidation and energy production and, instead, toward anabolic processes and biosynthesis.
PKM2 regulation and function
Starting with the observation that cellular metabolic pathways are influenced by growth-factor signaling, Christofk and colleagues have established a mechanism by which growth factors can regulate PK catalytic activity [9] and affect tumor growth [8] by signaling through protein tyrosine kinases. The authors used a clever proteomics screen to identify proteins that bind pTyr motifs. In this SILAC (stable isotope labeling of amino acids in cell culture) approach, cells were grown in either ‘heavy’ isotopic 13C-arginine and 13C-lysine or normal isotopic 12C-arginine and 12C-lysine. ‘Heavy’ lysates were passed over a pTyr peptide library affinity matrix, whereas ‘light’ lysates were passed over a corresponding unphosphorylated peptide library affinity matrix. After the elution of bound proteins, tandem mass spectrometry was used to identify proteins that bound preferentially to the pTyr matrix. Surprisingly, in addition to known pTyr-binding proteins, including STATs (signal transducer and activator of transcription) and GRB2 (growth factor receptor-bound protein 2), the authors identified PK as a pTyr-binding protein. Furthermore, they demonstrated that, of four PK isoforms (L, liver; R, red blood cell; M1, adult; M2, embryonic), only the M2 isoform could bind pTyr peptides.
pTyr binding had a marked influence on PKM2 function and might be important in establishing the aerobic glycolytic metabolism of the Warburg effect. Structural studies showed that PKM2 binding to pTyr residues caused the release of the PK allosteric activator fructose-1,6-bisphosphate and thereby decreased PKM2 enzymatic activity. This change in PK activity promoted an overall shift in cell metabolism to favor aerobic glycolysis with increased lactate production and decreased oxidative phosphorylation. Cells expressing a mutant form of PKM2 that is unable to bind pTyr peptides, however, failed to increase lactate production [9]. These findings are noteworthy because they indicate a link between cell growth and/or proliferation signals, which are often communicated through tyrosine kinase signaling pathways and are overactive in cancerous cells, and the changes in glucose metabolism observed in the Warburg phenotype.
PKM2 and cancer cell biology
The ability of PKM2 to be regulated by pTyr binding is, of course, only relevant for the Warburg effect and cancer biology to the extent that this isoform is expressed and active in cancer cells. The PKM2 isoform, normally thought to be embryonically restricted, is re-expressed in cancerous cells [10-12]. Indeed, whereas normal breast tissue expresses PKM1, tumors arising from the same tissue undergo a switch to express PKM2 [8]. Using RNAi knockdown and gene-replacement strategies the authors found that, whereas expression of either of these PK isoforms could rescue glucose metabolism and cell growth in the presence of normal oxygen and glucose levels, cells expressing PKM2 demonstrated a stronger glycolytic phenotype and showed a proliferative advantage in hypoxic conditions that could be more relevant for cancer development in vivo. Despite the reliance on the less efficient glycolytic generation of ATP in PKM2-expressing cells, ATP levels in PKM1- and PKM2-expressing cells were nevertheless identical, showing that, energetically, aerobic glycolysis need not be detrimental. This effect could result from an increased rate of glycolysis sufficient to generate normal ATP levels or through oxidation of an alternative fuel such as lipids or amino acids [13,14].
Aerobic glycolysis is proposed to enable greater availability of macromolecules for biosynthesis and cell growth [15]. The ability to promote aerobic glycolysis to support biosynthesis and maintain ATP levels indicates that PKM2 expression might enhance tumor growth in vivo. Indeed, the tumors formed by PKM2-expressing cancer cells grew more rapidly and were larger than those formed by PKM1-expressing cells in mouse xenograph experiments. Furthermore, whereas all of the recovered tumors retained expression of the exogenous overexpressed isoforms of PK (either M1 or M2), they all also re-expressed endogenous PKM2; none expressed PKM1 alone [8].
Although PKM2 can clearly contribute to the development of aerobic glycolysis and the Warburg effect, it nevertheless remains unclear precisely how this process occurs. The ultimate phenotype of aerobic glycolysis is the conversion of pyruvate to lactate, rather than the transport of pyruvate into the mitochondria for oxidation through the tricarboxylic acid cycle. PK, however, functions upstream of this bifurcation, converting phosphoenolpyruvate to pyruvate that can then undergo either of the two metabolic fates described earlier. PKM2 enzymatic activity, particularly when bound to pTyr residues, is substantially less than that of PKM1 [9]. This difference probably enables the diversion of glycolytic intermediates for macromolecular biosynthesis [16]. However, simple changes in the rate of pyruvate generation by different PK isoforms, such as those described by Christofk et al. [8,9] cannot easily explain why pyruvate is shifted toward lactate generation as opposed to oxidative phosphorylation. It is possible that other features of the different PK isoforms could influence pyruvate fate, such as their association with distinct protein complexes that include different combinations of enzymes or differences in intracellular localization.
Concluding remarks and future perspectives
The growth advantage observed in PKM2-expressing tumors and the selective pressure for PKM2 expression in tumors is striking. Indeed, there are clear metabolic benefits to using glycolysis for cancer cell growth and tumor formation (Figure 1). By decreasing cellular dependence on oxidative metabolism, PKM2 expression would better sustain cell growth in hypoxic environments. PKM2 might also provide cells with a growth advantage by enabling them greater flexibility in dividing glucose metabolites between energy production and anabolic processes such as lipid, nucleotide and amino acid synthesis. In addition, aerobic glycolysis participates in initiating anti-apoptotic signaling pathways in which glycogen synthase kinase-3 inhibition stabilizes the anti-apoptotic protein Mcl-1 (Myeloid cell leukemia sequence 1) and prevents the activation of the pro-apoptotic Bax (Bcl2-associated X protein) [17]. Thus, PKM2-mediated generation of the aerobic glycolytic phenotype, although wasteful in terms of glucose consumption and waste generation, could provide a variety of advantages for cancerous cells.
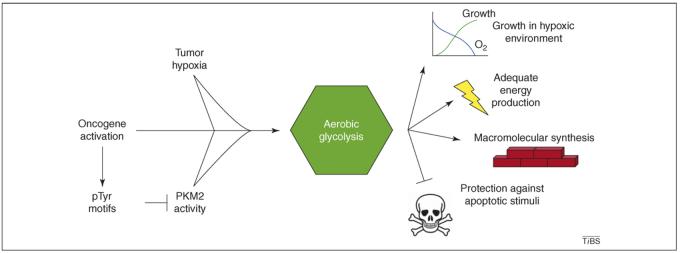
Figure 1.
Causes and potential benefits to cancerous cells of aerobic glycolysis. Factors that can promote increased glycolysis include: (i) hypoxia in the tumor microenvironment, which can lead to the upregulation of glycolytic genes; (ii) oncogene activation, which promotes glucose uptake and the generation of pTyr motifs; and (iii) pTyr-mediated PKM2 inhibition, which seems to shift pyruvate away from oxidative phosphorylation and toward lactate production through as yet unexplained mechanisms. This metabolic program might prove beneficial for tumor development by supporting cell growth and survival, especially in hypoxic environments, and by increasing cellular building blocks available for macromolecular biosynthesis.
The ultimate goal of cancer cell biology is to uncover aspects of cancer physiology that can be exploited for patient treatment. Warburg himself proposed that the altered metabolism of cancer cells might provide a means to treat cancer [1]. Nevertheless, metabolism is a ubiquitous process and attempts at finding specific approaches to target cancer-cell metabolism have been largely unsuccessful. The identification of PKM2 as a cancer cell-specific enzyme that is regulated by pTyr binding and is important for the aerobic glycolytic phenotype offers a potentially important new player in this approach. Understanding more about PKM2, such as how PKM2 expression and alternative splicing are regulated, which tyrosine phosphorylated proteins are important for PKM2 regulation in tumors, and how this affects pyruvate fate, could provide new directions for metabolic-based cancer therapies. After nearly 100 years, much remains to be learned about cancer-cell metabolism, but a better appreciation of PKM2 might provide a considerable advance in understanding and potentially exploiting the Warburg effect.
Acknowledgements
We thank Jon Coloff for helpful discussions. Work in our laboratory is supported by a grant from the NCI (R01-CA123350).
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Robey IF, et al. Hypoxia-inducible factor-1α and the glycolytic phenotype in tumors. Neoplasia. 2005;7:324–330. doi: 10.1593/neo.04430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plas DR, et al. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J. Biol. Chem. 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- 4.Wieman HL, et al. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol. Biol. Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes K, et al. Chronic myeloid leukaemia: an investigation into the role of Bcr-Abl-induced abnormalities in glucose transport regulation. Oncogene. 2005;24:3257–3267. doi: 10.1038/sj.onc.1208461. [DOI] [PubMed] [Google Scholar]
- 6.Bentley J, et al. Glucose transport regulation by p210 Bcr-Abl in a chronic myeloid leukaemia model. Br. J. Haematol. 2001;112:212–215. doi: 10.1046/j.1365-2141.2001.02428.x. [DOI] [PubMed] [Google Scholar]
- 7.Osthus RC, et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem. 2000;275:21797–21800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- 8.Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 9.Christofk HR, et al. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 10.Mazurek S, et al. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin. Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Dombrauckas JD, et al. Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry. 2005;44:9417–9429. doi: 10.1021/bi0474923. [DOI] [PubMed] [Google Scholar]
- 12.Koss K, et al. The metabolic marker tumour pyruvate kinase type M2 (tumour M2-PK) shows increased expression along the metaplasia-dysplasia-adenocarcinoma sequence in Barrett's oesophagus. J. Clin. Pathol. 2004;57:1156–1159. doi: 10.1136/jcp.2004.018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deberardinis RJ, et al. Brick by brick: metabolism and tumor cell growth. Curr. Opin. Genet. Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno-Sanchez R, et al. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 15.DeBerardinis RJ, et al. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Eigenbrodt E, et al. Double role for pyruvate kinase type M2 in the expansion of phosphometabolite pools found in tumor cells. Crit. Rev. Oncog. 1992;3:91–115. [PubMed] [Google Scholar]
- 17.Zhao Y, et al. Glycogen synthase kinase 3α and 3β mediate a glucose-sensitive antiapoptotic signaling pathway to stabilize Mcl-1. Mol. Cell. Biol. 2007;27:4328–4339. doi: 10.1128/MCB.00153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]