Abstract
Phytochemical analysis of Fijian populations of the green alga Tydemania expeditionis led to the isolation of two new unsaturated fatty acids, 3(″)-hydroxy-octadeca-4(E),6(Z),15(Z)-trienoic acid (1) and 3(″)-hydroxy-hexadeca-4(E),6(Z)-dienoic acid (2), along with the known 3(″)-hydroxy-octadeca-4(E),6(Z)-dienoic acid (4). Investigations of the red alga Hydrolithon reinboldii led to identification of a new glycolipid, lithonoside (3), and five known compounds, 15-tricosenoic acid, hexacosa-5,9-dienoic methyl ester, β-sitosterol, 10(S)-hydroxypheophytin A, and 10(R)-hydroxypheophytin A. The structures of 1-3 were elucidated by spectroscopic methods (1D and 2D-NMR spectroscopy and ESI-MS). Compounds 1, 2, and 4, containing conjugated double bonds, demonstrated moderate inhibitory activity against a panel of tumor cell lines (including breast, colon, lung, prostate and ovarian cells) with IC50 values ranging from 1.3 to 14.4 μM. The similar cell selectivity patterns of these three compounds suggest that they might act by a common, but unknown, mechanism of action.
Keywords: alga, cancer, marine, Hydrolithon reinboldii, Tydemania expeditionis, unsaturated fatty acid
1. Introduction
Unsaturated fatty acids are widely distributed in human foods including vegetable oils, meat, milk, and soy products (Jacobsen, 2004), fulfilling important physiological functions. Docosahexaenoic acid and arachidonic acid are important constituents in mammalian cell membranes (Sala-Vila et al., 2006) and are crucial to brain and eye development in human infants (Birch et al., 1997). Consumption of omega-3 and omega-6 fatty acids has been associated with reduced mortality from cardiovascular disease, suppressed arthritis-associated inflammation, and decreased risk of cancer (Simopoulos 2002). Specifically, arachidonic acid was shown to inhibit proliferation of chronic myeloid leukemia cells by inducing apoptotic cell death (Rizzo, 1999). However, the proliferation of prostate cancer cell lines (PC3 and LNCap) was stimulated by arachidonic acid due to the increased formation of 5-HETE, a 5-lipoxygenase product important to prostate cancer cells (Ghosh and Myers, 1998). In contrast, conjugated linoleic acids were reported to inhibit human prostate cancer and to reduce metastases of cancers to lung tissue (Cesano et al., 1998). These fatty acids were also reported to block growth and systemic spread of human breast cancer via mechanisms independent of the host immune system (Visonneau et al., 1998), perhaps by peroxidation of intracellular lipids (Devery et al., 2001).
Glycolipids, which incorporate both fatty acid and carbohydrate moieties, have also been shown to be active against tumor cells. For example, galactolipids with octadecatrienoyl residues were found to possess potent activity against murine leukemia and human colon adenocarcinoma cells (Jung et al., 1996), and nigricanosides A and B, ether-linked glycoglycerolipids, showed antimitotic activity against human breast and colon cancer cells (Williams et al., 2007). Glycolipids also have ecological functions such as herbivore deterrence in the brown seaweed Fucus vesiculosus (Deal et al., 2003) and inhibition of bacterial pathogens in the terrestrial plant Arabidopsis thaliana (Andersson et al., 2006).
Marine algae are a rich source of unsaturated fatty acids (Khotimchenko et al., 2002). During our ongoing effort to search for novel natural products as pharmaceutical leads, extracts of the green alga Tydemania expeditionis and of the red alga Hydrolithon reinboldii were found to be potently cytotoxic in an invertebrate toxicity assay. Herein, we report the isolation and structural elucidation of two novel unsaturated fatty acids (1-2) and a novel glycolipid (3) from these two species, and their inhibitory effects on 11 cancer cell lines.
2. Results and discussion
Guided by a rotifer (invertebrate) toxicity assay, investigation of the ethyl acetate-soluble fraction of T. expeditionis led to the isolation of two new fatty acids, 3(ξ)-hydroxy-octadeca-4(E),6(Z),15(Z)-trienoic acid (1) and 3(ξ)-hydroxy-hexadeca-4(E),6(Z)-dienoic acid (2) , along with the known 3(ξ)-hydroxy-octadeca-4(E),6(Z)-dienoic acid (4). Similarly, investigation of the cytotoxic hexane-soluble fraction of H. reinboldii led to a new glycolipid, lithonoside (3), along with five known compounds, 15-tricosenoic acid, hexacosa-5,9-dienoic acid methyl ester, β-sitosterol, 10 (S)-hydroxypheophytin A, and 10 (R)-hydroxypheophytin A.
Compound 1 was isolated as a colorless oil. HRESIMS analysis indicated a quasimolecular ion [M - H]− at m/z 293.2069, corresponding to a molecular formula of C18H30O3, with four units of unsaturation.
The 1H NMR spectrum of 1 (Table 1) indicated four conjugated olefinic protons at δ 5.62 (1H, dd, J= 6.5, 15.5 Hz, H-4), 6.49 (1H, dd, J= 11, 15.5 Hz, H-5), 5.95 (1H, t, J= 11Hz, H-6) and 5.41 (1H, dt, J= 8.0, 11 Hz, H-7), two non-conjugated olefinic protons at δ 5.35 (1H, m, H-15) and 5.46 (1H, m, H-16), an oxymethine proton at δ 4.10 (1H, dd, J= 6.5, 13 Hz, H-3), a methylene envelope at δ 1.32, and a terminal methyl group at δ 0.95 (3H, t, J= 7.5 Hz, H-18), which suggested a hydroxylated and unsaturated fatty acid skeleton. The optical rotation for 1 was zero, indicating that 1 is likely present as a racemic mixture, consistent with fatty acids previously reported (Stavri et al., 2006). The large coupling constant between H-4 and H-5 (15.5 Hz) indicated that these two protons were trans-oriented. In contrast, the 11 Hz coupling for both Δ6,7 and Δ15,16 indicated they were cis-oriented. Analysis of the 13C NMR and DEPT spectra of 1 revealed six olefinic carbon atoms, a methyl group, an oxymethine, and nine methylenes. The carboxyl group was not seen in the 13C NMR spectrum, but a clear correlation of H-2 at δ 2.25 to a carbonyl at 179.0 ppm was observed in the HMBC spectrum. Therefore, the carbon spectral data further confirmed the skeleton revealed by 1H NMR spectroscopy.
Table 1.
1H and 13C NMR spectral data of 1-2 (in CD3OD)
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δc DEPT | δH (mult., J in Hz) | δc DEPT | δH (mult., J in Hz) | |
| 1 | 179.0, s | 177.0, s | ||
| 2 | 35.3, t | 2.25 (2H, m) | 37.4, t | 2.34 (1H, m) |
| 1.52 (1H, m) | ||||
| 3 | 72.3, t | 4.10 (1H, dd, 6.5, 13) | 72.4, d | 4.06 (1H, dd, 6.5, 13) |
| 4 | 135.7, d | 5.62 (1H, dd, 6.5, 15.5) | 136.3, d | 5.60 (1H, dd, 6.5, 15) |
| 5 | 125.7, d | 6.49 (1H, dd, 11, 15.5) | 125.5, d | 6.49 (1H, dd, 11, 15) |
| 6 | 128.3, d | 5.95 (1H, t, 11) | 128.4, d | 5.96 (1H, t, 11) |
| 7 | 132.1, d | 5.41 (1H, dt, 8.0, 11) | 131.9, d | 5.41 (1H, m) |
| 8 | 27.6, t | 2.18 (2H, m) | 27.6, t | 2.20 (2H, m) |
| 9 | 25.7, t | 1.59 (2H, m) | 25.3, t | 1.60 (1H, m) |
| 1.41 (1H, m) | ||||
| 10 | 29.7, t | 1.32 (2H, m) | 29.8, t | 1.33 (2H, m) |
| 11 | 29.4, t | 1.32 (2H, m) | 29.6, t | 1.33 (2H, m) |
| 12 | 29.3, t | 1.32 21H, m) | 29.5, t | 1.33 21H, m) |
| 13 | 29.2, t | 1.32 (2H, m) | 29.1, t | 1.33 (2H, m) |
| 14 | 20.7, t | 2.05 (2H, m) | 32.0, t | 1.33 (2H, m) |
| 15 | 124.5, d | 5.35 (1H, m) | 22.7, t | 1.33 (2H, m) |
| 16 | 133.6, d | 5.46 (1H, m) | 13.4, t | 0.91 (3H, t, 7.5) |
| 17 | 20.7, t | 2.05 (2H, m) | ||
| 18 | 13.5, q | 0.95 (3H, t, 7.5) | ||
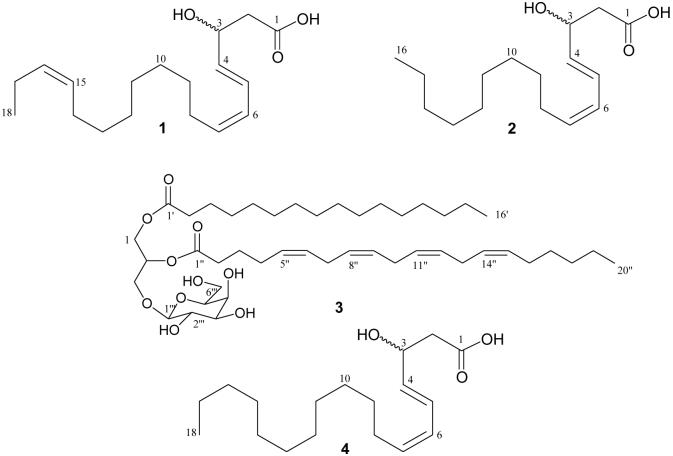
The full NMR assignments and connectivities of 1 were determined by 1H -1H COSY, HSQC, and HMBC spectroscopic data analysis. Some key HMBC correlations are shown in Fig. 1. The proton at δ 4.10 (H-3), attached to a carbon at δ 72.3, showed HMBC correlations to C-2, C-4, and C-5, indicating the location of the oxymethine at C-3. In the 1H -1H COSY spectrum, H-5 showed cross-peaks with H-6 and H-7, and H-8 was correlated to H-7 as well as to the methylene envelope at H-9—H-13. The location of the third double bond at C-15 and C-16 was revealed by the following HMBC correlations: H-18 → C-17 (δ 20.7) and C-16 (δ 133.6), and H-17→ C-16 and C-15 (δ 124.5). Accordingly, the structure of compound 1 was established to be 3(ξ)-hydroxy-octadeca-4(E),6(Z),15(Z)-trienoic acid.
Fig. 1.
Key HMBC correlations for 1.
Compound 2 was also isolated as a colorless oil. The HRESIMS analysis indicated a quasimolecular ion [M - H]− at m/z 267.1966, corresponding to a molecular formula of C16H28O3, possessing three units of unsaturation. Compared to the one separated and two conjugated double bonds of 1, the 1H NMR spectral data of 2 only suggested two conjugated double bonds, with olefinic proton signals at δ 5.60 (1H, dd, J= 6.5, 15 Hz, H-4), 6.49 (1H, dd, J= 11, 15 Hz H-5), 5.96 (1H, t, J= 11 Hz, H-6) and 5.41 (1H, m, H-7), which were confirmed by the four olefinic carbon signals at δ 136.3 (C-4), 125.5 (C-5), 128.4 (C-6) and 131.9 (C-7). The other signals were similar to those of 1. As with 1, the carboxyl group was not visible in the 13C NMR spectrum, but was revealed in the HMBC spectrum by a clear correlation of H-2 at δ 2.34 to a carbon at 177.0 ppm. Accordingly, the structure of compound 2 was established to be 3(ξ)-hydroxy-hexadeca-4(E),6(Z)-dienoic acid.
Compound 3 was isolated as a white powder from the cytotoxic hexane-soluble extract of the coralline red alga H. reinboldii. HRESIMS analysis indicated a quasimolecular ion [M + Na]+ at m/z 801.5503, corresponding to a molecular formula of C45H78O10. The 1H NMR spectrum (Table 2) showed four geminal protons attached to carbon atoms bearing an oxygen functionality at δ 4.38 (dd, J= 3.5, 12 Hz, H-1a), 4.19 (dd, J= 6.5, 12 Hz, H-1b), 3.73 (dd, J= 6.5, 11Hz, H-3a), and 3.88 (dd, J= 5.5, 11 Hz, H-3b) and a methine at 5.30 (1H, m, H-2), indicating the presence of a glycerol moiety, which was also confirmed by 13C and DEPT signals at δ 63.4 (t, C-1), 70.5 (d, C-2) and 68.8 (t, C-3).
Table 2.
1H and 13C NMR spectral data of 3 (in CDCl3)
| position | δH (mult., J in Hz) | δc DEPT | position | δH (mult., J in Hz) | δc DEPT |
|---|---|---|---|---|---|
| 1 | 4.38 (1H, dd, 3.5, 12) | 63.4, t | 10″ | 2.80 (2H, m) | 25.3, t |
| 4.19 (1H, dd, 6.5, 12) | |||||
| 2 | 5.30 (1H, m) | 70.5, d | 11″ | 5.35 (1H, m) | 128.7, d |
| 3 | 3.88 (1H, dd, 5.5, 11) | 68.8, t | 12″ | 5.35 (1H, m) | 128.5, d |
| 3.73 (1H, dd, 6.5, 11) | |||||
| 1′ | 175.0, s | 13″ | 2.80 (2H, m) | 25.1, t | |
| 2′ | 2.05 (2H, m) | 34.5, t | 14″ | 5.35 (1H, m) | 127.9, d |
| 3′ | 1.66 (2H, m) | 26.0, t | 15″ | 5.35 (1H, m) | 130.9, d |
| 4′-14′ | 1.2-1.3 (22H, m) | 29.5-30.1, t | 16″ | 2.01 (2H, m) | 27.6, t |
| 15′ | 1.34 (2H, m) | 23.0, t | 17″ | 1.67 (2H, m) | 30.6, t |
| 16′ | 0.85 (3H, t, 7) | 14.5, q | 18″ | 1.27 (2H, m) | 32.3, t |
| 1″ | 174.0, s | 19″ | 1.36 (2H, m) | 23.1, t | |
| 2″ | 2.31 (2H, m) | 34.7, t | 20″ | 0.87 (3H, t, 7) | 14.5, q |
| 3″ | 1.69 (2H, m) | 33.9, t | 1‴ | 4.26 (1H, d, 7.5) | 104.4, d |
| 4″ | 2.09 (2H, m) | 26.9, t | 2‴ | 3.63 (1H, m) | 72.1, d |
| 5″ | 5.35 (1H, m) | 129.4, d | 3‴ | 3.99 (1H, m) | 69.9, d |
| 6″ | 5.35 (1H, m) | 128.2, d | 4‴ | 3.53 (1H, m) | 74.9, d |
| 7″ | 2.80 (2H, m) | 25.4, t | 5‴ | 3.59 (1H, m) | 73.8, d |
| 8″ | 5.35 (1H, m) | 129.2, d | 6‴ | 3.96 (1H, m) | 63.2, d |
| 3.84 (1H, m) | |||||
| 9″ | 5.35 (1H, m) | 129.0, d |
The appearance of a triplet at δ 0.87 (J = 7.0 Hz, H-20″) in the 1H NMR spectrum of 3 was assigned to a terminal methyl of a fatty acid chain, and the multiplets at δ 2.31 (H-2″), 1.69 (H-3″) and 2.09 (H-4″) were due to the α, β and γ-methylenes, respectively, linked to the ester carbonyl functionality. These signals, together with the broad multiplet at δ 2.80 arising from allylic methylene protons and olefinic methine proton signals overlapping at δ 5.35, were assigned to an unsaturated acyl fatty chain. The eight carbon signals in the low-field region (δ 129.4, 128.2, 129.2, 129.0, 128.7, 128.5, 127.9 and 130.9) indicated four separated double bonds in this chain. The small coupling constants (< 11 Hz) revealed by the homonuclear J-spectrum (Derome, 1987; Sánchez-Sampedro et al., 2007) suggested a Z-configuration for all double bonds. Similarly, a triplet at δ 0.85 (J = 7.0 Hz, H-16′) belonging to the terminal methyl group, broad methylene signals at δ 1.20-1.30 due to (CH2)n of the aliphatic chain, and a multiplet at δ 2.05 (H-2′) associated with the α-methylene linked to the ester carbonyl functionality, were attributed to a saturated fatty acyl chain. The presence of two acyl fatty acids was reinforced by the MS/MS sodium adduct fragments m/z 497, which represented loss of eicosatetraenoic acid, and m/z 545, which represented loss of palmitic acid. Thus, the two chains were concluded to consist of eicosatetraenoyl and palmitoyl moieties. In the HMBC spectrum, the glycerol methylene proton at δ 4.19 (H-1b) was correlated to the ester carbonyl at δ 175.0 (C-1′), indicating linkage of the palmitoyl moiety to the glycerol portion at C-1, and the eicosatetraenoyl moiety was inferred to be connected at C-2.
The existence of a galactopyranosyl moiety was inferred from the anomeric protons at δ 4.26 (d, J = 7.5 Hz, H-1‴), and confirmed by the presence of a set of galactose carbon signals at δ 104.4 (d, C-1‴), 72.1 (d, C-2‴), 69.9 (d, C-3‴), 74.9 (d, C-4‴), 73.8(d, C-5‴), and 63.2 (d, C-2‴). The 7.5 Hz coupling of the anomeric proton supported the β-configuration for this sugar unit. In the HMBC spectrum, this anomeric proton was correlated to C-3 at δ 66.8, indicating the linkage of this sugar to the glycerol moiety.
The structure of 3 is reminiscent of galactolipids which have been found in both terrestrial plants (Cateni et al., 2003; Jung et al., 1996) and marine algae (Shao at al., 2002; Deal et al., 2003). The most closely related structure to 3 was 1-O-(palmitoyl)-2-O-(5,8,11,14,17-eicosapentaenoyl)-3-O-β-D-galactopyranosyl-glycerol, identified by spectral analysis of a glycolipid mixture from the red alga Chondria armata, with one trans and four cis double bonds in the acyl fatty chain (Al-Fadhli et al., 2006). Thus, compound 3 is a new galactosyl glycolipid identified to be 1-O-(palmitoyl)-2-O-(5Z, 8Z, 11Z, 14Z-eicosatetraenoyl)-3-O-β-D-galactopyranosyl-glycerol, and was accorded the trivial name lithonoside.
The structures of three known fatty acids, 3(ζ)-hydroxy-octadeca-4(E),6(Z)-dienoic acid (4) (Stavri et al., 2006), 15-tricosenoic acid (Carballeira et al., 1998), and hexacosa-5,9-dienoic methyl ester (Lam et al., 1989), and three non-fatty acids, β-sitosterol (Shang et al., 2000), 10(S)-hydroxypheophytin A (Nakatani et al., 1981), and 10(R)-hydroxypheophytin A (Nakatani et al., 1981), were identified by comparing their spectroscopic data with those of reported values.
Fatty acids 1, 2, and 4, containing conjugated double bonds, demonstrated modestly potent inhibitory activity against a panel of 12 breast, colon, lung, prostate, and ovarian tumor cells, with IC50 values in the ranges 1.3 to 14.4 μM (Fig. 2). Close examination of their cell selectivity patterns (Fig. 2) showed that all three compounds were more active against SHP-77 (lung), HCT-116 (colon), A2780/DDP-S (ovarian), and PC-3 and Du145 (both prostate) cell lines than against CCRF-CEM (leukemia), LNCap-FGCM (prostate), and MDA-MB-468 and MDA-MB-231(both breast) lines. The similar selectivity patterns of these compounds suggest they might act by a common mechanism of action. In contrast, galactolipid 3 showed more modest activity with a mean IC50 value of 19.8 μM, and 15-tricosenoic acid and hexacosa-5,9-dienoic methyl ester, with only separated double bonds, were inactive with mean IC50 values larger than 60 μM (individual cell line data not shown). In contrast to the antineoplastic activity, 3-4 demonstrated weak antimalarial activity (with IC50 values of 72 and 77 μM, respectively). All other compounds tested had IC50 values larger than 90 μM (data not shown).
Figure 2.
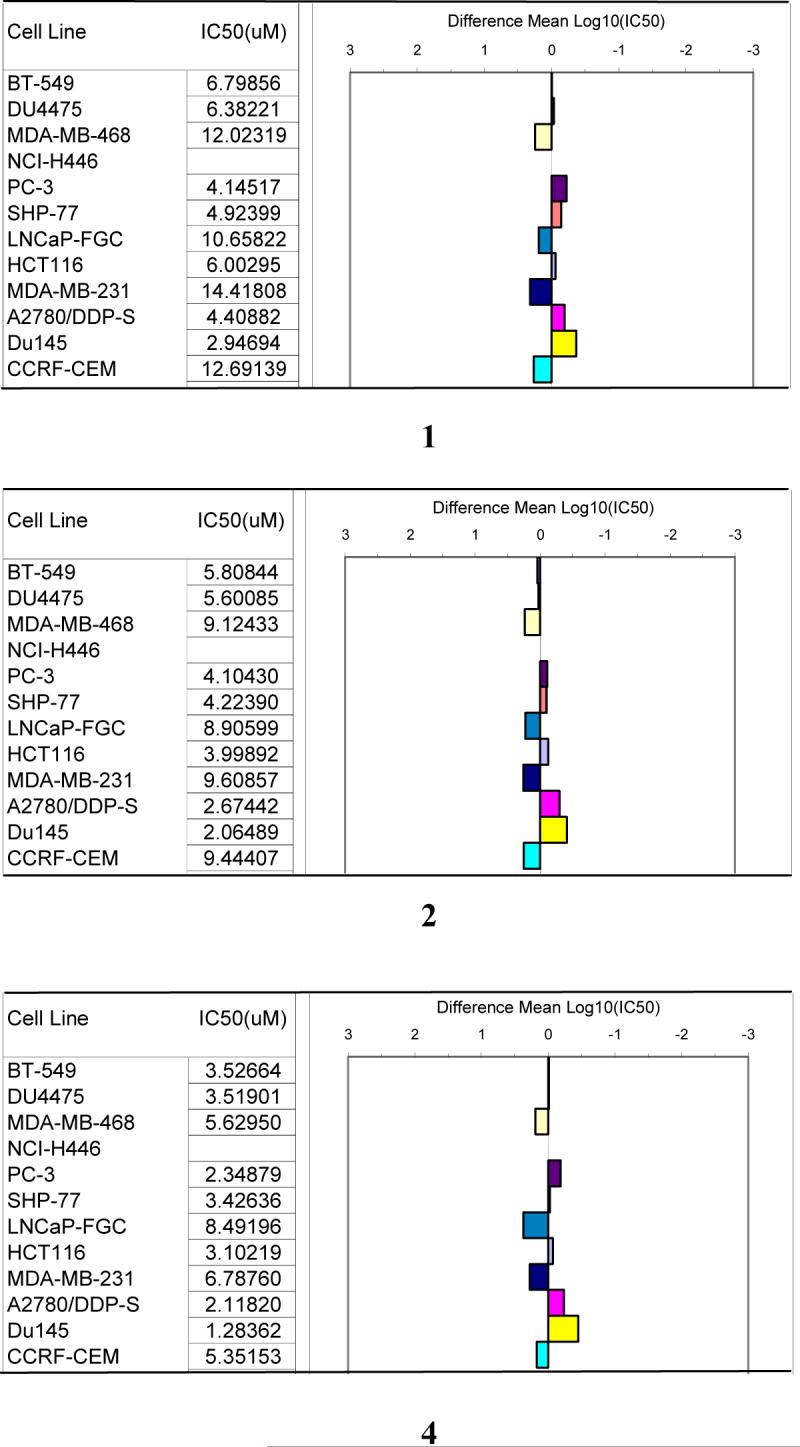
Cancer cell line selectivity patterns of 1, 2, and 4
3. Conclusions
From the green alga Tydemania expeditionis, we identified two novel antineoplastic fatty acids (1-2) as well as one structurally-related, previously-known fatty acid (4). Lithonoside (3), a novel glycolipid, is the first natural product reported from the coralline red algal genus Hydrolithon, possessing modest antineoplastic activity. Compounds 1, 2, and 4, containing conjugated double bonds and a hydroxyl group at C-3, demonstrated moderate inhibitory activity against a panel of tumor cell lines with IC50 values ranging from 1.3 to 14.4 μM, considerably more potent than conjugated linoleic acids (Ochoa, et al., 2004; Kim, et al., 2005). In constrast, 3 with a mean antineoplastic IC50 value of 19.8 μM, was less potent than recently discovered ether-linked glycoglycerolipids, e.g., nigricanosides A and B (Williams, et al., 2007).
4. Experimental
4.1 General
UV spectra were recorded with online HPLC-DAD. Optical rotations were measured on a Rudolph automatic polarimeter. NMR spectra (1H, 13C, DEPT, COSY, HSQC, and HMBC) were acquired on a Bruker DRX-500 instrument using a 5 mm broadband probe, and were referenced to the residual light solvent (δ 7.24 and 77.0 for chloroform; δ 3.31 and 49.0 for methanol). High-resolution mass spectra were generated by electrospray ionization with an Applied Biosystems QSTAR-XL hybrid quadrupole-time-of-flight tandem mass spectrometer and Analyst QS software. All commercial chemicals were reagent grade, except for the HPLC and Optima grade solvents used for HPLC and LC-MS, respectively (Fisher Scientific Co.). NMR solvents were purchased from Cambridge Isotope Laboratories.
4.2 Plant materials
T. expeditionis was collected at 7-26 m depth at Herald Pass, Kadavu Province, Fiji (18° 46.370' S, 178° 27.746' E) and was frozen at −20 °C until extraction (collection G-2004-06-45). Collection of H. reinboldii was made at 2 m depth in Denny's reef, Rewa Province, Fiji (18° 10.038' S, 178° 25.211' E); this plant was immediately immersed in methanol (collection G-2004-06-13). Specimens were identified by Dr. Posa Skelton at University of South Pacific, by comparison with morphological traits previously described (Littler and Littler, 2003), stored in aqueous formaldehyde, and deposited at the University of the South Pacific.
4.3 Rotifer (invertebrate) toxicity assay
The experiments were performed using procedures described previously (Snell, 2005).
4.4 Extraction and isolation guided by rotifer toxicity assay
The whole T. expeditionis plant (500 g, wet mass) was exhaustively extracted with MeOH and MeOH-CH2Cl2 (1:1). The solutions were combined, filtered, and concentrated under reduced pressure to afford a crude extract (17.9 g, yield 3.6% of wet mass), which was then partitioned between methanol/water (9:1) and hexane. Next, the aqueous fraction was rotary evaporated to remove methanol, and successively partitioned against ethyl acetate and n-butanol to eventually result in four fractions (hexane-, ethyl acetate-, butanol- and water-soluble). The bioactive ethyl acetate-soluble fraction was further separated by semi-preparative HPLC to afford compounds 1 (4.6 mg), 2 (3.2 mg), and 4 (5.5 mg). Purification of these compounds was performed on a Waters 2695 system equipped with 2996 diode-array UV detection and an Agilent Zorbax SB-C18 reversed-phase column (5 μm, 9.4 × 250 mm) with a gradient mobile phase (A: water, B: acetonitrile): 0-53 min, 10→70% B; 53-60 min: 70→100% B; and 60-63 min, 100→10% B; flow rate 3 ml/min and detection wavelengths 215 and 235 nm.
The methanol crude extract (2.0 g) of H. reinboldii was successively partitioned as described above to afford hexane- (0.193 g), ethyl acetate- (0.067 g), butanol- (0.222 g), and water-soluble fractions (1.088 g). The hexane-soluble fraction showed the most potent cytotoxicity, and so further fractionation was carried out (on 60 mg) by semi-preparative reversed-phase HPLC, affording lithonoside (3) (2.3 mg), 15-tricosenoic acid (1.0 mg), hexacosa-5,9-dienoic methyl ester (3.3 mg), β-sitosterol (5.6 mg), 10(S)-hydroxypheophytin A (1.3 mg), and 10(R)-hydroxypheophytin A (1.2 mg) respectively. Purification of these compounds was performed on the same system as above but an Alltech Alltima-C18 column (5 μm, 10 × 250 mm) with a gradient mobile phase.(A: water/methanol/ethyl acetate 20:65:15; B: water/methanol/ethyl acetate 10:30:60 ): 0-15 min, 60% B; 15-20 min: 60→100% B; and 20-35 min, 100% B; 35-40 min 100→60% B; flow rate 3 ml/min and detection wavelengths 215 and 265 nm.
3(ζ)-Hydroxy-octadeca-4(E),6(Z),15(Z)-trienoic acid (1)
Colorless oil; UV (acetonitrile/H2O) λmax 236 nm; 1H and 13C NMR data, see Table 1; ESI-MS m/z 293 [M-H]−, 275 [M-H-H2O]−; HRESI-MS m/z 293.2069M.[M-H]−; required 293.2117.
3(ζ)-Hydroxy-hexadeca-4(E),6(Z)-dienoic acid (2)
Colorless oil; UV (acetonitrile/H2O) λmax 234 nm; 1H and 13C NMR data, see Table 1; ESI-MS m/z 267 [M-H]−, 249 [M-H-H2O]−; HRESI-MS m/z 267.1966 [M-H]−; required 267.1960.
Lithonoside (3)
Colorless powder; [α]20D −66.0° (c 0.03 in CH3OH); 1H and 13C NMR data, see Table 2; ESI-MS/MS m/z 497 [M-C20H32O2+Na]+, m/z 545 [M-C16H32O2+Na]+, 801 [M+Na]+, HRESI-MS m/z 801.5499 [M+Na]+; required 801.5493.
4.5 Cancer cell growth assay
Natural products were tested against a panel of tumor cell lines: breast cancer (BT-549, DU4475, MDA-MB-468, MDA-MB-231), colon cancer (HCT116), lung cancer (NCI-H446 and SHP-77), prostate cancer (PC-3, LNcap-FGC, Du145), ovarian cancer (A2780/DDP-S) and leukemia (CCRF-CEM). The cells were seeded in 96-well plates and compounds added 24 h later. After 72 h of exposure, the cells were stained with (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS), leading to a reduced product from live cell metabolism, which was quantified spectrophotometrically (Lee et al., 2001). Dose response curves were generated and IC50 values calculated for each compound and cell line. A mean IC50 value was determined for the 12 cell line panel and converted to a log value, then the log of each individual IC50 value was subtracted from the mean log IC50 and that difference was plotted. Bars projecting to the right indicate cell lines more sensitive than the mean, whereas bars projecting to the left indicate cell lines less sensitive than the mean.
4.6 Malarial parasite growth inhibition assay
Antimalarial activity was tested using a SYBR Green-based whole-parasite proliferation assay, adapted from Smilkstein et al. (2004) and Bennett et al. (2004), as described by Lane et al. (2007). Plasmodium falciparum (3D7 strain MR4/ATCC, Manassas, VA) were cultured in human O+ erythrocytes as previously described (Trager and Jensen, 1976).
Acknowledgment
This research was supported by the U.S. National Institutes of Health's International Cooperative Biodiversity Groups program (grant U01-TW007401-01). Thanks are given to the Roko Tui Kadavu and Tui Dravuni, and to the Roko Tui Dreketi and Tui Suva, for their hospitality and for permission to collect in the reefs of Kadavu and Rewa Provinces, respectively. We are grateful to the government of the Fiji Islands for permission to export samples. The authors thank T. Myers, E. P. Stout, L. Mylacraine, M. Sharma, K. Feussner, R. Peterson, and K. Johnston for technical support, Dr. P. Skelton for identifying macroalgae, and T. Myers for helpful comments regarding the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Fadhli A, Wahidulla S, D'Souza L. Glycolipids from the red alga Chondria armata (Kütz.) Okamura. Glycobiology. 2006;16:902–915. doi: 10.1093/glycob/cwl018. [DOI] [PubMed] [Google Scholar]
- Andersson MX, Hamberg M, Kourtchenko O, Brunnstroem A, McPhail KL, Gerwick WH, Goebel C, Feussner I, Ellerstroem M. Oxylipin profiling of the hypersensitive response in Arabidopsis thaliana. Formation of a novel oxophytodienoic acid-containing galactolipid, arabidopside. E. J. Biol. Chem. 2006;281:31528–31537. doi: 10.1074/jbc.M604820200. [DOI] [PubMed] [Google Scholar]
- Bennett TN, Paguio M, Gligorijevic B, Seudieu C, Kosar AD, Davidson E, Roepe PD. Novel, rapid, and inexpensive cell-based quantification of antimalarial drug efficacy. Antimicrob. Agents Chemother. 2004;48:1807–1810. doi: 10.1128/AAC.48.5.1807-1810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EB, Birch D, Uauy R. Visual function and the essentiality of α-linolenic acid and docosahexaenoic acid in human infants. In: Yehuda S, Mostofsky DI, editors. Hand book of essential fatty acid biology. Human press; Totowa, New Jersey: 1997. pp. 183–199. [Google Scholar]
- Carballeira NM, Pagan M, Rodriguez AD. Identification and total synthesis of novel fatty acids from the Caribbean sponge Calyx podatypa. J. Nat. Prod. 1998;61:1049–1052. doi: 10.1021/np9801413. [DOI] [PubMed] [Google Scholar]
- Cateni F, Falsone G, Žilic J. Terpenoids and glycolipids from Euphorbiaceae. Mini Rev. Med. Chem. 2003;3:425–437. doi: 10.2174/1389557033487971. [DOI] [PubMed] [Google Scholar]
- Cesano A, Visonneau S, Scimeca JA, Kritchevsky D, Santoli D. Opposite effects of linoleic acid and conjugated linoleic acid on human prostatic cancer in SCID mice. Anticancer res. 1998;18:1429–34. [PubMed] [Google Scholar]
- Deal MS, Hay ME, Wilson D, Fenical W. Galactolipids rather than phlorotannins as herbivore deterrents in the brown seaweed Fucus vesiculosus. Oecologia. 2003;136:107–114. doi: 10.1007/s00442-003-1242-3. [DOI] [PubMed] [Google Scholar]
- Derome AE. Modern NMR techniques for chemistry research. Pergamon press; 1987. pp. 270–275. [Google Scholar]
- Devery R, Miller A, Stanton C. Conjugated linoleic acid and oxidative behavior in cancer cells. Biochem. Soc. Trans. 2001;29:341–344. doi: 10.1042/0300-5127:0290341. [DOI] [PubMed] [Google Scholar]
- Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc. Natl. Acad. Sci. USA. 1998;95:13182–13187. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen C. Developing polyunsaturated fatty acids as functional ingredients. In: Arnoldi A, editor. Functional foods, cardiovascular disease and diabetes. CRC Press; Cambridge, England: Woodhead Pub.: 2004. pp. 307–332. [Google Scholar]
- Jung JH, Lee H, Kang SS. Diacylglycerylgalactosides from Arisaema amurense. Phytochemistry. 1996;42:447–452. doi: 10.1016/0031-9422(95)00929-9. [DOI] [PubMed] [Google Scholar]
- Khotimchenko SV, Vaskovsky VE, Titlyanova TV. Fatty acids of marine algae from the Pacific coast of North California. Botanica Marina. 2002;45:17–22. [Google Scholar]
- Kim JH, Hubbard NE, Ziboh V, Erickson KL. Attenuation of breast tumor cell growth by conjugated linoleic acid via inhibition of 5-lipoxygenase activating protein. Biochim. Biophys. Acta. 2005;1736:244–250. doi: 10.1016/j.bbalip.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Lam WK, Hahn S, Ayanoglu E, Djerassi C. Phospholipid studies of marine organisms. 22. Structure and biosynthesis of a novel brominated fatty acid from a hymeniacidonid sponge. J. Org. Chem. 1989;54:3428–32. [Google Scholar]
- Lane AL, Stout EP, Hay ME, Prusak AC, Hardcastle K, Fairchild CR, Franzblau SG, Roch KL, Prudhomme J, Aalbersberg W, Kubanek J. Callophycoic acids and callophycols from the Fijian red alga Callophycus serratus. J. Org. Chem. 2007;72:7343–7351. doi: 10.1021/jo071210y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FYF, Borzilleri R, Fairchild CR, Kim SH, Long BH, Raventos-Suarez C, Vite GD, Rose WC, Kramer RA. BMS-247550: A novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin. Cancer Res. 2001;7:1429–1437. [PubMed] [Google Scholar]
- Littler DS, Littler MM. South Pacific Reef Plants. Offshore Graphics, Inc.; Washington, D.C.: 2003. pp. 46–47.pp. 258–259. [Google Scholar]
- Nakatani Y, Ourisson G, Beck JP. Chemistry and biochemistry of Chinese drugs.VII. Cytostatic pheophytins from silkworm excreta, and derived photocytotoxic pheophorbides. Chem. Pharm. Bull. 1981;29:2261–2269. doi: 10.1248/cpb.29.2261. [DOI] [PubMed] [Google Scholar]
- Ochoa JJ, Farquharson AJ, Grant I, Moffat LE, Heys SD, Wahle KWJ. Conjugated linoleic acids (CLAs) decrease prostate cancer cell proliferation: different molecular mechanisms for cis-9, trans-11 and trans-10, cis-12 isomers. Carcinogenesis. 2004;25:1185–1191. doi: 10.1093/carcin/bgh116. [DOI] [PubMed] [Google Scholar]
- Rizzo MT, Regazzi E, Garau D, Akard L, Dugan M, Boswell HS, Rizzoli V, Carlo-Stella C. Induction of apoptosis by arachidonic acid in chronic myeloid leukemia cells. Cancer Res. 1999;59:5047–5053. [PubMed] [Google Scholar]
- Sala-Vila A, Campoy C, Castellote AI, Garrido FJ, Rivero M, Rodriguez-Palmero M, Lopez-Sabater MC. Influence of dietary source of docosahexaenoic and arachidonic acids on their incorporation into membrane phospholipids of red blood cells in term infants. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2006;74:143–148. doi: 10.1016/j.plefa.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sánchez-Sampedro A, Kim HK, Choi YH, Verpoorte R, Corchete P. Metabolomic alterations in elicitor treated Silybum marianum suspension cultures monitored by nuclear magnetic resonance spectroscopy. J. Biotechnol. 2007;130:133–142. doi: 10.1016/j.jbiotec.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Shang M, Li J, Cai S, Li P, Xu L, Xu G. Chemical constituents of the fruit of Sinopodophyllum emodi. Zhongcaoyao. 2000;31:569–571. [Google Scholar]
- Shao ZY, Cai JN, Ye QZ, Guo YW. Crassicaulisine, a new sulphonoglycolipid from the red alga Chondria crassicaulis Harv. J. Asian Nat. Prod. Res. 2002;4:205–209. doi: 10.1080/10286020290024004. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine & Pharmacotherapy. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell TW. In: In Small-Scale Freshwater Environment Toxicity Test Methods. Blaise C, Ferard JF, editors. Kluwer; Dordrecht: 2005. [Google Scholar]
- Stavri M, Mathew KT, Gibbons S. Antimicrobial constituents of Scrophularia deserti. Phytochemistry. 2006;67:1530–1533. doi: 10.1016/j.phytochem.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Visonneau S, Cesano A, Tepper SA, Scimeca JA, Santoli D, Kritchevsky D. Conjugated linoleic acid suppresses the growth of human breast adenocarcinoma cells in SCID mice. Anticancer Res. 1997;17:969–973. [PubMed] [Google Scholar]
- Williams DE, Sturgeon CM, Roberge M, Andersen RJ. Nigricanosides A and B, Antimitotic glycolipids isolated from the green alga Avrainvillea nigricans collected in Dominica. J. Am. Chem. Soc. 2007;129:5822–5823. doi: 10.1021/ja0715187. [DOI] [PubMed] [Google Scholar]