Abstract
The first total synthesis for the sponge derived (5Z,9Z)-(±)-2-methoxy-5,9-octadecadienoic acid, an analog of taxoleic acid, was accomplished in seven steps and in a 10% overall yield. It was again corroborated that the best strategy to prepare these cis,cis dimethylene interrupted double bonds is the double-alkyne bromide coupling reaction of 1,5-hexadiyne, which provides the advantage of achieving a 100% cis stereochemical purity for both double bonds after hydrogenation under Lindlar conditions. The α-methoxy functionality was best prepared via the Mukaiyama reaction of (4Z,8Z)-heptadecadienal with trimethylsilyl cyanide and triethylamine followed by acid hydrolysis. Selective methylation of the hydroxyl group of (5Z,9Z)-(±)-2-hydroxy-5,9-octadecadienoic acid was achieved with sodium hydride/methyl iodide when tetrahydrofuran was used as solvent. Complete spectral data is presented, for the first time, for this unusual marine α-methoxylated fatty acid.
Keywords: Dimethylene interrupted fatty acids; Methoxylated fatty acids; 5,9-Octadecadienoic acids; Synthesis; Sponges
I. Introduction
The fatty acid 5,9-octadecadienoic acid (taxoleic acid) has been known for over 45 years but its biophysical and/or biological properties have been less studied than the more ubiquitous and more studied 9,12-octadecadienoic acid (linoleic acid). The 18:2Δ5,9 acid was first identified in the cellular slime mold Dictyostelium discoideum (Davidoff and Korn, 1963). However, it is particularly abundant in the triacylglycerols of seed oils (Lísa et al., 2007) such as from Teucrium depressum, Gingko biloba (Hierro et al., 1996), and particularly in conifer seed oils arising from Taxus baccata at concentrations as high as 12% (Madrigal and Smith, 1975). This interesting dimethylene-interrupted fatty acid has also been isolated from the phospholipids of marine organisms such as from the sponges Agelas sp. and Spongia tampa, where it occupies both the sn-1 and sn-2 positions in phosphatidylethanolamines, as well as from the anemone Stoichactis helianthus (Carballeira et al., 1994; Carballeira and Medina, 1994). The 18:2Δ5,9 acid has also been postulated as the biosynthetic precursor of the (Z,Z)-4,8-heptadecadiene, a possible alarm pheromone in the astigmatid mite Tortonia sp. (Kuwahara et al., 1995).
More recently, an α-methoxylated analog of the 18:2 Δ5,9 acid, namely the (5Z,9Z)-(±)-2-methoxy-5,9-octadecadienoic acid (1) was identified in the phospholipids of the Caribbean sponge Erylus goffrilleri, together with other analogs of longer chain lengths (Carballeira et al., 2007). Due to the low natural abundance of this methoxylated fatty acid in the sponge (ca. 0.2%) it was not possible to study its biophysical and biological properties, which remain speculative. Therefore, a practical synthetic approach towards this novel type of fatty acids would certainly accelerate the biological study of 1 and that of other similar analogs. In addition, a synthetic sample will help corroborate the structural assignment of the natural fatty acid and will facilitate the report, for the first time, of the complete spectral data so as to lay the foundation for the structural characterization of other similar analogs. These α-methoxylated fatty acids are known to have the R absolute configuration at the C-2 stereogenic center, but the absolute configuration of the naturally occurring title compound was not determined (Carballeira et al., 2007). Therefore, herein we report the first total synthesis for racemic 1, which was accomplished in seven steps and in a 10% overall yield.
2. Materials and methods
2.1 Instrumentation
1H NMR (300 MHz) and 13C NMR (75 MHz) were recorded on a Bruker DPX-300 spectrometer. 1H NMR chemical shifts are reported with respect to internal (CH3)4Si, 13C NMR chemical shifts are reported in parts per million relative to CDCl3 (77.0 ppm). GC/MS analyses were recorded at 70 eV using a Hewlett Packard 5972A MS ChemStation equipped with a 30 m × 0.25 mm special performance capillary column (HP-5MS) of polymethyl siloxane crosslinked with 5% phenyl methylpolysiloxane. IR spectra were recorded on a Nicolet 600 FT-IR spectrophotometer (Thermo-Nicolet, Madison, WI, USA). High resolution mass spectral data was performed at the Emory University Mass Spectrometry Center on a thermo LTQ-FTMS using flow injection analysis.
2.2 2-(3,7-Octadiynyl)-1,3-dioxolane (2)
Into a 50 mL round-bottomed flask at −78 °C containing dry THF (8 mL) was added 1,5-hexadiyne (50% in pentane, 1 mL, 5.1 mmol) followed by 2.5 M n-BuLi (1.86 mL, 4.6 mmol). The mixture was stirred at −78°C for 40 min, and then at rt for 15 min. The temperature was lowered to -70 °C and HMPA (3.57 mL, 20.5 mmol) (Caution! HMPA is toxic and safety precautions should be taken when handling this solvent) was added followed by the addition of 2-(2-bromoethyl)-1,3-dioxolane (0.55 mL, 4.6 mmol). The mixture was stirred for 24h and then washed with water (2 × 15 mL), extracted with ether (2 × 15 mL) and dried over Na2SO4, affording 0.6 g of 2 (73 % yield) as a colorless oil after purification by distillation (Kugelrohr) at 160 °C/3 mm Hg. 1H NMR (CDCl3, 300 MHz) δ: 4.97 (1H, t, J = 4.7 Hz, H-2), 3.95-3.85 (4H, m, -OCH2-), 2.35-2.27 (4H, m, -CH2-), 1.99 (1H, brs, H-8’), 1.81 (2H, m, -CH2-); 13C NMR (CDCl3, 75 MHz) δ: 103.3 (d), 83.0 (s), 80.2 (s), 78.5 (s), 69.0 (d, C-8), 64.9 (t), 33.1 (t), 19.0 (t), 18.8 (t), 13.7 (t); GC/MS (70 eV) m/z (relative intensity) 177 (M+-1, 1), 139 (14), 105 (3), 91 (8), 86 (6), 79 (4), 77 (6), 74 (4), 73 (100), 67 (5), 65 (8), 55 (2). HRMS (APCI) Calcd for C11H14O2 [M+H]+ 179.1066, found 179.1063.
2.3 2-(3,7-Hexadecadiynyl)-1,3-dioxolane (3)
To a solution of 2 (0.49 g, 2.7 mmol) in dry THF (8 mL) at −10 °C was added n-BuLi (2.5 M, 3 mL, 7.5 mmol). The reaction was stirred at 0 °C for 45 min. The temperature was lowered to −78 °C and HMPA (4 mL, 22.8 mmol) (Caution! HMPA is toxic and safety precautions should be taken when handling this solvent) was added followed by 1-bromooctane (0.57 mL, 3.3 mmol). The reaction mixture was stirred for 24h before washing with water (2 × 15 mL), extracting with ether (2 × 15 mL) and drying over Na2SO4, affording 0.51 g (65 % yield) of 3 as a colorless oil. 1H NMR (CDCl3, 300 MHz) δ: 4.97 (1H, t, J = 4.7 Hz, H-2), 3.95-3.85 (4H, m, -OCH2-), 2.32 (4H, m, -CH2-), 2.29 (2H, m, -CH2-), 2.13 (2H, brt, J = 6.3 Hz), 1.83 (2H, m, -CH2-), 1.45 (2H, m, -CH2-), 1.35 (2H, m, -CH2-), 1.25 (10H, brs, -CH2-), 0.85 (3H, t, J = 6.4 Hz, -CH3); 13C NMR (CDCl3, 75 MHz) δ: 103.3 (d, C-2), 81.2 (s), 79.8 (s), 79.1 (s), 78.6 (s), 64.9 (t), 33.2 (t), 31.8 (t), 29.2 (t), 29.1 (t), 29.0 (t), 28.8 (t), 22.7 (t), 19.5 (t), 19.4 (t), 18.7 (t), 14.1 (q), 13.7 (t); GC/MS (70 eV) m/z (relative intensity) 290 (M+, 1), 289 (1), 262 (2), 191 (5), 177 (9), 139 (38), 133 (7), 131 (4), 119 (9), 117 (9), 115 (5), 105 (10), 99 (6), 95 (6), 91 (20), 86 (9), 81 (5), 79 (11), 77 (11), 73 (100), 67 (14), 65 (11), 57 (5), 55 (12). HRMS (APCI) Calcd for C19H30O2 [M+H]+ 291.2318, found 291.2317.
2.4 2-[(3Z,7Z)-3,7-Hexadecadienyl]-1,3-dioxolane (4)
Into a two necked round-bottomed flask containing Lindlar’s catalyst (0.52 g) (Fluka) were added a solution of 3 (0.2 g, 0.7 mmol) in hexane (3 mL) and quinoline (cat). The mixture was stirred under a hydrogen atmosphere for 24h. The reaction mixture was filtered and the quinoline was removed by distillation at 130 °C/ 3 mm Hg. The reaction afforded 0.14 g of 4 (70 % yield) as a colorless oil and 100% stereopurity as confirmed by capillary gas chromatography. 1H NMR (CDCl3, 300 MHz) δ: 5.43-5.33 (4H, m, -CH=CH-) 4.86 (1H, t, J = 4.9 Hz, H-2), 3.99-3.80 (4H, m, -OCH2-), 2.16 (2H, m), 2.08 (2H, m), 2.01 (4H, m), 1.71 (2H, m), 1.25 (12H, brs, -CH2-), 0.88 (3H, t, J = 6.2 Hz, -CH3); 13C NMR (CDCl3, 75 MHz) δ: 130.5 (d), 130.0 (d), 129.0 (d), 128.9 (d), 104.1 (d, C-2), 64.9 (t), 33.8 (t), 31.9 (t), 29.7 (t), 29.5 (t), 29.3 (t), 27.3 (t), 22.7 (t), 21.9 (t), 14.1 (q); GC/MS (70 eV) m/z (relative intensity) 294 (M+, 1), 293 (1), 198 (2), 155 (3), 141 (17), 128 (10), 125 (7), 119 (5), 99 (46), 86 (21), 81 (12), 80 (9), 79 (23), 77 (5), 73 (100), 69 (14), 68 (6), 67 (25), 57 (8), 55 (28). HRMS (APCI) Calcd for C19H35O2 [M+H]+ 295.2631, found 295.2625.
2.5 (4Z,8Z)-Heptadecadienal (5)
To a solution of 4 (0.082 g, 0.3 mmol) in acetone (0.07 mL) was added HCl (conc.) (0.03 mL) and water (0.041 mL). The mixture was refluxed at 60°C for 3h. The reaction mixture was then extracted with ether (2 × 10 mL) and dried over MgSO4 affording 0.07g of 5 (95 % yield) as a colorless oil; IR (NaCl) νmax 3007, 2925, 2854, 2715, 1728 (C=O), 1455, 1377, 1260, 1096, 723 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 9.77 (1H, t, J = 1.5, CHO), 5.43-5.33 (4H, m, -CH=CH-), 2.47 (2H, m), 2.37 (2H, m), 2.08 (2H, m), 2.01 (4H, m), 1.25 (12H, brs, -CH2-), 0.88 (3H, t, J = 6.2 Hz, -CH3); 13C NMR (CDCl3, 75 MHz) δ: 202.1 (d, C-1), 130.9 (d), 130.6 (d), 128.7 (d), 127.5 (d), 43.8 (t), 31.9 (t), 31.6 (t), 29.7 (t), 29.5(t), 29.3(t), 27.3(t), 27.1(t), 22.7 (t), 20.1(t), 14.1(q); GC/MS (70 eV) m/z (relative intensity) 250 (M+, 1), 232 (1), 207 (2), 206 (6), 152 (6), 137 (7), 135 (6), 124 (8), 123 (7), 121 (8), 119 (9), 111 (7), 110 (8), 109 (10), 108 (7), 97 (59), 96 (21), 95 (22), 94 (12), 93 (11), 92 (6), 91 (21), 85 (4), 84 (15), 83 (42), 82 (18), 81 (30), 80 (24), 79 (83), 77 (26), 71 (14), 70 (10), 69 (83), 68 (23), 67 (89), 66 (10), 57 (30), 55 (100).
2.6 (5Z,9Z)-(±)-2-Trimethylsilyloxy-5,9-octadecadienonitrile (6)
To a solution of 5 (0.07g, 0.3 mmol) in CH2Cl2 (5 mL) at 0 °C was added trimethylsilyl cyanide (TMSCN) (0.056 mL, 0.42 mmol), and then triethylamine (catalytic amounts). The mixture was stirred for 3h. The solvent was removed in vacuo and the crude reaction mixture was washed with water (2 × 10 mL), extracted with ether (2 × 10 mL) and dried over Na2SO4, affording 0.075 g of 6 (77 % yield), which was used as such for the next step without further purification; 1H NMR (CDCl3, 300 MHz) δ: 5.43-5.33 (4H, m), 4.4 (1H, t, J = 6.5), 2.20 (2H, m), 2.08 (2H, m), 2.01 (4H, m), 1.25 (12H, brs, -CH2-), 0.88 (3H, t, J = 6.2 Hz, -CH3), 0.2 (9H, s, -OSi(CH3)3); 13C NMR (CDCl3, 75 MHz) δ: 131.4 (d), 130.7 (d), 128.7 (d), 127.2 (d), 119.9 (s,C-1), 60.8 (d, C-2), 36.1 (t), 31.9 (t), 31.6 (t), 29.7 (t), 29.5 (t), 29.3 (t), 27.4 (t), 27.3 (t), 27.1 (t), 22.7 (t), 22.3 (t), 14.1 (q), -0.39 (q, -Si(CH3)3) GC/MS (70 eV) m/z (relative intensity) 349 (M+, 15), 334 (13), 322 (9), 321 (28), 308 (6), 236 (6), 232 (7), 222 (6), 209 (6), 208 (7), 206 (10), 180 (8), 171 (8), 168 (12), 155 (15), 153 (12), 152 (14), 144 (10), 142 (16), 133 (10), 128 (12), 127 (32), 119 (14), 116 (36), 106 (17), 101 (62), 100 (13), 97 (32), 95 (16), 93 (14), 91 (19), 84 (26), 83 (36), 82 (12), 81 (27), 80 (36), 79 (60), 77 (19), 75 (38), 73 (100), 69 (52), 68 (12), 67 (61), 59 (19), 57 (41), 55 (96).
2.7 (5Z,9Z)-(±)-2-Hydroxy-5,9-octadecadienoic acid (7)
To a solution of 6 (0.075 g, 0.2 mmol) in 2-methyltetrahydrofuran (3.2 mL) was added concentrated HCl (1.3 mL). The solution was stirred at 60°C for 24 h. The reaction mixture was extracted with ether (2 × 10 mL) and dried over MgSO4 affording 0.05 g, as a colorless oil, of 7 (75% yield) after purification by Kugelrohr distillation at 160 °C/3 mmHg. 1H NMR (CDCl3, 300 MHz) δ: 5.45-5.33 (4H, m, -CH=CH-), 4.20 (1H, m, H-2), 2.20 (2H, m), 2.08 (2H, m), 2.01 (4H, m), 1.83 (2H, m), 1.27 (12H, brs, -CH2-), 0.87 (3H, t, J = 6.0 Hz, –CH3); 13C NMR (CDCl3, 75 MHz) δ: 175.0 (s, C-1), 130.8 (d), 130.6 (d), 128.9 (d), 128.3 (d), 69.9 (d, C-2), 36.5 (t), 34.3 (t), 31.9 (t), 29.7 (t), 29.5 (t), 29.3 (t), 28.4 (t), 27.3 (t), 27.2 (t), 25.4 (t), 22.6 (t), 14.1 (q). HRMS (ESI) Calcd for C18H31O3 [M-H]+ 295.2273, found 295.2278.
2.8 (5Z,9Z)-(±)-2-methoxy-5,9-octadecadienoic acid (1)
To a solution of NaH (0.014 g, 0.6 mmol) in dry THF (3 mL) under argon was added a solution of 7 (0.05 g, 0.17 mmol) in THF (2 mL). The reaction was stirred for 10 min, and then methyl iodide (0.03 mL, 0.5 mmol) was added dropwise at 0°C. The reaction was stirred at rt for 2h. Then, HCl (conc.) was added until the pH was acidic. The mixture was extracted with ether (2 × 10 mL) and dried over Na2SO4. The product was purified by silica gel column chromatography first eluting with hexane/ether (9:1), and then with ether affording 0.033 g of 1 (62 % yield) as a colorless oil. IR (NaCl) νmax 3500-2500 (-OH), 3007, 2925, 2854, 1715 (C=O), 1463, 1377, 1126, 722 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 5.45-5.33 (4H, m, -CH=CH-), 3.78 (1H, brt, J = 5.8 Hz, H-2), 3.45 (3H, s, -OCH3), 2.20 (2H, m), 2.08 (2H, m), 2.01 (4H, m), 1.83 (2H, m), 1.27 (12H, brs, -CH2-), 0.87 (3H, t, J = 6.0 Hz, -CH3); 13C NMR (CDCl3, 75 MHz) δ: 177.2 (s, C-1), 130.8 (d, C-10), 130.5 (d, C-6), 128.9 (d, C-9), 128.1 (d, C-5), 79.4 (d, C-2), 58.3 (q, -OCH3), 32.3 (t), 31.9 (t), 30.9 (t), 29.7 (t), 29.5 (t), 29.3 (t), 27.3 (t), 27.26 (t), 27.2 (t), 22.7 (t), 22.6 (t), 14.1 (q, -CH3); GC/MS (70 eV) m/z (relative intensity) for the methyl ester derivative as a single peak in capillary gas chromatography; 324 (M+, 2), 292 (6), 266 (2), 265 (11), 234 (2), 233 (11), 221 (2), 193 (2), 179 (6), 171 (3), 166 (6), 165 (7), 152 (7), 151 (5), 140 (7), 139 (36), 138 (12), 135 (10), 134 (6), 121 (14), 120 (5), 119 (10), 111 (39), 109 (12), 107 (21), 105 (11), 104 (100), 97 (18), 96 (11), 95 (22), 94 (8), 93 (20), 92 (6), 91 (15), 89 (10), 83 (18), 82 (15), 81 (59), 80 (30), 79 (83), 77 (15), 75 (17), 71 (21), 69 (31), 68 (12), 67 (62), 59 (17), 58 (36), 57 (26), 56 (10), 55 (70). HRMS (ESI) Calcd for C19H33O3 [M-H]+ 309.2424, found 309.2434.
3. Results and discussion
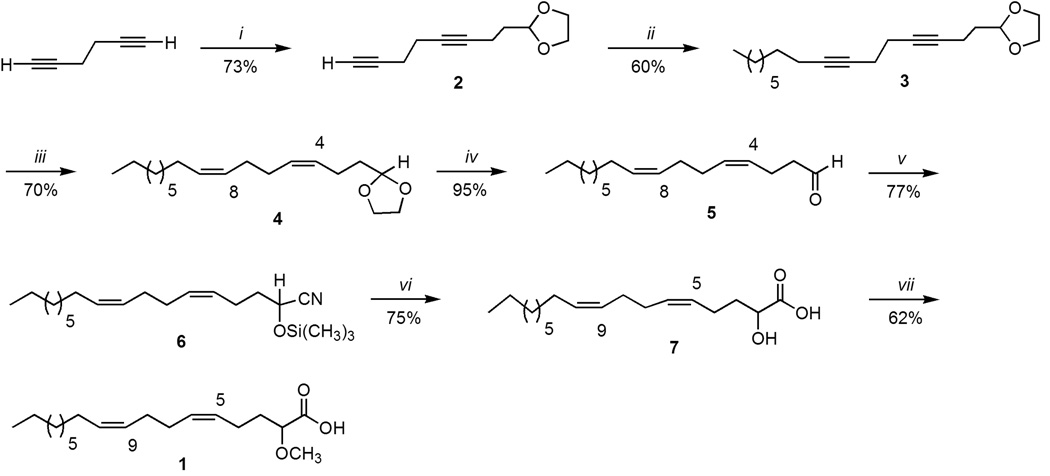
The synthesis of 1 requires the construction of a cis,cis dimethylene interrupted Δ5,9 diunsaturation as well as the introduction of a methoxy functionality α to the carboxylic acid. Several syntheses for Δ5,9 fatty acids with different chain lengths have been reported, including one for a (5Z,9Z)-2-methoxyhexacosa-5,9-dienoic acid (Mena et al., 1984; Reyes and Carballeira, 1997; Kulkarni et al., 2004). A key strategy has been alkyne-bromide coupling followed by hydrogenation under Lindlar conditions to introduce one of the cis double bonds, while the other cis double bond was assembled using the Wittig reaction. However, the problem with this methodology is that, at best, a 95% stereochemical purity is obtained for the cis double bond arising from the Wittig reaction. A double-alkyne bromide coupling reaction starting with 1,5-hexadiyne followed by hydrogenation under Lindlar conditions provides the advantage that a 100% stereochemical purity can be obtained for the two cis double bonds. Such an approach, also applicable to the synthesis of other dimethylene interrupted fatty acids, was originally utilized in the synthesis of the 18:2Δ5,9 acid (Lam and Lie Ken Jie, 1976) and we have successfully used it in the synthesis of a 16:2 Δ5,9 acid (Carballeira et al., 2002). Therefore, for the synthesis of 1 we chose a double-alkyne bromide coupling reaction using the 1,5-hexadiyne (which is now commercially available) as the starting material.
The synthesis of 1 started with commercially available 1,5-hexadiyne (50% in pentane), which was coupled with the 2-(2-bromoethyl)-1,3-dioxolane using n-Buli in THF-HMPA at −78°C, which resulted in the 2-(3,7-octadiynyl)-1,3-dioxolane (2) in 73% yield. Important in this step was the control of the stoichiometry of the reactants as well as the temperature of deprotonation to ensure monoalkylation. In the second step of the synthesis the dioxolane 2 was alkylated with 1-bromooctane under similar reaction conditions as the first step resulting in the 2-(3,7-hexadecadiynyl)-1,3-dioxolane (3) in 60% yield. The third step was the catalytic hydrogenation of diyne 3 under Lindlar (5% palladium on calcium carbonate, poisoned with lead) conditions using hexane as solvent, which afforded the 2-[(3Z,7Z)-3,7-hexadecadienyl]-1,3-dioxolane (4) in 70% yield with a 100% cis,cis double bond stereopurity as confirmed by 13C-NMR spectroscopy and capillary gas chromatography. In the hydrogenation step it was important to control the amount of hydrogen since side products, resulting from over hydrogenation, might be obtained. The dioxolane in 4 was then removed under acidic conditions (HCl) using acetone/water as solvent and heating at 60°C for 3h, which resulted in 95% yield of (4Z,8Z)-heptadecadienal (5). Aldehyde 5 is an interesting compound on its own, since it could be a yet to be identified metabolite arising from taxoleic acid in conifer seeds. An analogy can be made with linoleic acid, where the aldehyde (8Z,11Z)-8,11-heptadecadienal occurs in the essential oil of the marine green alga Ulva pertusa and also in the essential oil from the fern Matteuccia struthiopteris (Miyazawa et al., 2007; Akakabe et al., 2005). The (8Z,11Z)-8,11-heptadecadienal has been suggested as arising from the α-hydroxylation of linoleic acid followed by decarboxylation and subsequent loss of one carbon atom (Kajiwara et al., 1989). This same biosynthetic degradation scenario could be possible for taxoleic acid resulting in aldehyde 5. In fact, the (Z,Z)-4,8-heptadecadiene has been postulated to arise from taxoleic acid (Kuwahara et al., 2005). Therefore, having aldehyde 5 as well as its spectral data at hand will help in its future identification.
With aldehyde 5 at hand, it was then envisaged that the α-methoxy functionality could be achieved by cyanohydrination of 5 followed by hydrolysis and methylation. In the synthesis of the (5Z,9Z)-2-methoxyhexacosa-5,9-dienoic acid the authors used sodium cyanide for the cyanohydrination reaction (Kulkarni et al., 2004), but in our hands we have found that trimethylsilyl cyanide in a Mukaiyama reaction works better for this purpose (Carballeira et al., 2003). Therefore, aldehyde 5 was reacted with trimethylsilyl cyanide in dichloromethane and catalytic amounts of triethylamine at 0°C, which afforded the (5Z,9Z)-(±)-2-trimethylsilyloxy-5,9-octadecadienonitrile (6) in 77% yield. The nitrile 6 was then converted, in 75% yield, into the (5Z,9Z)-(±)-2-hydroxy-5,9-octadecadienoic acid (7) by acid hydrolysis of 6 in 2-methyltetrahydrofuran (2-MeTHF) as solvent and heating at 60°C for 24h. The use of 2-MeTHF proved to be a better solvent than tetrahydrofuran (THF) for this reaction, not only because it is a greener alternative (made from 2-furaldehyde which is derived from renewable resources such as corncobs), but because it facilitated the separation of the product during work-up due to the immiscibility of the 2-Me-THF with water. The final acid 1 was obtained in the last step (62% yield) through the methylation of 7 with sodium hydride and methyliodide in THF as solvent. The choice of THF as solvent (a borderline solvent for a SN2 reaction with a dielectric constant of ca. 7.5) was important so as to ensure selective methylation of the hydroxyl group (over the carboxyl group) due to the greater nucleophilicity of the intermediate alkoxide over that of the competing carboxylate. For example, the Swain and Scott nucleophilic constant (nCH3I) for a carboxylate is around 4.3, while that for an alkoxide is around 6.3 (Pearson and Songstad, 1967). We should mention that if we use the more polar solvent DMSO in this methylation reaction (an aprotic solvent that greatly favors the SN2 reaction with a dielectric constant of ca. 47) methylation occurs at both the hydroxyl group and the carboxyl group (Carballeira et al., 2003).
Despite the fact that the stereochemistry at the α-carbon was not elucidated in the natural acid 1, a capillary gas chromatography GC co-injection of the methyl ester of 1 with the fatty acid methyl ester mixture from E. goffrilleri confirmed the structure of the natural acid as 1. The structure of the natural fatty acid was elucidated using mass spectrometry of its corresponding pyrrolidide derivative (Carballeira et al., 2007).
The most significant absorption in the NMR spectrum of acid 1 was observed for the carbons and hydrogens bearing the methoxy functionality. For example, the methoxy protons resonated at δ 3.45 ppm and the methoxy carbon was observed at δ 58.3 ppm, while the methine α-hydrogen resonated at δ 3.78 ppm, the methine α-carbon at δ 79.4 ppm, and the carbonyl at δ 177.2 ppm. These 1H NMR and 13C NMR displacements are similar to the ones observed for saturated 2-methoxylated fatty acids (Carballeira et al., 2003). However, in contrast to the saturated analogs, the 13C NMR spectrum of acid 1 also displays the typical absorptions of a dimethylene interrupted Δ5,9 fatty acid, i.e., the carbons C-5 and C-9 resonate at δ 128 ppm, while the C-6 and C-10 carbons resonate at δ 130 ppm (Mena et al., 1984).
Scheme 1. Synthesis of (5Z,9Z)-(±)-2-methoxy-5,9-octadecadienoic acid (1).
i) n-BuLi, THF-HMPA, 2-(2-bromoethyl)-1,3-dioxolane,−78°C, 5h; ii) n-BuLi, THF-HMPA, 1-bromooctane, −10°Cto −78°C; iii) H2, Lindlar/hexane, 24h; iv) HCl (conc), acetone/H20, 60°C, 3h; v) TMSCN, Et3N,CH2Cl2, 0°C, 3h; vi) HCl (conc.), 2-Me-THF, 60°C, 24h; vii) NaH-CH3I, THF, 3h.
Acknowledgements
This work was supported by a grant from the SCORE program of the National Institutes of Health (Grant No. S06GM08102). R. O’Neill thanks the NIH–RISE program for a graduate fellowship, while D. Silva thanks the NIH-MARC program for an undergraduate fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akakabe Y, Washizu K, Matsui K, Kajiwara T. Concise synthesis of (8Z,11Z,14Z)-8,11,14-heptadecatrienal, (7Z,10Z,13Z)-7,10,13-hexadecatrienal, and (8Z,11Z)-heptadecadienal, components of the essential oil of marine green alga Ulva pertusa. Biosci.Biotechnol. Biochem. 2005;69:1348–1352. doi: 10.1271/bbb.69.1348. [DOI] [PubMed] [Google Scholar]
- Carballeira NM, Emiliano A, Morales R. Positional distribution of octadecadienoic acids in sponge phosphatidylethanolamines. Lipids. 1994;29:523–525. doi: 10.1007/BF02578251. [DOI] [PubMed] [Google Scholar]
- Carballeira NM, Medina JR. New delta 5,9 fatty acids in the phospholipids of the sea anemone Stoichactis helianthus. J. Nat. Prod. 1994;57:1688–1695. doi: 10.1021/np50114a011. [DOI] [PubMed] [Google Scholar]
- Carballeira NM, Betancourt JE, Orellano EA, González FA. Total synthesis and biological evaluation of (5Z,9Z)-5,9-hexadecadienoic acid, an inhibitor of human topoisomerase I. J. Nat. Prod. 2002;65:1715–1718. doi: 10.1021/np0202576. [DOI] [PubMed] [Google Scholar]
- Carballeira NM, Cruz H, Orellano EA, González FA. The first total synthesis of the marine fatty acid (±)-2-methoxy-13-methyltetradecanoic acid: a cytotoxic fatty acid to leukemia cells. Chem. Phys. Lipids. 2003;126:149–153. doi: 10.1016/s0009-3084(03)00110-5. [DOI] [PubMed] [Google Scholar]
- Carballeira NM, Oyola D, Vicente J, Rodriguez AD. Identification of novel α- methoxylated phospholipid fatty acids in the Caribbean sponge Erylus goffrilleri. Lipids. 2007;42:1047–1053. doi: 10.1007/s11745-007-3110-0. [DOI] [PubMed] [Google Scholar]
- Davidoff F, Korn ED. Fatty acid and phospholipid composition of the cellular slime mold, Dictyostelium discoideum. J. Biol. Chem. 1963;238:3199–3209. [PubMed] [Google Scholar]
- Hierro MTG, Robertson G, Christie WW, Joh YG. The fatty acid composition of the seeds of Ginkgo biloba. J. Am. Oil Chem. Soc. 1996;73:575–579. [Google Scholar]
- Kajiwara T, Yoshikawa H, Matsui K, Hatanaka A, Kawai T. Specificity of the enzyme system producing long chain aldehydes in the green alga, Ulva pertusa. Phytochemistry. 1989;28:407–409. [Google Scholar]
- Kulkarni BA, Sharma A, Gamre S, Chattopadhyay S. Synthesis of the marine compound (2R,5Z,9Z)-2-methoxyhexacosa-5,9-dienoic acid via a lipase-catalyzed resolution and a novel O-alkylation protocol. Synthesis. 2004:595–599. [Google Scholar]
- Kuwahara Y, Samejima M, Sakata T, Kurosa K, Sato M, Matsuyama S, Suzuki T. Chemical ecology of Astigmatid mites XLIV. Identification of (Z,Z,Z)-5,9,12-octadecatrienoic acid as possible biosynthetic precursors of new hydrocarbons (Z,Z,Z)-4,8,11-heptadecatriene and (Z,Z)-4,8-heptadecadiene found in the Astigmatid mite, Tortonia sp. Appl. Entomol. Zool. 1995;30:433–441. [Google Scholar]
- Lam CH, Lie Ken Jie MSF. Fatty acids VII. The gas-liquid chromatographic properties of all dimethylene interrupted methyl cis, cis-octadecadienoates. J. Chromatogr. 1976;117:365–374. doi: 10.1016/0021-9673(76)80013-1. [DOI] [PubMed] [Google Scholar]
- Lísa M, Holèapek M, Øezanka T, Kabátová N. High-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry and gas chromatography-flame ionization detection characterization of Δ5-polyenoic fatty acids in triacylglycerols from conifer seed oils. J. Chromatogr. A. 2007;1146:67–77. doi: 10.1016/j.chroma.2007.01.122. [DOI] [PubMed] [Google Scholar]
- Madrigal RV, Smith CR., Jr Taxus baccata seed oil: A new source of cis-5,cis-9-octadecadienoic acid. Lipids. 1975;10:502–504. doi: 10.1007/BF02532438. [DOI] [PubMed] [Google Scholar]
- Mena PL, Pilet O, Djerassi C. Phospholipid studies of marine organisms. 7. Stereospecific synthesis of (5Z,9Z)-, (5Z,9E)-, (5E,9Z)-and (5E,9E)-5,9-hexacosadienoic acid. J. Org. Chem. 1984;49:3260–3264. [Google Scholar]
- Miyazawa M, Horiuchi E, Kawata J. Components of the essential oil from Matteuccia struthiopteris. J. Oleo Sci. 2007;56:457–461. doi: 10.5650/jos.56.457. [DOI] [PubMed] [Google Scholar]
- Pearson RG, Songstad J. Application of the principle of hard and soft acids and bases to organic chemistry. J. Am. Chem. Soc. 1967;89:1827–1836. [Google Scholar]
- Reyes ED, Carballeira NM. Total synthesis of the antimicrobial fatty acid (5Z,9Z)-14-methylpentadeca-5,9-dienoic acid and its longer-chain analog (5Z,9Z)-24-methylpentacosa-5,9-dienoic acid. Synthesis. 1997:1195–1198. [Google Scholar]