Abstract
The balance of contaminant risk and nutritional benefit from maternal prenatal fish consumption for child cognitive development is not known. Using data from a prospective cohort study of 341 mother-child pairs, authors studied associations of maternal 2nd trimester fish intake and erythrocyte mercury levels with child age 3 year scores on the Peabody Picture Vocabulary Test (PPVT) and Wide-Range Assessment of Visual Motor Abilities (WRAVMA). Mean maternal total fish intake was 1.5 (SD 1.4) servings/month, and 40 (12%) of mothers consumed > 2 weekly fish servings. Mean (SD) maternal mercury was 3.8 (3.8) ng/g. After adjustment using multivariable linear regression, higher fish intake was associated with better child cognitive test performance, and higher mercury levels with poorer test scores. Associations strengthened with inclusion of both fish and mercury: effect estimates (95% CI) for fish intake > 2 servings/week vs. never were 2.2 (−2.6, 7.0) for PPVT and 6.4 (2.0, 10.8) for WRAVMA; and for mercury in the top decile, −4.5 (−8.5, −0.4) for PPVT and −4.6 (−8.3, −0.9) for WRAVMA. Fish consumption <= 2 weekly servings was not associated with a benefit. Dietary recommendations for pregnant women should incorporate the nutritional benefits as well as the risks of fish intake.
Keywords: Pregnancy, Fishes, Mercury, Child Development, n-3 fatty acids
Fish and other seafood may contain beneficial nutrients such as n-3 fatty acids as well as harmful contaminants such as mercury. Methyl mercury is a demonstrated neurotoxicant to which the fetal brain is particularly sensitive (1, 2). A well-designed cohort study in the Faroe Islands has found that prenatal exposure to organic mercury from maternal fish and pilot whale consumption during pregnancy was associated with subtle neurodevelopmental deficits in childhood, such as poorer performance on some tests of language and intelligence, (3, 4). In a cohort from the Seychelle Islands, however, investigators did not find evidence for a neurodevelopmental risk from prenatal methyl mercury exposure resulting from ocean fish consumption (5). However, those studies did not examine the overall relationship of fish intake with child cognitive development. In 2001, the US Environmental Protection Agency recommended that pregnant women avoid consuming fish types high in mercury, and limit their total fish intake to no more than two 6 oz. servings per week (6). Following the advisory, some pregnant women decreased their fish intake (7).
Fish and other seafood serve as the primary dietary source for elongated polyunsaturated n-3 fatty acids including docosahexaenoic acid (DHA), an integral structural component of the brain. Pre- or early postnatal DHA supplementation may improve child development (8–11). As most pregnant women do not consume adequate dietary DHA, limiting fish consumption might further reduce access to this essential nutrient (12, 13).
The overall effect of fish consumption during pregnancy, incorporating the risks as well as the benefits, remains uncertain. A few recent studies, as well as a re-analysis of data from the Faroe Islands cohort, have found that on balance, maternal fish intake is associated with improved child cognitive development (14–16). In the present study, we used prospectively collected information on maternal diet and mercury levels during pregnancy to examine the risks and benefits of maternal prenatal fish intake on child development at age 3 years.
MATERIALS AND METHODS
Population and study design
Subjects were participants in Project Viva, a prospective pre-birth cohort study (13, 14). Mothers provided informed consent at study recruitment, and the human subjects committee of Harvard Pilgrim Health Care approved all protocols. Of 2128 women who delivered a live singleton infant, 1579 were eligible for the age 3-year visit because they had completed a prenatal dietary questionnaire and had not disenrolled. We had information on maternal fish intake, stored maternal blood samples, and child age 3 year cognitive test results from 896 mother-child pairs. Available funding allowed measurement of erythrocyte mercury from 341 mothers. We selected women with an available maternal hair sample (n=98), and with preterm or SGA birth (n= 45) to ensure that the prevalence of these characteristics in the group of women studied in the present analysis closely mirrored the prevalence in the overall cohort. We also assayed a random sample of 198 of the remaining 753.
Compared with the 1238 mothers not included in this study, the 341 mothers included had similar fish intake (1.5 vs. 1.7 servings/week), were slightly older (32.6 vs. 31.9 years), more likely to be white (82 percent vs. 65 percent), better educated (41 percent vs. 30 percent with a graduate degree), less likely to smoke (8 percent vs. 13 percent), and had higher PPVT scores (108.8 vs. 104.6). Included children had similar gestation length (39.4 vs. 39.5 weeks) and fetal growth (0.24 vs. 0.18), but longer breastfeeding duration (7.0 vs. 6.0).
Exposures
Maternal fish intake
At the second trimester study visit, participants completed a semiquantitative food frequency questionnaire, which we modified for pregnancy from a well-validated instrument used in several large cohort studies (17, 18). We previously calibrated this questionnaire against erythrocyte levels of elongated n-3 fatty acids (19). The questionnaire quantified average frequency of consumption of over 140 foods and beverages during the previous three months, including four questions on intake of fish: “canned tuna fish (3–4 oz.)”; “shrimp, lobster, scallops, clams (1 serving)”; “dark meat fish, e.g. mackerel, salmon, sardines, bluefish, swordfish (3–5 oz.)”; and “other fish, e.g. cod, haddock, halibut (3–5 oz.)”. Six frequency response options ranged from “never/less than 1 per month” to “1 or more servings per day.” We combined responses to the four questions to estimate average total fish intake.
Red blood cell mercury
At the second trimester visit, we obtained blood specimens in vacutainer tubes containing ethylenediaminetetraacetic acid. We centrifuged tubes at 2000 rpm for 10 minutes at 4°C to separate plasma from erythrocytes, which we then washed with chilled saline. We stored erythrocyte aliquots at −70°C. Because of inhomogeneity of the erythrocyte sample, we diluted the thawed sample 1:1 by weight with deionized water,,froze samples overnight, then centrifuged thawed samples at 30,000 RPM for 30 minutes at 20°C (Beckman L8-M Ultracentrifuge with TI-70) to remove cell membranes. We performed total mercury assays using the Direct Mercury Analyzer 80 (Milestone Inc., Monroe, CT). Results were reported as mercury content in the original red cell sample. The detection limit is 0.5 ng/ml of sample, and percent recovery for QC standards are ~90–110 percent. Reproducibility for duplicate analysis was ~20 percent.
Fatty acids
We summed contributions to intake of the elongated marine fatty acids DHA and eicosapentaenoic acid (EPA) from all foods and supplements. We also estimated intake of DHA+EPA from fish alone. To obtain estimates of fatty acid content, we used the Harvard nutrient database, which is based on US Department of Agriculture publications as well as other published sources and personal communications, and has been used in other studies of n-3 fatty acid intake (13, 20, 21).
We also analyzed the fatty acid content of packed maternal erythrocytes with gas-liquid chromatography following a single thaw, to determine levels of DHA and EPA. Methods have been described in detail elsewhere (22, 23). Coefficients of variation were 6.5 percent for DHA and 4 percent for EPA.
Child Age 3 year Cognitive Outcomes
Trained research assistants administered two cognitive tests to enrolled children, either in the child’s home or in a research office. The Peabody Picture Vocabulary Test (PPVT) evaluates receptive vocabulary among children aged 2½ years and older, based on a nationally stratified reference sample (24). PPVT scores are strongly correlated (r>=0.90) with verbal and full scale intelligence quotient (IQ) on the Wechsler Intelligence Scale for Children-III (24). Mothers also completed the PPVT. The Wide Range Assessment of Visual Motor Ability (WRAVMA) (25) evaluates three domains of visual motor development: visual-spatial (matching test), visual-motor (drawing test), and fine motor skills (pegboard test), which are used to generate a total standard score. This test has norms for children ages 3 years and older, is moderately correlated with IQ (r~0.60), and is sensitive to other neurotoxicants such as lead (25, 26). We calculated child age at testing in months and days.
Covariates
Using interviews and questionnaires we collected self-reported maternal race/ethnicity, age, education, parity, smoking, alcohol use, marital status, household income, pre-pregnancy weight and height, and paternal education. We determined a western diet score according to intake of red and processed meats, refined grains, desserts, and snack foods, and a prudent diet score according to intake of fruits, vegetables, legumes, fish, and poultry (27). We obtained child sex, birth weight, and delivery date from the medical record. We determined birth weight for gestational age z-value (“fetal growth”) based on US national reference data (28). We collected hair samples at delivery from 211 mothers (14), of whom 98 are included in the present analysis. Mothers reported duration of breastfeeding and the child’s primary language on postpartum questionnaires. We obtained clinical screening lead levels for 139 children.
Statistical analysis
We studied bivariate associations of participant characteristics with maternal fish intake using t tests and chi square analysis. We examined associations among the primary exposures using Spearman correlation and simple linear regression.
We used multivariate linear regression to examine associations of participant characteristics and exposures of interest with child cognitive test scores. We categorized fish intake according to current guidelines (29) as never, <= 2, and > 2 servings per week. We categorized mercury at the top decile or below, as our measure of total mercury in packed erythrocytes does not easily translate to categorization according to the benchmark dose for whole blood mercury (2). We analyzed associations of fish intake and mercury levels individually with each outcome, and then included both measures in prediction models.
We began collecting maternal hair samples in February 2002, and continued through the end of the study. During this period, 409 participants delivered, of whom 302 were approached for collection of a hair sample, 34 were ineligible (hair too short or in braids), and 211 of the remaining 270 (78%) consented to provide a hair sample. In the subset of mothers with hair collected at delivery, 10 percent had hair mercury levels > 1.2 ppm (14). Thus, we anticipate that erythrocyte mercury in the top decile of our population likely corresponds to a hair mercury > 1.2 ppm, the level used to determine the US EPA reference dose (2). Ninety-eight women with hair mercury results also had blood available for mercury assay.
In secondary analyses, we studied fish intake and mercury levels simultaneously, using the following 5 categories: low mercury/fish intake > 2 weekly servings; low mercury/fish <= 2 weekly servings; mercury top decile/fish intake > 2 weekly servings; mercury top decile/fish intake <= 2 weekly servings; and never fish intake. Exclusion of the 1 individual who reported never consuming fish but was in the top decile of mercury levels did not alter results. We also studied intake of canned tuna fish, fish types excluding canned tuna, and fish types excluding shellfish. We also modeled dietary and erythrocyte levels of DHA+EPA and maternal blood mercury as continuous predictors.
We included covariates that were of a priori interest as independent predictors of child cognition, namely maternal age, pre-pregnancy body mass index (BMI), prenatal smoking and alcohol use, race/ethnicity, marital status, and education; paternal education; and child sex, fetal growth, gestation length, duration of breastfeeding, primary language, and age at testing. We modeled all covariates as presented in table 1 and table 2; we also included missing categories for maternal education (2%), smoking (1%), and paternal education (5%). Additional adjustment for household income, maternal western or prudent dietary pattern, depression at 6 months postpartum, child BMI, or test administrator did not substantially change estimates for fish or mercury, so we did not include these factors in our final models. We performed all analyses using SAS version 9.1 (SAS Institute, Cary, NC).
TABLE 1.
Parental and child characteristics according to maternal 2nd trimester fish intake among 341 mother-child pairs in Project Viva.
| Maternal 2nd Trimester Fish Intake | ||||
|---|---|---|---|---|
| Maternal Characteristics | Overall n=341 | Never 47 (14%) | <= 2 servings/week 254 (74%) | >2 servings/week 40 (12%) |
| Mean (SD) or % | ||||
| Age (years) | 32.6 (4.7) | 31.7 (4.8) | 32.8 (4.6) | 32.3 (4.7) |
| Race/ethnicity | ||||
| Black | 6% | 2% | 7% | 8% |
| Hispanic | 2% | 0% | 3% | 3% |
| Other | 9% | 13% | 9% | 5% |
| White | 82% | 85% | 82% | 85% |
| Education | ||||
| High school | 6% | 6% | 5% | 8% |
| Some college | 14% | 13% | 15% | 10% |
| College graduate | 40% | 49% | 37% | 43% |
| Graduate degree | 41% | 32% | 43% | 40% |
| Partner status | ||||
| Married/cohabiting | 96% | 96% | 96% | 93% |
| Single | 4% | 4% | 4% | 8% |
| PPVT score | 108.8 (14.3) | 109.0 (16.4) | 109.5 (13.9) | 103.6 (14.2) |
| Smoking in pregnancy | ||||
| Former | 22% | 24% | 21% | 23% |
| During pregnancy | 8% | 17% | 6% | 10% |
| Never | 70% | 59% | 73% | 67% |
| Alcohol | ||||
| Any during pregnancy | 76% | 81% | 76% | 70% |
| None | 24% | 19% | 24% | 30% |
| Pre-pregnancy BMI | ||||
| < 25 kg/m2 | 70% | 77% | 70% | 65% |
| 25–30 kg/m2 | 20% | 17% | 19% | 30% |
| 30+ kg/m2 | 10% | 6% | 11% | 5% |
| Mercury (ng/g) | 3.8 (3.8) | 1.9 (2.3) | 3.9 (3.8) | 5.6 (4.5) |
| Mercury top decile | 10% | 2% | 10% | 23% |
| DHA+EPA from fish (mg/day) | 128 (128) | 0 (0) | 122 (97) | 318 (160) |
| DHA+EPA total from diet (mg/day) | 149 (154) | 22 (77) | 148 (142) | 301 (159) |
| Maternal 2nd Trimester Fish Intake | ||||
| Paternal and child Characteristics | Overall n=341 | Never 47 (14%) | <= 2 servings/week 254 (74%) | >2 servings/week 40 (12%) |
| Mean (SD) or % | ||||
| Paternal Education | ||||
| High School | 23% | 27% | 21% | 27% |
| College Graduate | 41% | 33% | 42% | 41% |
| Graduate Degree | 36% | 40% | 36% | 32% |
| Birth order | ||||
| Firstborn | 54% | 66% | 54% | 43% |
| Not firstborn | 46% | 34% | 46% | 58% |
| Gestation length (wk) | 39.4 (1.8) | 39.2 (2.5) | 39.5 (1.7) | 39.4 (1.4) |
| Fetal growth (z value) | 0.24 (1.0) | 0.29 (0.9) | 0.23 (1.0) | 0.25 (1.0) |
| Sex | ||||
| Boy | 49% | 45% | 52% | 38% |
| Girl | 51% | 55% | 48% | 63% |
| Breastfeeding (mos) | 7.0 (4.5) | 7.2 (4.4) | 7.0 (4.5) | 6.8 (4.7) |
| Age at outcome (mos) | 38.4 (2.2) | 38.9 (3.2) | 38.4 (2.0) | 38.5 (1.9) |
| Primary language | ||||
| English | 95% | 98% | 94% | 93% |
| Other | 5% | 2% | 6% | 8% |
TABLE 2.
Associations of parental and child characteristics with child test scores at age 3 years
| Characteristics | Peabody Picture Vocabulary Test (PPVT) | Wide Range Assessment of Visual Motor Abilities (WRAVMA) | ||
|---|---|---|---|---|
| Maternal | Beta Coefficient* | 95% Confidence Interval | Beta Coefficient* | 95% Confidence Interval |
| Age (years) | 0.4 | 0.1, 0.7 | 0.6 | 0.1, 1.1 |
| Race/ethnicity | ||||
| Black | −11.2 | −16.8, −5.6 | −2.3 | −7.9, 3.3 |
| Hispanic | −15.4 | −24.0, −6.8 | −7.7 | −16.2, 0.7 |
| Other | −1.6 | −6.3, 3.1 | 1.5 | −2.7, 5.8 |
| White | Ref | |||
| Education | ||||
| High school | −7.4 | −14.9, 0.2 | −5.5 | −12.4, 1.3 |
| Some college | −3.0 | −7.4, 1.4 | −1.5 | −5.6, 2.5 |
| College graduate | −3.3 | −6.1, −0.5 | −1.9 | −4.5, 0.7 |
| Graduate degree | Ref | Ref | ||
| Partner status | ||||
| Married/cohabiting | Ref | Ref | ||
| Single | 6.4 | −10.3, 23.2 | −4.8 | −20.0, 10.5 |
| PPVT score | 0.12 | 0.02, 0.23 | 0.0 | 0.0, 0.1 |
| Smoking in pregnancy | ||||
| Former | −3.2 | −6.2, −0.3 | 1.2 | −1.5, 3.9 |
| During pregnancy | −0.6 | −5.5, 4.2 | 2.9 | −1.7, 7.4 |
| Never | Ref | Ref | ||
| Alcohol | ||||
| Any during pregnancy | 2.8 | −0.2, 5.8 | 0.8 | −2.0, 3.6 |
| None | Ref | Ref | ||
| Pre-pregnancy BMI | ||||
| < 25 kg/m2 | Ref | Ref | ||
| 25–30 kg/m2 | −1.9 | −4.9, 1.2 | −1.2 | −4.0, 1.6 |
| 30+ kg/m2 | 5.9 | 1.7, 10.0 | 0.1 | −3.7, 3.9 |
| Characteristics | Peabody Picture Vocabulary Test (PPVT) | Wide Range Assessment of Visual Motor Abilities – Total (WRAVMA) | ||
| Beta Coefficient* | 95% Confidence Interval | Beta Coefficient* | 95% Confidence Interval | |
| Paternal Education | ||||
| High School | −3.4 | −7.2, 0.4 | −3.5 | −7.0, 0.1 |
| College Graduate | −1.2 | −4.1, 1.7 | 1.5 | −1.2, 4.1 |
| Graduate Degree | Ref | Ref | ||
| Birth order | ||||
| Firstborn | 4.8 | 2.1, 7.4 | 0.9 | −1.5, 3.3 |
| Not firstborn | Ref | Ref | ||
| Gestation length (wk) | 0.05 | −0.6, 0.7 | −0.1 | −0.7, 0.6 |
| Fetal growth (z value) | 2.4 | 1.1, 3.7 | 0.8 | −0.4, 2.0 |
| Sex | ||||
| Boy | Ref | Ref | ||
| Girl | 1.5 | −0.9, 4.0 | 4.5 | 2.3, 6.8 |
| Breastfeeding (mos) | 0.1 | −0.2, 0.4 | −0.1 | −0.4, 0.2 |
| Age at outcome (mos) | 0.7 | 0.1, 1.3 | 0.6 | 0.1, 1.1 |
| Primary language | ||||
| English | Ref | Ref | ||
| Other | −11.6 | −18.0, −5.3 | −2.2 | −8.1, 3.6 |
Estimates from multivariable linear regression models, adjusted for all characteristics in the table and also for maternal fish intake and mercury levels.
RESULTS
Mean maternal fish intake was 1.5 (SD 1.4, range 0–7.5) servings/week, and 40 (12 percent) of mothers consumed more than 2 weekly fish servings, whereas 47 (14 percent) never consumed fish (table 1). Mean erythrocyte total mercury was 3.8 (SD 3.8, range 0.03–21.9) ng/g, with 35 mothers above the 90th percentile of 9.1 ng/g. Mean (SD) child age at testing was 38.4 (2.2) months, and child test scores were 105.7 (13.8) for the PPVT, 99.9 (10.3) for drawing, 99.8 (10.3) for pegboard, 107.8 (14.1) for matching, and 103.2 (10.5) for WRAVMA total score.
Maternal fish intake was directly correlated with erythrocyte total mercury (spearman r = 0.42, p < 0.0001), with an unadjusted increase of 0.94 (95 percent confidence interval [CI]: 0.66, 1.21) ng/g mercury for each weekly fish serving. The likelihood of being in the top decile of erythrocyte mercury was 2 percent in those who never consumed fish but 23 percent in those who consumed fish more than twice weekly (table 1). Otherwise, maternal or child characteristics did not significantly differ according to maternal fish intake (p> 0.05 for all characteristics in table 1). Among 98 mothers with available data, mean hair mercury was 0.53 (SD 0.47, range 0–2.3) mcg/g. Hair mercury was correlated with erythrocyte total mercury (Spearman r =0.46, p<0.0001) and with fish intake (r=0.49, p<0.0001). An increase of 1 ppm of hair mercury was associated with an increase in erythrocyte mercury of 4.5 (95 percent CI: 3.3, 5.8) ng/g.
Participant characteristics were generally associated with child test scores in the anticipated directions (table 2). For example, scores were higher among children who were born with higher fetal growth, who were girls, who were firstborn, and who were older at testing (table 2).
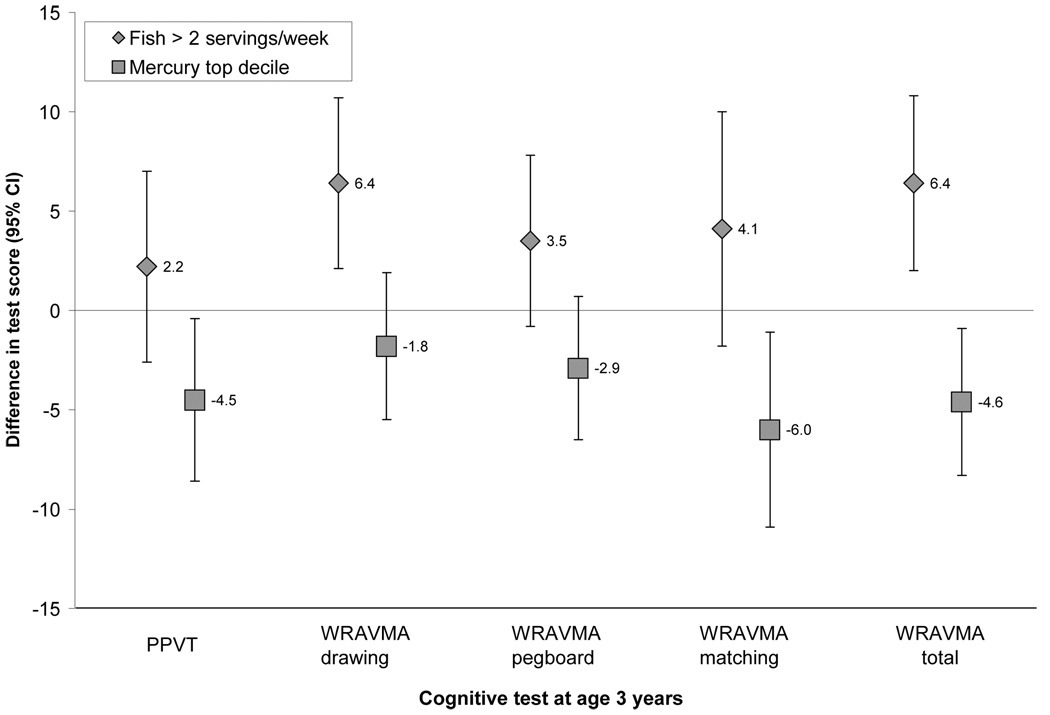
After adjustment for parent and child characteristics, maternal fish intake > 2 weekly servings, compared with never, was directly associated with higher child WRAVMA drawing and total scores (table 3). Associations strengthened with adjustment for mercury levels, with the largest effects seen for the WRAVMA drawing (6.4, 95 percent CI: 2.1, 10.7) and total (6.4, 95 percent CI: 2.0, 10.8) scores, and generally positive associations also seen on the other tests (table 3 and figure). We saw no evidence for an advantage of fish consumption at or below 2 weekly servings, compared with never (table 3). Exclusion of the four participants who reported taking prenatal fish oil supplements did not materially change results. The interaction between fish intake and breastfeeding duration was not significant (p=0.08 for PPVT model and p=0.38 for WRAVMA model).
TABLE 3.
Associations of maternal fish intake during pregnancy* with child cognitive test results at age 3 years among 341 mother-child pairs in Project Viva.
| Age 3 cognitive test | Unadjusted mean score | Adjusted for child age and sex | Multivariable† | Additionally adjusted for erythrocyte mercury | |||
|---|---|---|---|---|---|---|---|
| Beta | 95% CI | Beta | 95% CI | Beta | 95% CI | ||
| PPVT | |||||||
| Fish > 2 svg/wk | 106.3 | −1.5 | −7.3, 4.4 | 1.2 | −3.5, 6.0 | 2.2 | −2.6, 7.0 |
| Fish <= 2 svg/wk > never | 105.2 | −2.2 | −6.5, 2.2 | −2.1 | −5.7, 1.4 | −1.8 | −5.4, 1.8 |
| Fish never | 107.5 | 0 | Referent | 0 | Referent | 0 | Referent |
| WRAVMA drawing | |||||||
| Fish > 2 svg/wk | 104.0 | 5.1 | 0.9, 9.4 | 6.0 | 1.8, 10.2 | 6.4 | 2.1, 10.7 |
| Fish <= 2 svg/wk > never | 99.5 | 1.3 | −1.8, 4.5 | 1.2 | −2.0, 4.4 | 1.3 | −1.8, 4.5 |
| Fish never | 98.5 | 0 | Referent | 0 | Referent | 0 | Referent |
| WRAVMA pegboard | |||||||
| Fish > 2 svg/wk | 103.2 | 3.2 | −1.2, 7.5 | 2.9 | −1.4, 7.1 | 3.5 | −0.8, 7.8 |
| Fish <= 2 svg/wk > never | 99.2 | −0.5 | −3.7, 2.6 | −0.7 | −3.9, 2.4 | −0.5 | −3.7, 2.7 |
| Fish never | 100.1 | 0 | Referent | 0 | Referent | 0 | Referent |
| WRAVMA matching | |||||||
| Fish > 2 svg/wk | 107.9 | 0.6 | −5.4, 6.5 | 2.8 | −3.1, 8.6 | 4.1 | −1.8, 10.0 |
| Fish <= 2 svg/wk > never | 108.0 | 1.4 | −3.1, 5.8 | 1.8 | −2.6, 6.3 | 2.3 | −2.1, 6.7 |
| Fish never | 107.0 | 0 | Referent | 0 | Referent | 0 | Referent |
| WRAVMA total | |||||||
| Fish > 2 svg/wk | 106.4 | 3.7 | −0.7, 8.1 | 5.3 | 0.9, 9.6 | 6.4 | 2.0, 10.8 |
| Fish <= 2 svg/wk > never | 102.8 | 0.7 | −2.5, 4.0 | 1.1 | −2.2, 4.4 | 1.5 | −1.8, 4.7 |
| Fish never | 100.1 | 0 | Referent | 0 | Referent | 0 | Referent |
Of 341 participants, 47 (14%) reported never consuming fish, 254 (74%) reported <= 2 weekly servings, and 40 (12%) reported > 2 weekly servings
adjusted for child sex, age at testing, fetal growth, gestation length, breastfeeding duration, birth order, and primary language; maternal PPVT score, age, pre-pregnancy BMI, race/ethnicity, education, marital status, alcohol use and smoking during pregnancy; and paternal education.
FIGURE 1.
Associations of maternal 2nd trimester fish intake (> 2 weekly servings vs. never) and erythrocyte mercury levels (top decile vs. below) with child cognitive test results at age 3 years. Effect estimates are adjusted for each other as well as for parent and child characteristics. CI = confidence interval. PPVT = Peabody Picture Vocabulary Test. WRAVMA = Wide Range Assessment of Visual Motor Ability.
The 28 mothers (8 percent) who reported eating canned tuna at least twice weekly had children with higher scores on the PPVT (3.7, 95 percent CI: −0.9, 8.3) and WRAVMA total (5.6, 95 percent CI: 1.4, 9.8), compared with the 130 women (38 percent) who reported never eating tuna fish. The 11 mothers (3 percent) who reported consuming more than 2 weekly fish servings of fish other than canned tuna had children with higher scores on the WRAVMA total (6.1, 95 percent CI: −0.7, 12.8) but not on the PPVT (−1.4, 95 percent CI: −8.9, 6.1), compared with the 97 (28 percent) who reported eating no fish excluding tuna. Effect estimates for intake of the three fish types excluding shellfish were 4.3 (95 percent CI: −0.5, 9.0) for PPVT and 5.9 (95 percent CI: 1.6, 10.3) for WRAVMA total score.
Among children for whom we obtained clinical lead results, lead levels were not correlated with either maternal fish consumption (Spearman r = −0.01, p= 0.88) or with mercury levels (r=0.03, p=0.76). Additional adjustment for lead level in multivariable models did not alter effect estimates for fish or mercury on child cognitive test scores (results not shown).
Mean intake of DHA+EPA from fish was 128 (SD 128, range 0–843) mg/day, and mean intake from all sources was 149 (SD 154, range 0–1605) mg/day. For each 100 mg of maternal daily DHA+EPA intake from fish, children had PPVT scores that were 0.5 (95 percent CI: −0.5, 1.5) points higher, and WRAVMA total scores that were 1.1 (0.1, 2.0) points higher. Neither intake of DHA+EPA from all dietary sources, nor the DHA+EPA content of maternal erythrocytes, was associated with child cognition (data not shown).
Higher maternal erythrocyte mercury levels were associated with worse child test performance, with stronger associations after adjustment for fish intake (table 4). We observed the strongest adverse associations of mercury levels with the PPVT (−4.5, 95 percent CI: −8.5, −0.4), WRAVMA matching (−6.0, 95 percent CI: −10.9, −1.1), and WRAVMA total (−4.6, 95 percent CI: −8.3, −0.9) tests, with associations that were somewhat less strong but still suggestive of an inverse relationship for the WRAVMA drawing and pegboard tests (table 4 and figure).
TABLE 4.
Associations of maternal 2nd trimester erythrocyte mercury levels with child cognitive test results at age 3 years among 341 mother-child pairs in Project Viva.
| Unadjusted mean score | Adjusted for child age and sex | Multivariable* | Additionally adjusted for fish intake | ||||
|---|---|---|---|---|---|---|---|
| Beta | 95% CI | Beta | 95% CI | Beta | 95% CI | ||
| PPVT | |||||||
| Mercury top decile | 100.9 | −5.3 | −10.1, −0.5 | −4.0 | −8.0, 0.05 | −4.5 | −8.5, −0.4 |
| Mercury <90th %ile | 106.2 | 0 | Referent | 0 | Referent | 0 | Referent |
| Per ng/g mercury | −0.5 | −0.9, −0.2 | −0.4 | −0.7, −0.05 | −0.4 | −0.8, −0.1 | |
| WRAVMA drawing | |||||||
| Mercury top decile | 99.7 | −0.2 | −3.8, 3.4 | −0.7 | −4.4, 3.0 | −1.8 | −5.5, 1.9 |
| Mercury <90th %ile | 99.9 | 0 | Referent | 0 | Referent | 0 | Referent |
| Per ng/g mercury | 0.2 | −0.04, 0.5 | 0.2 | −0.1, 0.5 | 0.1 | −0.2, 0.4 | |
| WRAVMA pegboard | |||||||
| Mercury top decile | 99.3 | −0.5 | −4.1, 3.0 | −2.2 | −5.8, 1.4 | −2.9 | −6.5, 0.7 |
| Mercury <90th %ile | 99.8 | 0 | Referent | 0 | Referent | 0 | Referent |
| Per ng/g mercury | 0.2 | −0.04, 0.5 | 0.1 | −0.2, 0.4 | 0.03 | −0.3, 0.3 | |
| WRAVMA matching | |||||||
| Mercury top decile | 102.1 | −6.3 | −11.1, −1.5 | −5.4 | −10.2, −0.5 | −6.0 | −10.9, −1.1 |
| Mercury <90th %ile | 108.5 | 0 | Referent | 0 | Referent | 0 | Referent |
| Per ng/g mercury | −0.2 | −0.6, 0.1 | −0.1 | −0.5, 0.3 | −0.2 | −0.6, 0.2 | |
| WRAVMA total | |||||||
| Mercury top decile | 100.1 | −3.4 | −7.0, 0.2 | −3.5 | −7.2, 0.2 | −4.6 | −8.3, −0.9 |
| Mercury <90th %ile | 103.5 | 0 | Referent | 0 | Referent | 0 | Referent |
| Per ng/g mercury | 0.05 | −0.2, 0.3 | 0.03 | −0.3, 0.3 | −0.06 | −0.4, 0.2 | |
adjusted for child sex, age at testing, fetal growth, gestation length, breastfeeding duration, birth order, and primary language; maternal PPVT score, age, pre-pregnancy BMI, race/ethnicity, education, marital status, alcohol use and smoking during pregnancy; and paternal education.
We next examined maternal fish intake and mercury levels simultaneously. Compared with children whose mothers reported never consuming fish, children of women with mercury below the top decile but fish intake above 2 weekly servings had higher WRAVMA total scores (5.9 points, 95 percent CI: 1.0, 10.9). Children whose mothers consumed more than 2 weekly fish servings and with mercury in the top decile also had somewhat higher WRAVMA scores, whereas children of mothers with fish intake up to 2 weekly servings and mercury in the top decile had somewhat lower WRAVMA scores (table 5).
TABLE 5.
Child age 3 year WRAVMA total score according to maternal prenatal fish intake and mercury levels
| Mercury <= 90th %ile | Mercury top decile | |||||
|---|---|---|---|---|---|---|
| Fish intake | N | Estimate* | 95% CI | N | Estimate* | 95% CI |
| > 2 weekly servings | 31 | 5.9 | 1.0, 10.9 | 9 | 4.1 | −3.4, 11.7 |
| <= 2 weekly servings | 229 | 1.8 | −1.8, 5.3 | 25 | −4.2 | −9.6, 1.2 |
| Never | 47 | 0 | (referent) | |||
Estimates adjusted for child sex, age at testing, fetal growth, gestation length, breastfeeding duration, birth order, and primary language; maternal PPVT score, age, pre-pregnancy BMI, race/ethnicity, education, marital status, alcohol use and smoking during pregnancy; and paternal education.
DISCUSSION
In this US cohort with moderate fish consumption, pregnant women who ate more fish had higher erythrocyte mercury levels. Among their children, higher prenatal mercury exposure was associated with lower developmental test scores at age 3 years. Nevertheless, we observed no overall adverse effect upon child development with higher maternal fish intake. Rather, maternal fish intake more than twice a week was associated with improved performance on tests of language and visual motor skills.
These results support our earlier findings among a smaller subset of our study population we assessed at age 6 months with a single outcome (14). The present results derive from a larger sample of children whom we assessed at age 3 years with well-validated developmental tests that are correlated with IQ. Our findings show broadly consistent results for tests of language as well as visual spatial, visual motor, and fine motor skills, suggesting that the benefit of fish consumption spans these developmental domains.
These results also extend findings from other populations that have suggested benefits from maternal prenatal n-3 fatty acid or fish intake. Children of women randomized to receive prenatal fish oil supplementation had higher mental processing scores and better eye-hand coordination (8, 9). In a recent publication of data from the United Kingdom, Hibbeln et al. (15) found that mothers who ate >= 3 fish servings per week had children who were less likely to have suboptimal scores on tests of IQ and behavior at 7–8 years of age, but did not observe any benefit of seafood intake below compared with above 3 servings/week. In that study there was no measure of mercury, although in an earlier publication from a subset of the same population, umbilical cord tissue Hg levels were not associated with development in infancy (30). Additionally, in a re-analysis of data from the Faroe Islands cohort, which has much higher seafood intake and mercury exposure, Budtz-Jorgensen et al.(16) reported that maternal fish intake during pregnancy was associated with somewhat higher performance on all 7 outcomes studied at ages 7 and 14 years. Similar to our findings, in that study the effects of fish and mercury were each strengthened with mutual adjustment.
We observed associations of mercury levels with child cognition at exposure levels substantially lower than in populations previously studied. Our findings suggest that no lower threshold exists for the adverse effects of prenatal mercury exposure.
In previous cohort studies, biomarkers of mercury exposure included mercury in maternal hair collected at or after delivery (4, 5, 14, 31), and in umbilical cord blood (4). In Project Viva, we did not retain cord blood erythrocytes, and we collected hair from a small number of mothers. We measured total mercury in maternal erythrocytes based on evidence that over 90 percent of the total blood mercury resides in the erythrocytes (2), and that about 95 percent of the mercury in red blood cells is methyl mercury with some of the inorganic mercury derived from demethylated methyl mercury (32). Maternal 1st or 2nd trimester blood mercury is highly correlated with blood mercury collected at delivery, and with cord blood levels (33–37). Thus, good evidence exists that the sample we used is a suitable proxy for fetal methyl mercury exposure.
Advantages of the present study include the assessment of maternal diet during pregnancy with a validated dietary questionnaire, well-established outcome measures, and measurement of a number of parent and child characteristics including maternal PPVT scores. Although women in our population were generally well educated and employed, their fish and dietary DHA intake was similar to that reported in other general populations in North America (12, 38, 39). Nevertheless this study has several limitations. It is possible that unmeasured confounding may account for at least part of the observed findings. In particular, we did not assess home environment. We did not measure other contaminants that may be found in fish such as polychlorinated biphenyls (PCB’s) Accounting for the harms of PCB’s would be expected to suggest an even stronger benefit of the nutrients in fish, but might weaken estimates for mercury. It is possible that women in other populations who consume fish types with different amounts of nutrients and contaminants may experience less of a benefit or even perhaps overall harm from higher prenatal fish intake. In addition, we did not measure other neurodevelopmental domains, such as overall intelligence, that might be differently associated with the nutrients or toxicants in fish.
We have previously reported that mothers in Project Viva consumed less fish after dissemination of a federal mercury advisory in January 2001, and reduced their intake even of fish types not included in the advisory (7). Subsequent advisories and expert reviews have emphasized the nutritional benefits of fish (29, 40), but have continued to recommend that pregnant women consume no more than 2 weekly fish servings (29). Our finding that the benefit of fish intake is strengthened with adjustment for mercury levels suggests that if mercury contamination were not present, the cognitive benefits of fish intake would be greater. Maternal consumption of fish lower in mercury and reduced environmental mercury contamination would allow for stronger benefits of fish intake. Recommendations for fish consumption during pregnancy should take into account the nutritional benefits of fish as well as the potential harms from mercury exposure.
Grants and Acknowledgements
We appreciate the invaluable assistance with the mercury assays we received from Rebecca Doigan, Dr. Innocent Jayawardena, Kelly Konopacki, Nicola Lupoli, and Dr. Chinweike Ukomadu.
This project was supported by grants from the National Institutes of Health (HD34568, HL68041, HD44807, ES00002, P01ES012874), and by Harvard Medical School and the Harvard Pilgrim Health Care Foundation.
List of abbreviations
- BMI
body mass index
- CI
confidence interval
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- IQ
intelligence quotient
- PPVT
Peabody Picture Vocabulary Test
- SD
standard deviation
- US
United States
- WRAVMA
Wide-Range Assessment of Visual Motor Abilities
Footnotes
Conflict of interest statement:
Dr. Gillman has received grant support from Mead Johnson Nutritionals. Dr. Bellinger served as a member of an expert panel for a study conducted by the Harvard Center for Risk Analysis evaluating the benefits and risks of seafood consumption, with funding from the National Food Producers Association. None of the other authors has any conflict of interest to report.
References
- 1.Amin-Zaki L, Elhassani S, Majeed MA, Clarkson TW, Doherty RA, Greenwood M. Intra-uterine methylmercury poisoning in Iraq. Pediatrics. 1974;54:587–595. [PubMed] [Google Scholar]
- 2.Goyer R, Aposhian V, Arab L, et al. Toxicological effects of methylmercury. Washington, D.C.: National Academy Press; 2000. [Google Scholar]
- 3.Grandjean P, Weihe P, Burse VW, et al. Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicol Teratol. 2001;23:305–317. doi: 10.1016/s0892-0362(01)00155-6. [DOI] [PubMed] [Google Scholar]
- 4.Debes F, Budtz-Jorgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol. 2006;28:536–547. doi: 10.1016/j.ntt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Myers GJ, Davidson PW, Cox C, et al. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. [Accessed July 17, 2007.: FDA, January 12, 2001];Consumer advisory: An important message for pregnant women and women of childbearing age who may become pregnant about the risks of mercury in fish. Available at: http://vm.cfsan.fda.gov/~dms/admehg.html.
- 7.Oken E, Kleinman KP, Berland WE, Simon SR, Rich-Edwards JW, Gillman MW. Decline in fish consumption among pregnant women after a national mercury advisory. Obstet Gynecol. 2003;102:346–351. doi: 10.1016/S0029-7844(03)00484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111:e39–e44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 9.Dunstan JA, Simmer K, Dixon G, Prescott SL. Cognitive assessment at 21/2 years following fish oil supplementation in pregnancy: a randomized controlled trial. Arch Dis Child Fetal Neonatal Ed. 2007 doi: 10.1136/adc.2006.099085. In press. [DOI] [PubMed] [Google Scholar]
- 10.Werkman SH, Carlson SE. A randomized trial of visual attention of preterm infants fed docosahexaenoic acid until nine months. Lipids. 1996;31:91–97. doi: 10.1007/BF02522417. [DOI] [PubMed] [Google Scholar]
- 11.Willatts P, Forsyth JS, DiModugno MK, Varma S, Colvin M. Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet. 1998;352:688–691. doi: 10.1016/s0140-6736(97)11374-5. [DOI] [PubMed] [Google Scholar]
- 12.Denomme J, Stark KD, Holub BJ. Directly quantitated dietary (n-3) fatty acid intakes of pregnant Canadian women are lower than current dietary recommendations. J Nutr. 2005;135:206–211. doi: 10.1093/jn/135.2.206. [DOI] [PubMed] [Google Scholar]
- 13.Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: results from a US pregnancy cohort. Am J Epidemiol. 2004;160:774–783. doi: 10.1093/aje/kwh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oken E, Wright RO, Kleinman KP, et al. Maternal fish consumption, hair mercury, and infant cognition in a U.S. Cohort. Environ Health Perspect. 2005;113:1376–1380. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibbeln JR, Davis JM, Steer C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 16.Budtz-Jorgensen E, Grandjean P, Weihe P. Separation of risks and benefits of seafood intake. Environ Health Perspect. 2007;115:323–327. doi: 10.1289/ehp.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 18.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 19.Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol. 2004;14:754–762. doi: 10.1016/j.annepidem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 20.US Department of Agriculture Agricultural Research Service. USDA Nutrient Database for standard reference, release 13. Nutrient Data Laboratory Home Page. 1999 Available at: http://www.nal.usda.gov/fnic/foodcomp.
- 21.Hu FB, Bronner L, Willett WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–1821. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 22.Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr. 2007;86:74–81. doi: 10.1093/ajcn/86.1.74. [DOI] [PubMed] [Google Scholar]
- 23.Baylin A, Kim MK, Donovan-Palmer A, et al. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol. 2005;162:373–381. doi: 10.1093/aje/kwi213. [DOI] [PubMed] [Google Scholar]
- 24.Dunn LM, Dunn LM. Examiner's manual for the Peabody Picture Vocabulary Test. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 25.Adams W, Sheslow D. Wide Range Assessment of Visual Motor Abilities. Wilmington, DE: Wide Range, Inc.; 1995. [Google Scholar]
- 26.Bellinger DC, Hu H, Kalaniti K, et al. A pilot study of blood lead levels and neurobehavioral function in children living in Chennai, India. Int J Occup Environ Health. 2005;11:138–143. doi: 10.1179/oeh.2005.11.2.138. [DOI] [PubMed] [Google Scholar]
- 27.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243–249. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 28.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Department of Health and Human Services. [Accessed July 20, 2004., 2004];What you need to know about mercury in fish and shellfish. 2004 March; Available at: http://www.cfsan.fda.gov/~dms/admehg3.html.
- 30.Daniels JL, Longnecker MP, Rowland AS, Golding J. Fish intake during pregnancy and early cognitive development of offspring. Epidemiology. 2004;15:394–402. doi: 10.1097/01.ede.0000129514.46451.ce. [DOI] [PubMed] [Google Scholar]
- 31.Crump KS, Kjellstrom T, Shipp AM, Silvers A, Stewart A. Influence of prenatal mercury exposure upon scholastic and psychological test performance: benchmark analysis of a New Zealand cohort. Risk Anal. 1998;18:701–713. doi: 10.1023/b:rian.0000005917.52151.e6. [DOI] [PubMed] [Google Scholar]
- 32.Berglund M, Lind B, Bjornberg KA, Palm B, Einarsson O, Vahter M. Inter-individual variations of human mercury exposure biomarkers: a cross-sectional assessment. Environ Health. 2005;4:20. doi: 10.1186/1476-069X-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrissette J, Takser L, St-Amour G, Smargiassi A, Lafond J, Mergler D. Temporal variation of blood and hair mercury levels in pregnancy in relation to fish consumption history in a population living along the St. Lawrence River. Environ Res. 2004;95:363–374. doi: 10.1016/j.envres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Vahter M, Akesson A, Lind B, Bjors U, Schutz A, Berglund M. Longitudinal study of methylmercury and inorganic mercury in blood and urine of pregnant and lactating women, as well as in umbilical cord blood. Environ Res. 2000;84:186–194. doi: 10.1006/enrs.2000.4098. [DOI] [PubMed] [Google Scholar]
- 35.Ask K, Akesson A, Berglund M, Vahter M. Inorganic mercury and methylmercury in placentas of Swedish women. Environ Health Perspect. 2002;110:523–526. doi: 10.1289/ehp.02110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakamoto M, Kubota M, Liu XJ, Murata K, Nakai K, Satoh H. Maternal and fetal mercury and n-3 polyunsaturated fatty acids as a risk and benefit of fish consumption to fetus. Environ Sci Technol. 2004;38:3860–3863. doi: 10.1021/es034983m. [DOI] [PubMed] [Google Scholar]
- 37.Ramirez GB, Cruz MC, Pagulayan O, Ostrea E, Dalisay C. The Tagum study I: analysis and clinical correlates of mercury in maternal and cord blood, breast milk, meconium, and infants' hair. Pediatrics. 2000;106:774–781. doi: 10.1542/peds.106.4.774. [DOI] [PubMed] [Google Scholar]
- 38.Kris-Etherton PM, Taylor DS, Yu-Poth S, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179S–188S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 39.Innis SM, Elias SL. Intakes of essential n-6 and n-3 polyunsaturated fatty acids among pregnant Canadian women. Am J Clin Nutr. 2003;77:473–478. doi: 10.1093/ajcn/77.2.473. [DOI] [PubMed] [Google Scholar]
- 40.Nesheim M, Yaktine A. Seafood Choices: Balancing benefits and risks. Washington, DC: The National Academies Press; 2007. [Google Scholar]