Abstract
Two chemically mutagenized agerminative variants of Candida albicans were used to immunize mice against challenge with highly virulent cells of the parent strain. Although both mutants (Vir- 3 and Vir- 13) resulted in nonlethal infection and could be recovered from mouse organs for many days after the intravenous inoculation of 10(7) to 10(6) cells, significant protection to systemic challenge with virulent C. albicans was induced by only one (Vir- 3) of the two variants. Anticandidal resistance in Vir- 3-infected mice was associated with the occurrence in vivo of strong delayed-type hypersensitivity to Candida antigen, detection in vitro of highly fungicidal effector macrophages, and presence in the serum of a large proportion of Candida-reactive antibodies of the immunoglobulin G2a isotype. Bulk cultures of purified CD4+ lymphocytes from mice infected with either mutant were compared for their ability to produce gamma interferon (IFN-gamma), interleukin-2 (IL-2), IL-4, and IL-6 in vitro. After stimulation with specific antigen, CD4+ cells from Vir- 3-immunized mice released large amounts of the Th1-specific cytokines, IFN-gamma and IL-2, at a time when CD4+ cells from Vir- 13-infected mice predominantly secreted the characteristic Th2 cytokines, IL-4 and IL-6. These results were confirmed by quantitative analysis of cytokine-producing Th1 and Th2 cells. In addition, only mice infected with Vir- 3 displayed a high frequency of CD8+ cells with the potential for in vitro lysis of yeast-primed bone marrow macrophages. Purified CD4+ cells from Vir- 3-infected mice, but not a mixture of these cells with CD4+ lymphocytes from mice infected with Vir- 13, could adoptively transfer delayed-type hypersensitivity reactivity onto naive mice. Taken together, these data suggest that both Th1 and Th2 CD4+ lymphocytes may be activated during experimental C. albicans infection in mice.
Full text
PDF


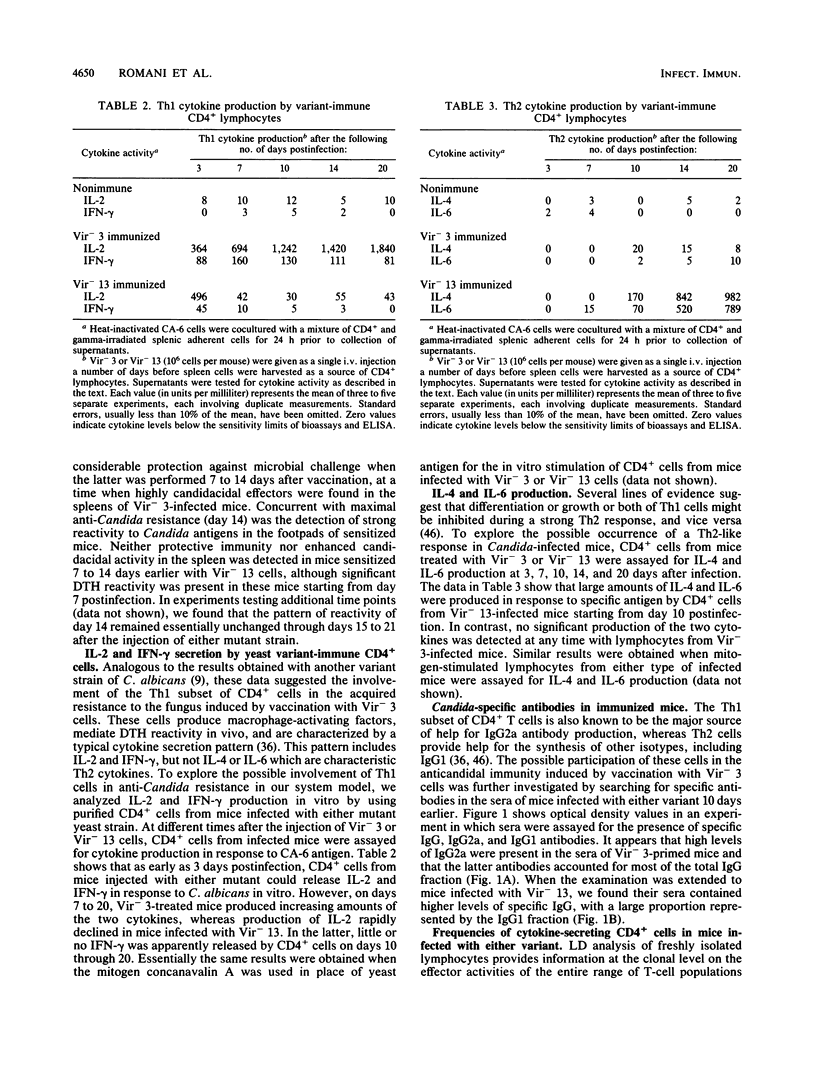
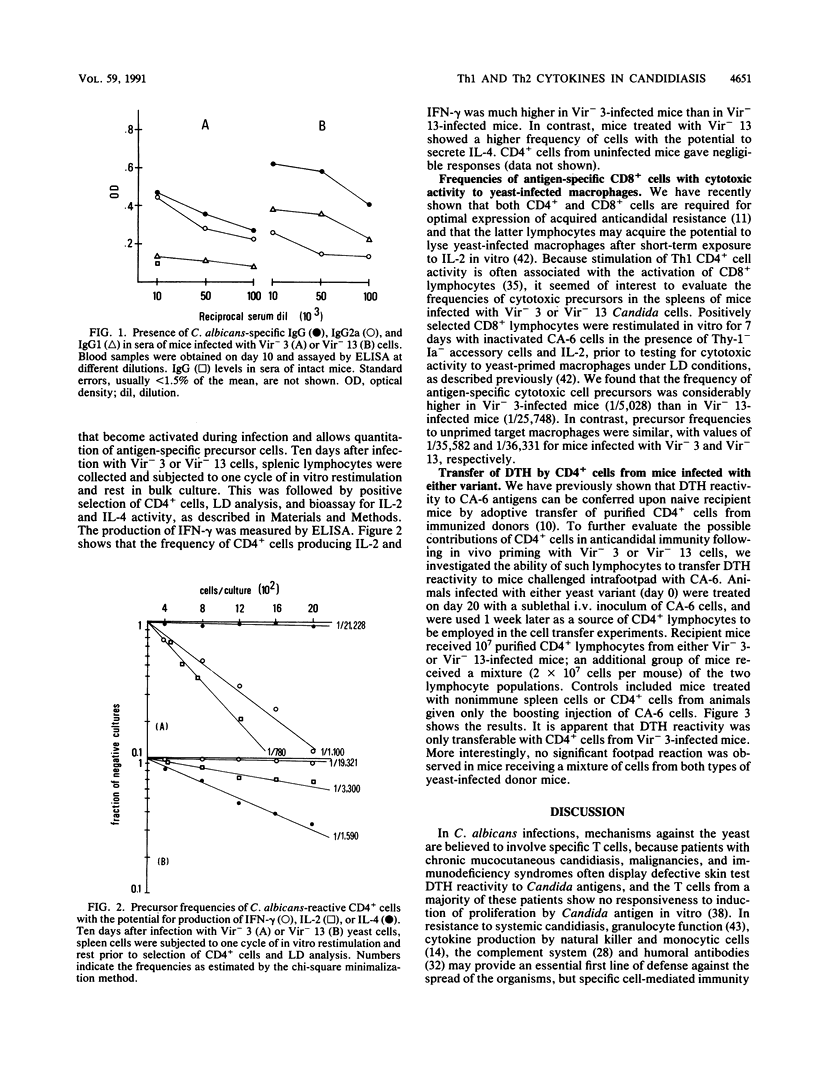



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman R. B., Papadimitriou J. M., Ott A. K., Warmington J. R. Antigens and immune responses in Candida albicans infection. Immunol Cell Biol. 1990 Feb;68(Pt 1):1–13. doi: 10.1038/icb.1990.1. [DOI] [PubMed] [Google Scholar]
- Ashman R. B., Papadimitriou J. M. What's new in the mechanisms of host resistance to Candida albicans infection? Pathol Res Pract. 1990 Aug;186(4):527–534. doi: 10.1016/S0344-0338(11)80477-2. [DOI] [PubMed] [Google Scholar]
- Baccarini M., Bistoni F., Puccetti P., Garaci E. Natural cell-mediated cytotoxicity against Candida albicans induced by cyclophosphamide: nature of the in vitro cytotoxic effector. Infect Immun. 1983 Oct;42(1):1–9. doi: 10.1128/iai.42.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Vecchiarelli A., Cenci E., Puccetti P., Marconi P., Cassone A. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect Immun. 1986 Feb;51(2):668–674. doi: 10.1128/iai.51.2.668-674.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Verducci G., Perito S., Vecchiarelli A., Puccetti P., Marconi P., Cassone A. Immunomodulation by a low-virulence, agerminative variant of Candida albicans. Further evidence for macrophage activation as one of the effector mechanisms of nonspecific anti-infectious protection. J Med Vet Mycol. 1988;26(5):285–299. doi: 10.1080/02681218880000401. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Anaissie E. J. Chronic systemic candidiasis. Eur J Clin Microbiol Infect Dis. 1989 Oct;8(10):855–857. doi: 10.1007/BF01963770. [DOI] [PubMed] [Google Scholar]
- Boon T. Antigenic tumor cell variants obtained with mutagens. Adv Cancer Res. 1983;39:121–151. doi: 10.1016/s0065-230x(08)61034-9. [DOI] [PubMed] [Google Scholar]
- Cantorna M. T., Balish E. Mucosal and systemic candidiasis in congenitally immunodeficient mice. Infect Immun. 1990 Apr;58(4):1093–1100. doi: 10.1128/iai.58.4.1093-1100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci E., Bartocci A., Puccetti P., Mocci S., Stanley E. R., Bistoni F. Macrophage colony-stimulating factor in murine candidiasis: serum and tissue levels during infection and protective effect of exogenous administration. Infect Immun. 1991 Mar;59(3):868–872. doi: 10.1128/iai.59.3.868-872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci E., Romani L., Vecchiarelli A., Puccetti P., Bistoni F. Role of L3T4+ lymphocytes in protective immunity to systemic Candida albicans infection in mice. Infect Immun. 1989 Nov;57(11):3581–3587. doi: 10.1128/iai.57.11.3581-3587.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci E., Romani L., Vecchiarelli A., Puccetti P., Bistoni F. T cell subsets and IFN-gamma production in resistance to systemic candidosis in immunized mice. J Immunol. 1990 Jun 1;144(11):4333–4339. [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- De Libero G., Kaufmann S. H. Antigen-specific Lyt-2+ cytolytic T lymphocytes from mice infected with the intracellular bacterium Listeria monocytogenes. J Immunol. 1986 Oct 15;137(8):2688–2694. [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Richards A. L., Friedman H. Tumor necrosis factor induction by Candida albicans from human natural killer cells and monocytes. J Immunol. 1988 Dec 1;141(11):4047–4052. [PubMed] [Google Scholar]
- Domer J. E. Intragastric colonization of infant mice with Candida albicans induces systemic immunity demonstrable upon challenge as adults. J Infect Dis. 1988 May;157(5):950–958. doi: 10.1093/infdis/157.5.950. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R., Sanders V. M., Mosmann T. R., Vitetta E. S. Lymphokine-mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J Exp Med. 1988 Aug 1;168(2):543–558. doi: 10.1084/jem.168.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Garner R. E., Childress A. M., Human L. G., Domer J. E. Characterization of Candida albicans mannan-induced, mannan-specific delayed hypersensitivity suppressor cells. Infect Immun. 1990 Aug;58(8):2613–2620. doi: 10.1128/iai.58.8.2613-2620.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger D. K., Domer J. E., Moser S. A., McQuitty J. T., Jr Experimental murine candidiasis: pathological and immune responses in T-lymphocyte-depleted mice. Infect Immun. 1978 Sep;21(3):729–737. doi: 10.1128/iai.21.3.729-737.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum L. A., Horowitz J. B., Woods A., Pasqualini T., Reich E. P., Bottomly K. Autocrine growth of CD4+ T cells. Differential effects of IL-1 on helper and inflammatory T cells. J Immunol. 1988 Mar 1;140(5):1555–1560. [PubMed] [Google Scholar]
- Grohmann U., Romani L., Binaglia L., Fioretti M. C., Puccetti P. Intrasplenic immunization for the induction of humoral and cell-mediated immunity to nitrocellulose-bound antigen. J Immunol Methods. 1991 Mar 1;137(1):9–15. doi: 10.1016/0022-1759(91)90388-v. [DOI] [PubMed] [Google Scholar]
- Hurtrel B., Langrange P. H., Michel J. C. Absence of correlation between delayed-type hypersensitivity and protection in experimental systemic candidiasis in immunized mice. Infect Immun. 1981 Jan;31(1):95–101. doi: 10.1128/iai.31.1.95-101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Carding S., Jones B., Murray J., Portoles P., Rasmussen R., Rojo J., Saizawa K., West J., Bottomly K. CD4+ T cells: specificity and function. Immunol Rev. 1988 Jan;101:39–80. doi: 10.1111/j.1600-065x.1988.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Jenkinson H. F., Shepherd M. G. A mutant of Candida albicans deficient in beta-N-acetylglucosaminidase (chitobiase). J Gen Microbiol. 1987 Aug;133(8):2097–2106. doi: 10.1099/00221287-133-8-2097. [DOI] [PubMed] [Google Scholar]
- Lee K. W., Balish E. Systemic candidosis in silica-treated athymic and euthymic mice. Infect Immun. 1983 Sep;41(3):902–907. doi: 10.1128/iai.41.3.902-907.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan L., Wadsworth E., Calderone R. Candida albicans C3d receptor, isolated by using a monoclonal antibody. Infect Immun. 1988 Aug;56(8):1981–1986. doi: 10.1128/iai.56.8.1981-1986.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Virulence for mice of a proteinase-secreting strain of Candida albicans and a proteinase-deficient mutant. J Gen Microbiol. 1983 Feb;129(2):431–438. doi: 10.1099/00221287-129-2-431. [DOI] [PubMed] [Google Scholar]
- Mahanty S., Greenfield R. A., Joyce W. A., Kincade P. W. Inoculation candidiasis in a murine model of severe combined immunodeficiency syndrome. Infect Immun. 1988 Dec;56(12):3162–3166. doi: 10.1128/iai.56.12.3162-3166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M., Snoddy C. B., Fromtling R. A. Comparative pathogenicity of auxotrophic mutants of Candida albicans. Can J Microbiol. 1984 Jan;30(1):31–35. doi: 10.1139/m84-005. [DOI] [PubMed] [Google Scholar]
- Matthews R., Burnie J., Smith D., Clark I., Midgley J., Conolly M., Gazzard B. Candida and AIDS: evidence for protective antibody. Lancet. 1988 Jul 30;2(8605):263–266. doi: 10.1016/s0140-6736(88)92547-0. [DOI] [PubMed] [Google Scholar]
- Miyake T., Takeya K., Nomoto K., Muraoka S. Cellular elements in the resistance to candida infection in mice. I. Contribution of T lymphocytes and phagocytes at various stages of infection. Microbiol Immunol. 1977;21(12):703–725. doi: 10.1111/j.1348-0421.1977.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Moll H., Röllinghoff M. Resistance to murine cutaneous leishmaniasis is mediated by TH1 cells, but disease-promoting CD4+ cells are different from TH2 cells. Eur J Immunol. 1990 Sep;20(9):2067–2074. doi: 10.1002/eji.1830200927. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Murphy J. W. Immunity to fungi. Curr Opin Immunol. 1989;2(3):360–367. doi: 10.1016/0952-7915(89)90142-8. [DOI] [PubMed] [Google Scholar]
- Powers G. D., Abbas A. K., Miller R. A. Frequencies of IL-2- and IL-4-secreting T cells in naive and antigen-stimulated lymphocyte populations. J Immunol. 1988 May 15;140(10):3352–3357. [PubMed] [Google Scholar]
- Rifkind D., Marchioro T. L., Schneck S. A., Hill R. B., Jr Systemic fungal infections complicating renal transplantation and immunosuppressive therapy. Clinical, microbiologic, neurologic and pathologic features. Am J Med. 1967 Jul;43(1):28–38. doi: 10.1016/0002-9343(67)90146-5. [DOI] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Immunity to Candida albicans. Microbiol Rev. 1980 Dec;44(4):660–682. doi: 10.1128/mr.44.4.660-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L., Mocci S., Cenci E., Rossi R., Puccetti P., Bistoni F. Candida albicans-specific Ly-2+ lymphocytes with cytolytic activity. Eur J Immunol. 1991 Jun;21(6):1567–1570. doi: 10.1002/eji.1830210636. [DOI] [PubMed] [Google Scholar]
- Ruthe R. C., Andersen B. R., Cunningham B. L., Epstein R. B. Efficacy of granulocyte transfusions in the control of systemic candidiasis in the leukopenic host. Blood. 1978 Sep;52(3):493–498. [PubMed] [Google Scholar]
- Shepherd M. G. Pathogenicity of morphological and auxotrophic mutants of Candida albicans in experimental infections. Infect Immun. 1985 Nov;50(2):541–544. doi: 10.1128/iai.50.2.541-544.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd M. G., Sullivan P. A. The production and growth characteristics of yeast and mycelial forms of Candida albicans in continuous culture. J Gen Microbiol. 1976 Apr;93(2):361–370. doi: 10.1099/00221287-93-2-361. [DOI] [PubMed] [Google Scholar]
- Street N. E., Mosmann T. R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991 Feb;5(2):171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Van Snick J., Vink A., Cayphas S., Uyttenhove C. Interleukin-HP1, a T cell-derived hybridoma growth factor that supports the in vitro growth of murine plasmacytomas. J Exp Med. 1987 Mar 1;165(3):641–649. doi: 10.1084/jem.165.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli A., Mazzolla R., Farinelli S., Cassone A., Bistoni F. Immunomodulation by Candida albicans: crucial role of organ colonization and chronic infection with an attenuated agerminative strain of C. albicans for establishment of anti-infectious protection. J Gen Microbiol. 1988 Sep;134(9):2583–2592. doi: 10.1099/00221287-134-9-2583. [DOI] [PubMed] [Google Scholar]



