Abstract
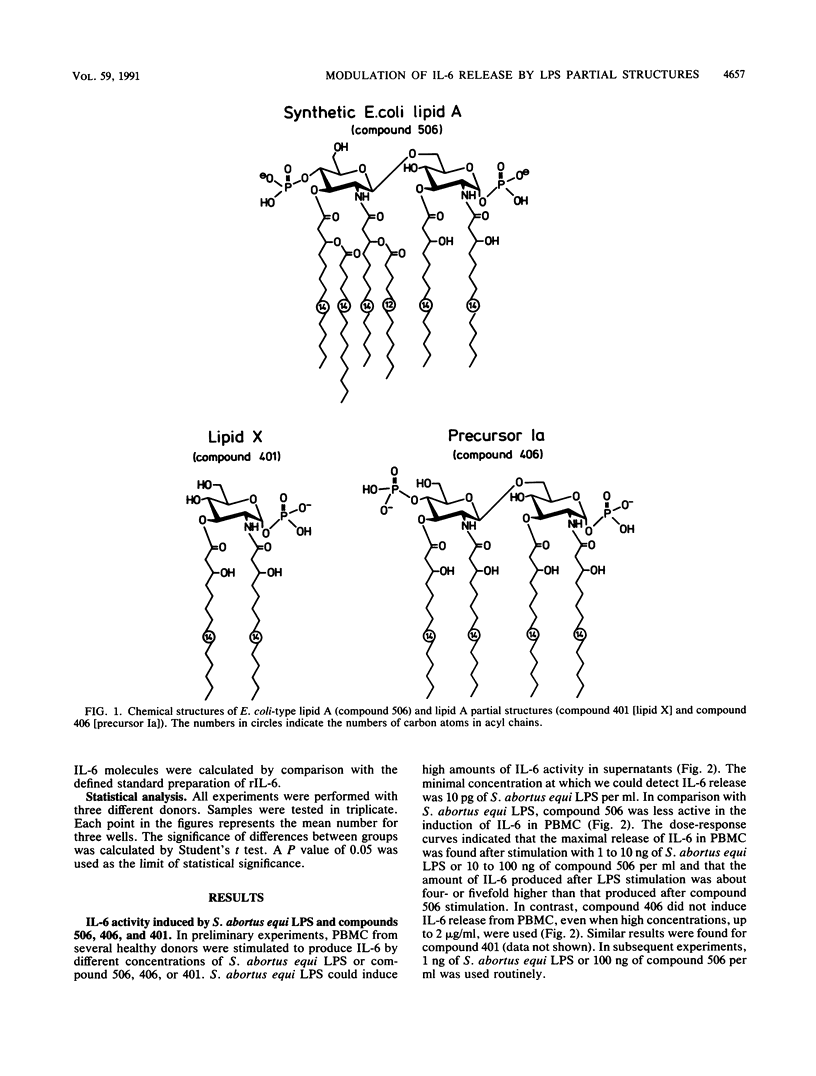
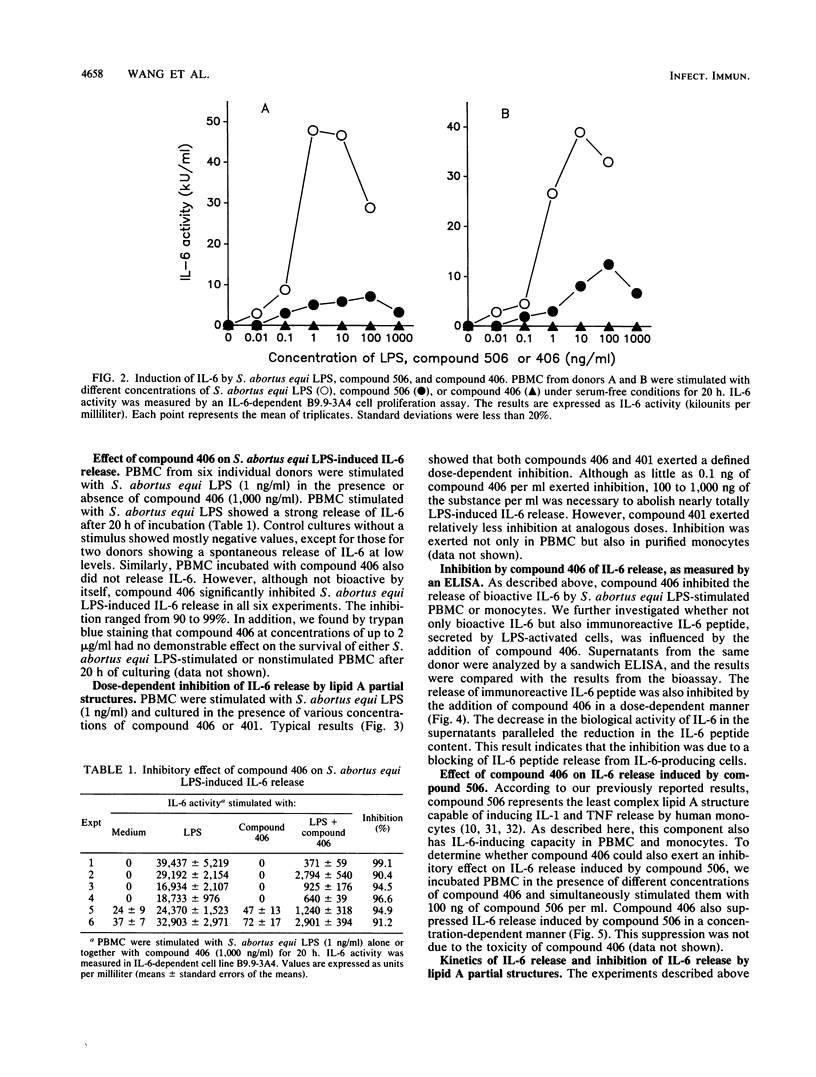
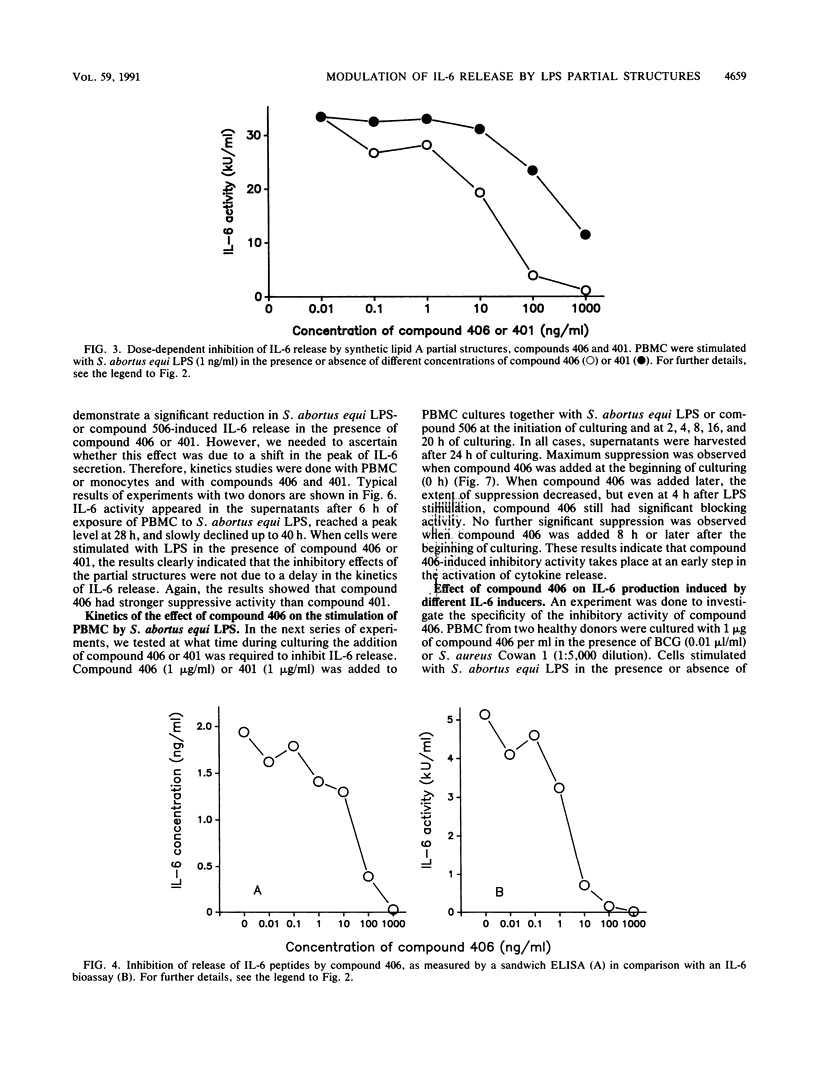
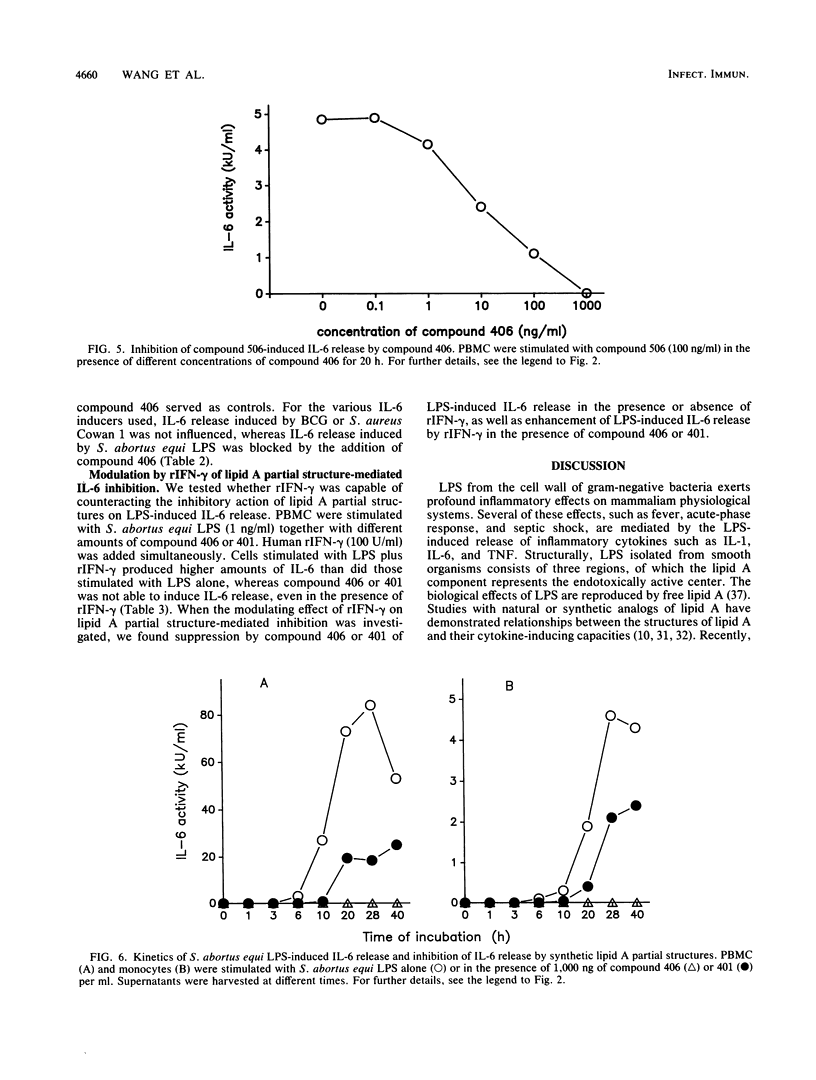
The effect of two synthetic lipid A partial structures, compound 406 (or LA-14-PP, identical in structure to the lipid A precursor, known as Ia or IVa) and compound 401 (lipid X), on the in vitro modulation of endotoxin (lipopolysaccharide)-induced interleukin-6 production by human blood mononuclear cells was investigated. Lipopolysaccharide of Salmonella abortus equi and synthetic Escherichia coli-type lipid A (compound 506, or LA-15-PP) had potent interleukin-6-inducing capacities. The maximum release of interleukin-6 was found after stimulation with 1 to 10 ng of lipopolysaccharide or 10 to 100 ng of synthetic E. coli-type lipid A per ml. Both synthetic lipid A partial structures (compounds 406 and 401) failed to induce interleukin-6 release. However, they inhibited lipopolysaccharide- or lipid A-induced interleukin-6 production in a dose-dependent manner. Inhibition was found not only in mononuclear cells but also in purified monocytes and was not due to a shift in the kinetics of cytokine production. Suppression was manifested in the early stage of interleukin-6 production. Inhibition was also found in the presence of recombinant gamma interferon, indicating that compound 406 and recombinant gamma interferon act in different, independent pathways. Our data, therefore, indicate that the inhibition of interleukin-6 production by lipid A partial structures may help elucidate the mechanism of interaction of the lipid A component of lipopolysaccharide with immune cells in the inflammatory reaction during gram-negative infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Akira S., Hirano T., Taga T., Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990 Aug;4(11):2860–2867. [PubMed] [Google Scholar]
- Aschauer H., Grob A., Hildebrandt J., Schuetze E., Stuetz P. Highly purified lipid X is devoid of immunostimulatory activity. Isolation and characterization of immunostimulating contaminants in a batch of synthetic lipid X. J Biol Chem. 1990 Jun 5;265(16):9159–9164. [PubMed] [Google Scholar]
- Bendtzen K. Interleukin 1, interleukin 6 and tumor necrosis factor in infection, inflammation and immunity. Immunol Lett. 1988 Nov;19(3):183–191. doi: 10.1016/0165-2478(88)90141-1. [DOI] [PubMed] [Google Scholar]
- Brade H., Brade L., Rietschel E. T. Structure-activity relationships of bacterial lipopolysaccharides (endotoxins). Current and future aspects. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Apr;268(2):151–179. doi: 10.1016/s0176-6724(88)80001-4. [DOI] [PubMed] [Google Scholar]
- Böhle A., Nowc C., Ulmer A. J., Musehold J., Gerdes J., Hofstetter A. G., Flad H. D. Elevations of cytokines interleukin-1, interleukin-2 and tumor necrosis factor in the urine of patients after intravesical bacillus Calmette-Guerin immunotherapy. J Urol. 1990 Jul;144(1):59–64. doi: 10.1016/s0022-5347(17)39366-7. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Calandra T., Baumgartner J. D., Grau G. E., Wu M. M., Lambert P. H., Schellekens J., Verhoef J., Glauser M. P. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis. 1990 May;161(5):982–987. doi: 10.1093/infdis/161.5.982. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Belin D., Vassalli J. D., de Kossodo S., Vassalli P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986 Dec 1;164(6):2113–2118. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feist W., Ulmer A. J., Musehold J., Brade H., Kusumoto S., Flad H. D. Induction of tumor necrosis factor-alpha release by lipopolysaccharide and defined lipopolysaccharide partial structures. Immunobiology. 1989 Oct;179(4-5):293–307. doi: 10.1016/S0171-2985(89)80036-1. [DOI] [PubMed] [Google Scholar]
- Flad H. D. Induction of IL-1 by lipopolysaccharide (LPS) and its modulation by synthetic lipid A precursor Ia. Lymphokine Res. 1990 Winter;9(4):557–560. [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of a standardized lipopolysaccharide from salmonella abortus equi (Novo-Pyrexal). Zentralbl Bakteriol Orig A. 1979 Apr;243(2-3):226–244. [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Girardin E., Grau G. E., Dayer J. M., Roux-Lombard P., Lambert P. H. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988 Aug 18;319(7):397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- Golenbock D. T., Will J. A., Raetz C. R., Proctor R. A. Lipid X ameliorates pulmonary hypertension and protects sheep from death due to endotoxin. Infect Immun. 1987 Oct;55(10):2471–2476. doi: 10.1128/iai.55.10.2471-2476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich P. C., Castell J. V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990 Feb 1;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P. The role of IFN-gamma in the pathology of experimental endotoxemia. J Immunol. 1990 Nov 1;145(9):2920–2924. [PubMed] [Google Scholar]
- Helfgott D. C., Tatter S. B., Santhanam U., Clarick R. H., Bhardwaj N., May L. T., Sehgal P. B. Multiple forms of IFN-beta 2/IL-6 in serum and body fluids during acute bacterial infection. J Immunol. 1989 Feb 1;142(3):948–953. [PubMed] [Google Scholar]
- Heremans H., Van Damme J., Dillen C., Dijkmans R., Billiau A. Interferon gamma, a mediator of lethal lipopolysaccharide-induced Shwartzman-like shock reactions in mice. J Exp Med. 1990 Jun 1;171(6):1853–1869. doi: 10.1084/jem.171.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssiau F. A., Bukasa K., Sindic C. J., Van Damme J., Van Snick J. Elevated levels of the 26K human hybridoma growth factor (interleukin 6) in cerebrospinal fluid of patients with acute infection of the central nervous system. Clin Exp Immunol. 1988 Feb;71(2):320–323. [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Takahashi I., Ikeda T., Otsuka K., Shimauchi H., Kasai N., Mashimo J. Synthetic lipid A with endotoxic and related biological activities comparable to those of a natural lipid A from an Escherichia coli re-mutant. Infect Immun. 1985 Jul;49(1):225–237. doi: 10.1128/iai.49.1.225-237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach N. L., Yee E., Munford R. S., Raetz C. R., Harlan J. M. Lipid IVA inhibits synthesis and release of tumor necrosis factor induced by lipopolysaccharide in human whole blood ex vivo. J Exp Med. 1990 Jul 1;172(1):77–84. doi: 10.1084/jem.172.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C., Hildebrandt J., Schütze E., Rosenwirth B., Proctor R. A., Liehl E., Stütz P. Immunostimulatory, but not antiendotoxin, activity of lipid X is due to small amounts of contaminating N,O-acylated disaccharide-1-phosphate: in vitro and in vivo reevaluation of the biological activity of synthetic lipid X. Infect Immun. 1991 Jul;59(7):2351–2358. doi: 10.1128/iai.59.7.2351-2358.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loppnow H., Brade H., Dürrbaum I., Dinarello C. A., Kusumoto S., Rietschel E. T., Flad H. D. IL-1 induction-capacity of defined lipopolysaccharide partial structures. J Immunol. 1989 May 1;142(9):3229–3238. [PubMed] [Google Scholar]
- Loppnow H., Brade L., Brade H., Rietschel E. T., Kusumoto S., Shiba T., Flad H. D. Induction of human interleukin 1 by bacterial and synthetic lipid A. Eur J Immunol. 1986 Oct;16(10):1263–1267. doi: 10.1002/eji.1830161013. [DOI] [PubMed] [Google Scholar]
- Loppnow H., Libby P., Freudenberg M., Krauss J. H., Weckesser J., Mayer H. Cytokine induction by lipopolysaccharide (LPS) corresponds to lethal toxicity and is inhibited by nontoxic Rhodobacter capsulatus LPS. Infect Immun. 1990 Nov;58(11):3743–3750. doi: 10.1128/iai.58.11.3743-3750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C. The case for specific lipopolysaccharide receptors expressed on mammalian cells. Microb Pathog. 1989 Dec;7(6):389–398. doi: 10.1016/0882-4010(89)90019-3. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Riedo F. X., Munford R. S., Campbell W. B., Reisch J. S., Chien K. R., Gerard R. D. Deacylated lipopolysaccharide inhibits plasminogen activator inhibitor-1, prostacyclin, and prostaglandin E2 induction by lipopolysaccharide but not by tumor necrosis factor-alpha. J Immunol. 1990 May 1;144(9):3506–3512. [PubMed] [Google Scholar]
- Shalaby M. R., Waage A., Aarden L., Espevik T. Endotoxin, tumor necrosis factor-alpha and interleukin 1 induce interleukin 6 production in vivo. Clin Immunol Immunopathol. 1989 Dec;53(3):488–498. doi: 10.1016/0090-1229(89)90010-x. [DOI] [PubMed] [Google Scholar]
- Starnes H. F., Jr, Pearce M. K., Tewari A., Yim J. H., Zou J. C., Abrams J. S. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-alpha challenge in mice. J Immunol. 1990 Dec 15;145(12):4185–4191. [PubMed] [Google Scholar]
- Tahri-Jouti M. A., Mondange M., Le Dur A., Auzanneau F. I., Charon D., Girard R., Chaby R. Specific binding of lipopolysaccharides to mouse macrophages--II. Involvement of distinct lipid a substructures. Mol Immunol. 1990 Aug;27(8):763–770. doi: 10.1016/0161-5890(90)90085-e. [DOI] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Beutler B., Kirkland T. N. Diphosphoryl lipid A from Rhodopseudomonas sphaeroides ATCC 17023 blocks induction of cachectin in macrophages by lipopolysaccharide. Infect Immun. 1989 Apr;57(4):1336–1338. doi: 10.1128/iai.57.4.1336-1338.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge J. E., Bowersox O., Tribble H., Lee S. H., Shepard H. M., Liggitt D. Toxicity of tumor necrosis factor is synergistic with gamma-interferon and can be reduced with cyclooxygenase inhibitors. Am J Pathol. 1987 Sep;128(3):410–425. [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Cerami A. Cachectin: a hormone that triggers acute shock and chronic cachexia. J Infect Dis. 1988 Mar;157(3):413–420. doi: 10.1093/infdis/157.3.413. [DOI] [PubMed] [Google Scholar]
- Ulmer A. J., Flad H. D. Functional relationships of human T-lymphocytes and interleukins: I. Different accessory cell requirements of human T-lymphocyte subpopulations for generation of interleukin-2. Immunobiology. 1982 May;161(5):476–487. doi: 10.1016/S0171-2985(82)80050-8. [DOI] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. H., Feist W., Herzbeck H., Brade H., Kusumoto S., Rietschel E. T., Flad H. D., Ulmer A. J. Suppressive effect of lipid A partial structures on lipopolysaccharide or lipid A-induced release of interleukin 1 by human monocytes. FEMS Microbiol Immunol. 1990 Oct;2(3):179–185. doi: 10.1111/j.1574-6968.1990.tb03517.x. [DOI] [PubMed] [Google Scholar]
- Wolvekamp M. C., Marquet R. L. Interleukin-6: historical background, genetics and biological significance. Immunol Lett. 1990 Mar-Apr;24(1):1–9. doi: 10.1016/0165-2478(90)90028-o. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Zabel P., Wolter D. T., Schönharting M. M., Schade U. F. Oxpentifylline in endotoxaemia. Lancet. 1989 Dec 23;2(8678-8679):1474–1477. doi: 10.1016/s0140-6736(89)92929-2. [DOI] [PubMed] [Google Scholar]



