Abstract
The rDEN4Δ30-200,201 is a live attenuated DENV-4 vaccine candidate specifically designed to further attenuate the rDEN4Δ30 parent virus. In the present study, 28 healthy adult volunteers were randomized to receive either 105 plaque-forming unit (PFU) of vaccine (20) or placebo (8) as a single subcutaneous injection. Volunteers were evaluated for safety every other day for 16 days. Serum neutralizing antibody titer against DEN4 was determined at study day 28, 42, and 180. The vaccine infected all vaccinees and was well tolerated without inducing alanine aminotransferase (ALT) elevations. Although virus was not recovered from the serum of any vaccinee, moderate levels of neutralizing antibody were induced in all volunteers. Thus the restricted replication of rDEN4Δ30-200,201 previously documented in animal models was confirmed in humans. The rDEN4Δ30-200,201 is a promising candidate and can be considered for inclusion in a tetravalent dengue virus (DENV) vaccine.
INTRODUCTION
Dengue is an emerging infectious disease that has become increasingly prevalent over the past several decades; it is now the medically most important arboviral infection worldwide with greater than 1.2 million cases reported in 2002 among more than 70 countries.1 Children continue to bear most of the dengue-associated burden of disease, which is estimated to be as high as 616,000 disability adjusted life years.2 Dengue viruses (DENV) are efficiently transmitted by the Aedes aegypti mosquito and exist as four distinct serotypes (DENV-1, DENV-2, DENV-3, and DENV-4). Each serotype is capable of causing the full spectrum of disease, which includes undifferentiated febrile illness, classic dengue fever (DF), and life-threatening dengue hemorrhagic fever/shock syndrome (DHF/DSS).3 Although long-term homotypic immunity is generated after infection with a single DENV serotype, long-term heterotypic protection is not induced.4–6 In addition, pre-existing immunity to one DENV serotype has been identified as a risk factor for more severe disease upon secondary, heterotypic infection.7,8 For these reasons, a potential DENV vaccine must induce long-lived protective immunity against all four DENV serotypes. On the basis of the success of the live attenuated yellow fever vaccine, live attenuated DENV vaccine candidates have been developed, and many are in advanced stages of clinical evaluation.9
We are currently developing a tetravalent DENV vaccine based on the attenuation provided by a 30-nucleotide deletion (Δ30) in the 3′ untranslated region (UTR) of DENV.10 Several of the monovalent vaccine components have been evaluated in clinical trials to determine which are most appropriate for inclusion in a tetravalent DENV vaccine.11–14 Vaccine candidate rDEN4Δ30, the first such vaccine tested, was evaluated at doses ranging from 105 plaque-forming unit (PFU) to 101 PFU.11,14 The vaccine was found to be safe, infectious, and highly immunogenic at all doses tested; however, asymptomatic rash, neutropenia, and elevations in serum alanine aminotransferase (ALT) levels were noted in some vaccinees given at a dose of 105 PFU. The elevation of serum ALT level was dependent on the dose of vaccine administered; it was predominantly observed at the 105 PFU dose cohort and only rarely at lower doses. Asymptomatic rash and neutropenia were observed at similar frequencies at all doses tested. Transient elevations in serum liver enzymes are also seen in vaccinees infected with other live attenuated DENV vaccine candidates,15–18 however, the levels observed in vaccinees are generally much lower than those observed in humans experiencing DF or DHF/DSS. Furthermore, the hepatomegaly that is observed in some patients19–22 has not been observed in vaccinees. Hepatotropism is a natural feature of DENV infection of humans,20,23,24 and the rDEN4Δ30 vaccine candidate likely exhibited residual asymptomatic hepatotoxicity at the 105 PFU dose. Although this was an asymptomatic reaction to vaccine, we sought to generate a derivative of the rDEN4Δ30 vaccine candidate that would lack this hepatotoxicity, even at a high dose of 105 PFU.
Using a molecular genetic approach, we sought to reduce the mild reactogenicity observed with rDEN4Δ30 by developing attenuated mutants of rDEN4Δ30 with decreased replication in HuH-7 human hepatocarcinoma cells, which serve as our surrogate for human liver cells. Paired charge-to-alanine mutagenesis of the DENV-4 NS5 polymerase gene was conducted by mutating the eighty pairs of charged amino acids present in DENV-4 NS5 to Ala-Ala.25 One such mutation at amino acid positions 200 and 201 was found to restrict replication of DENV-4 in severe combined immunodeficiency (SCID) mice bearing HuH-7 tumors. The rDEN4Δ30-200,201 vaccine candidate manifested a 250- and 40-fold reduction in peak viremia compared with DENV-4 wild-type virus and the rDEN4Δ30 parent virus, respectively.25,26 Because viremia in the SCID-HuH-7 mice is thought to arise primarily from replication in the transplanted human liver cells, attenuation in this model suggests altered tropism for human liver cells. The rDEN4Δ30-200,201 was also highly attenuated in rhesus macaques when compared with both wild-type DENV-4 and rDEN4Δ30.26 Although 4 of 4 macaques infected with rDEN4Δ30 developed viremia, none of the macaques infected with rDEN4Δ30-200,201 were viremic. Despite this lack of viremia, the macaques developed serum neutralizing antibody titers that were comparable to those induced by rDEN4Δ30. In addition, the mutation at amino acid 200 and 201 in the NS5 protein each independently contributed to the attenuation phenotype of rDEN4-200,201, indicating that a virus with two of these mutations should be phenotypically stable because both mutated codons would require two nucleotide substitutions each to change the alanine substitution to the original charged amino acid residue present in the wild type virus.26
Because of its favorable pre-clinical profile, rDEN4Δ30-200,201 was considered a suitable vaccine candidate for evaluation in human volunteers. Here we present the results of a Phase I clinical trial of rDEN4Δ30-200,201 in healthy, flavivirus-naive adults. Vaccine candidate rDEN4Δ30-200,201 is more attenuated in humans than its rDEN4Δ30 parent and does not induce an elevation in serum ALT levels. Despite an absence of viremia after administration of rDEN4Δ30-200,201, the virus elicits a moderate level of neutralizing antibody.
SUBJECTS, MATERIALS, AND METHODS
Regulatory oversight
This phase I, randomized, double-blind, placebo-controlled study was conducted at the Center for Immunization Research (CIR) at The Johns Hopkins Bloomberg School of Public Health under an investigational new drug application (BB-IND 12670) reviewed by the U.S. Food and Drug Administration. The clinical protocol, protocol amendments, informed consent form, advertisements, and other study-related documents were reviewed and approved by the Western Institutional Review Board. The clinical protocol was reviewed and approved by the Johns Hopkins University Institutional Biosafety Committee. A Data and Safety Monitoring Board was convened by the study sponsor National Institutes of Allergy and Infectious Diseases (NIAID) for periodic review of all study data. The clinical protocol and study design were developed by CIR and NIAID investigators. Data analysis was performed at the CIR.
Study population
Healthy adult male and non-pregnant female volunteers were recruited from the metropolitan Balti-more, Maryland area. Informed consent was obtained from each volunteer in accordance with the Code of Federal Regulations (CFR21, Part 50). Healthy volunteers between the ages of 18 and 50 were enrolled if they met the following eligibility criteria: normal findings during physical examination; negative for antibodies to all dengue viruses, yellow fever virus, West Nile virus, St. Louis encephalitis virus, Japanese encephalitis virus, human immunodeficiency virus, and hepatitis C virus; negative for hepatitis B surface antigen; normal values for complete blood count (CBC) with differential, serum aspartate aminotransferase (AST), ALT, total bilirubin, alkaline phosphatase, creatinine, creatine phosphokinase (CPK), prothrombin time (PT), partial thromboplastin time (PTT), and urinalysis. Additional safety-related exclusion criteria were also applied. Female volunteers were required to have a negative result on a urine pregnancy test at least three days prior to vaccination and on the day of vaccination and to agree to use contraception or abstain from sexual intercourse for the duration of the study.
Study design and clinical monitoring
Twenty-eight healthy adult volunteers meeting the above eligibility criteria were enrolled in the study. On study day 0, volunteers reported to the CIR out-patient clinic and were randomly assigned to receive 105 PFU of rDEN4Δ30-200,201 vaccine virus or placebo (vaccine diluent) given as a single 0.5 mL subcutaneous injection. Twenty volunteers received vaccine and 8 volunteers received placebo. Volunteers were monitored for immediate adverse reactions related to the vaccine for at least 30 minutes after vaccination. Each volunteer was given a digital thermometer and a diary card to record their oral temperature three times per day for the next 16 days.
Clinical assessments were performed every other day through study day 16 and again on study days 21, 28, 42, and 180. Blood was drawn at each assessment for detection of viremia through study day 16 and for antibody assay on study days 0, 28, 42, and 180. Prior to vaccination, baseline CBC with differential, ALT, CPK, creatinine, and coagulation studies were obtained. After vaccination, a CBC with differential and ALT levels were obtained every other day through study day 16. Serum for ALT testing was also collected again on study day 21. Creatine phosphokinase and creatinine levels were obtained on study days 4, 8, 12, 16, and 21. Coagulation studies were performed on study days 4, 8, 12, 16, and 28. During clinic visits, a medical provider performed a focused physical examination to evaluate for local reactogenicity, skin rash, petechiae, conjunctival or oral erythema, photophobia, lymphadenopathy, abdominal tenderness, and other abnormalities. Volunteers were also questioned about headaches, nausea, malaise, arthralgia, myalgia, retro-orbital pain, and photophobia, in addition to other health-related events. All adverse events were graded for intensity and relationship to vaccination. Adverse events were graded as mild (easily tolerated), moderate (required an intervention or interfered with daily activity), or severe (prevented daily activity). Abnormal hematology and serum chemistry findings were also graded as mild, moderate, or severe as described below. All adverse events and abnormal clinical findings were collected for the duration of the study and were followed to resolution. Dengue-like illness was defined as infection associated with fever (oral temperature ≥ 100.4°F) and two or more of the following clinical signs: moderate headache lasting ≥ 12 hours, moderate photophobia lasting ≥ 12 hours, or moderate generalized myalgia lasting ≥ 12 hours. Study staff were blinded to vaccination status until all volunteers completed study on day 42.
Vaccine virus
The rDEN4Δ30-200,201 vaccine virus is a live attenuated recombinant virus derived from the rDEN4Δ30 vaccine candidate, which contains a 30-nucleotide (nt) deletion in the 3′ UTR of the genome. The wild-type parent DENV-4 from which rDEN4Δ30 and rDEN4Δ30-200,201 were derived is DENV-4 strain 814669 (Dominica 1981). A full-length complementary DNA (cDNA) copy of rDEN4Δ30, p4Δ30 (GenBank accession no. AY376438), was used as the parent cDNA to create the rDEN4Δ30-200,201 virus. The rDEN4Δ30-200,201 virus was generated by changing NS5 codons 200 and 201 (Lys-His to Ala-Ala) in cDNA plasmid p4Δ30, as previously described.26
Sequence analysis of the previously generated and tested preparation of rDEN4Δ30-200,201 revealed that the virus genome contained adventitious coding mutations in the NS3 gene (Gly103 → Arg) and in the NS5 gene (Val182 → Ile), and both were not present in the p4Δ30-200,201 cDNA used to generate the virus. The p4Δ30-200,201 cDNA that was used to generate the clinical lot evaluated in the present study was modified to contain these two adventitious mutations to ensure that the viruses evaluated in the pre-clinical and clinical studies were identical in sequence.
The seed virus for the production of rDEN4Δ30-200,201 was produced in the Laboratory of Infectious Disease (LID) at the NIAID. A clinical lot of live recombinant DEN4Δ30-200,201 vaccine candidate was produced and safety tested at Charles River Laboratories Biopharmaceutical Services (Malvern, PA) with current Good Manufacturing Practices (GMP). Prior to administration, the vaccine was diluted to 105.3 PFU/mL with safety-tested L-15 medium, and 0.5 mL was drawn up in a 1-mL syringe labeled with the volunteer number. Placebo doses consisted of 0.5 mL of L-15 medium.
Virus quantitation
The level of viremia was determined using a standard plaque assay as previously described.11 Briefly, serum or plasma was diluted 10-fold in tissue culture medium and placed in duplicate wells of Vero cell monolayers. After virus was absorbed for 1 hour at 37°C, a methyl-cellulose overlay was added, and the cells were incubated for five days at 37°C. Virus plaques were then identified by immunoperoxidase staining with anti-DENV-4 antibody. Virus was also amplified by inoculating plasma or serum directly onto Vero cell monolayers and incubating for five days. Tissue culture fluids were then titrated for virus as described above.
Serologic assessment
Antibody response to DENV-4 was determined by 60% plaque reduction neutralization titer (PRNT60) assay on Vero cell monolayers as previously described.11 The PRNT60 was determined for serum samples collected from volunteers on study days 0, 28, 42, and 180. Seroconversion to DENV-4 was defined as a ≥ 4-fold rise in serum neutralizing antibody titer to the wild type DENV-4 parent virus (DENV-4 strain 814669, Dominica 1981) at study day 28 or 42, compared with the pre-vaccination PRNT60 titer. Antibody titers against DENV-4 induced by the rDEN4Δ30-200,201 vaccine were then directly compared with those induced by the rDEN4Δ30 parent virus. For this comparison, a separate PRNT60 assay was performed using serum samples originally collected on study day 42 from the 20 volunteers inoculated with 105 PFU of the rDEN4Δ30 vaccine11 and sera collected on study day 42 in the present study. The durability of the antibody response was determined by measurement of the PRNT60 at study day 180.
Data analysis
The purpose of this study was to describe differences in immune responses and the frequency of solicited adverse events rather than to test formal statistical hypotheses. Baseline characteristics and frequency of vaccine-related adverse events, graded by severity, were compared between vaccine and placebo groups, and additionally compared with a historical group of volunteers who received the rDEN4Δ30 parent vaccine virus at a dose of 105 PFU.11 Statistical significance was determined according to Fischer’s exact test using JMP 7 software version 5.0.1.2 (SAS Institute, Cary, NC). In addition, the entire cohort was analyzed for all adverse events by severity and relationship and reported with 95% confidence intervals.
RESULTS
Demographics
Twenty-eight healthy volunteers, 19 to 49 years of age, were enrolled in this study. The mean age for the vaccine group was 38.6 years compared with 37.1 years for the placebo group (P = 0.6). There was no significant difference in gender or ethnicity between the vaccine and placebo groups. Sixty percent of vaccinees were female. Thirteen volunteers in the vaccine group were African American, six were Caucasian, and one volunteer self-identified as multiracial. Twenty-seven volunteers completed the study and one volunteer was removed from the study shortly after study day 42 because of non-compliance with the study protocol. Data from this volunteer is included in all safety and serologic analysis with the exception of the day 180 antibody titer calculation. An additional volunteer revealed that she was pregnant at her day 180 visit. On the basis of information obtained from this volunteer’s obstetrician, it was determined that the date of conception was prior to study day 28. As specified by the clinical protocol, this volunteer’s immunogenicity data is not included in the analysis. However, this volunteer’s safety data has been included.
Local reactogenicity
Local reactogenicity, which may include injection site tenderness, erythema, and induration, was assessed 30 minutes post-vaccination and at each follow up study visit through study day 12. Only two vaccine recipients and one placebo recipient experienced any local reactogenicity, which was limited to mild tenderness at the injection site lasting no longer than 2 days. On physical examination, none of the volunteers were noted to have injection site erythema or induration.
Solicited adverse events
The vaccine was well tolerated by all vaccine recipients. There were no serious adverse events reported and none of the volunteers experienced a systemic dengue-like illness. There was no difference in the frequency or severity of adverse events including fever, headache, photophobia, malaise, nausea, myalgia, or arthralgia between the vaccine group and the placebo group (P = 0.865 for sum of all adverse events). The frequency of all solicited adverse events is presented in Table 1. The most commonly reported adverse event was headache. Six vaccinees and two placebo recipients experienced a headache. Four vaccinees experienced headaches that were mild in intensity and two vaccinees developed a headache of moderate intensity. During physical examinations on study days 5–7 it was noted that four vaccinees (20%) had a mild maculopapular rash that was non-pruritic and similar in character to that observed in recipients of the rDEN4Δ30 parent virus.11,14 The rash appeared on the abdomen, chest, back, and proximal upper extremities and was not found on any placebo recipient. The rash did not extend above the neck area. The duration of rash for all vaccinees was 10 days.
Table 1.
The number of volunteers vaccinated with rDEN4Δ30-200,201 or placebo that experienced a solicited adverse event
| rDEN4Δ30-200,201 (%) N = 20 | Placebo (%) N = 8 | |
|---|---|---|
| Fever | 1 (5) | 0 (0) |
| Headache | 6 (30) | 2 (25) |
| Arthralgia | 1 (5) | 1 (12.5) |
| Myalgia | 0 (0) | 0 (0) |
| Rash | 4 (20) | 0 (0) |
| Nausea | 2 (10) | 0 (0) |
| Retro-orbital pain | 0 (0) | 0 (0) |
| Photophobia | 0 (0) | 0 (0) |
| Malaise | 0 (0) | 0 (0) |
| Neutropenia | 2 (10) | 2 (25) |
| ↑ALT* | 0 (0) | 0 (0) |
| ↑PT† | 1 (5) | 0 (0) |
| ↓Platelets‡ | 1 (5) | 0 (0) |
| ↓Hemoglobin§ | 5 (25) | 1 (12.5) |
| ↑CPK¶ | 2 (10) | 0 (0) |
↑ Alanine aminotransferase (ALT) is defined as > 1.25 × upper limit of normal (ULN).
↑ Prothrombin time (PT) is defined as above the ULN.
↓ Platelet count is defined as < 120,000/mm3.
↓ Hemoglobin (Hgb) is defined as < 11.0 gm/dL.
↑ Creatine phosphokinase (CPK) is defined as > 4 × ULN.
One vaccinee recorded an elevated temperature (100.6°F) on one measurement on study day 15. The other temperatures recorded that day were normal. The volunteer had no other symptoms at the time of the temperature elevation and reported that he felt well at the time.
Hematological and serum chemistry abnormalities were uncommon. No vaccine recipient developed an ALT elevation during the course of the study. A transient neutropenia (defined as < 1,500/mm3) occurred in two vaccinees and two placebo recipients. One vaccine recipient developed neutropenia on three separate occasions (study days 3 through 9; 12 through 14; and 16 through 21). The nadir of the absolute neutrophil count was 800/mm3 (moderate) on study day 4 and the neutrophil count increased to 1,100/mm3 (mild) by study day 5. This same vaccinee also developed a mild [< 1.24 × upper limit of normal (ULN)] elevation in PT on study days 3 through 12 and again on study days 16 and 21. The volunteer had no evidence of bleeding or bruising and maintained a normal platelet count throughout the study. No other vaccinee had abnormal coagulation studies. The platelet count of one volunteer met criteria for thrombocytopenia (< 120,000/mm3) as it dropped from 128,000/mm3 on day 0 prior to vaccination and to 115,000/mm3 on day 5. The platelet count at the next visit had returned to 129,000/mm3. There was no evidence of petechiae or bleeding in this volunteer. Five vaccinees developed mild anemia (Hgb = 10g – 11.5 g/dL) that occurred between study days 8 through 16. All hemoglobin levels returned to normal by the next study visit. One placebo recipient also developed a mild anemia over the 16-day acute follow-up period. The probability of developing anemia was significantly greater in those volunteers with a baseline hemoglobin of ≤ 13 gm/dL (P = 0.0248), but was not significantly related to receipt of vaccine (P = 0.1578). Two placebo recipients developed a mild neutropenia, one lasting nine days and the other lasting 4 days.
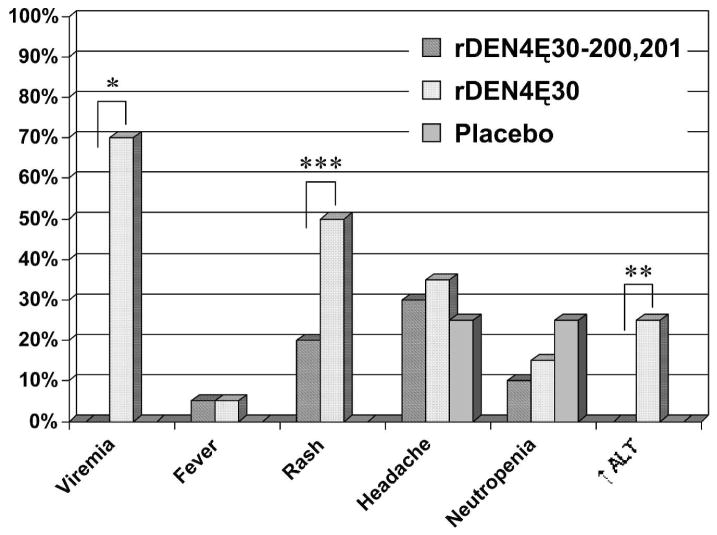
The incidence of rash, neutropenia, and ALT elevation were lower in recipients of rDEN4Δ30-200,201 compared with volunteers who received rDEN4Δ30 in a previous study (Figure 1). The difference in the frequency of rash and ALT elevation between the two groups was significant (P = 0.05 for rash and P = 0.02 for ALT elevation). The incidence of rash or neutropenia was not significantly different between rDEN4Δ30-200,201 vaccinees and placebo recipients.
Figure 1.
Frequency of selected clinical signs from volunteers receiving 105 plaque-forming unit (PFU) of rDEN4Δ30-200,201 vaccine candidate, 105 PFU of rDEN4Δ30 vaccine candidate,11 or placebo. The frequency of viremia, rash, and serum alanine aminotransferase (ALT) elevation were significantly lower among recipients of the rDEN4Δ30-200,201 vaccine compared with recipients of the rDEN4Δ30 vaccine (*, P = 0.0001; **, P = 0.02; ***, P = 0.05).
Viremia
Viremia was not detected in any vaccine recipient during the acute follow-up period. Viremia was not detectable by either direct titration of serum or after attempts to amplify virus by passage in tissue culture. Therefore, as shown in Table 2, the mean serum virus titer was at least 10-fold less in rDEN4Δ30-200,201 vaccinees compared with rDEN4Δ30 vaccinees. Although 70% of vaccinees who received 105 PFU of rDEN4Δ30 had detectable viremia, none of the 20 vaccinees who received rDEN4Δ30-200,201 were viremic (P < 0.0001). The absence of viremia should preclude transmission by mosquito.
Table 2.
rDEN4Δ30-200,201 is immunogenic despite its high level of attenuation
| Mean serum neutralizing antibody titer† (PRNT60)
|
|||||||
|---|---|---|---|---|---|---|---|
| Vaccine virus | N | % of volunteers with viremia | Mean peak virus titer ± SE* (log10 PFU/mL serum) | Day 28 | Day 42 | Day 180 | % Seroconversion|| |
| rDEN4Δ30-200,201 | 20 | 0 | < 0.5 | 100 | 79‡ | 22§ | 100 |
| rDEN4Δ30** | 20 | 70 | 1.6 ± 0.1 | 567 | 399 | ND¶ | 100 |
Calculated only for viremic volunteers. The lower limit of detection is 0.5 log10 plaque-forming unit (PFU)/mL serum.
Plaque reduction (60%) neutralization titer (PRNT60 expressed as reciprocal geometric mean). Serum samples collected during the course of each clinical trial were assayed together after study day 180 (rDEN4Δ30-200,201) or study day 42 (rDEN4Δ30). PRNT60 values for rDEN4Δ30 are as previously reported.14
N = 19. One vaccinee was eliminated from the immunological analysis because of pregnancy prior to study day 28.
N = 18. An additional volunteer was eliminated from the study prior to study day 180.
Not determined. Serum was not collected on day 180.
Percent seroconversion is defined as a 4-fold or greater increase in serum neutralizing antibody to DENV-4 on day 28 or day 42.
Serological response
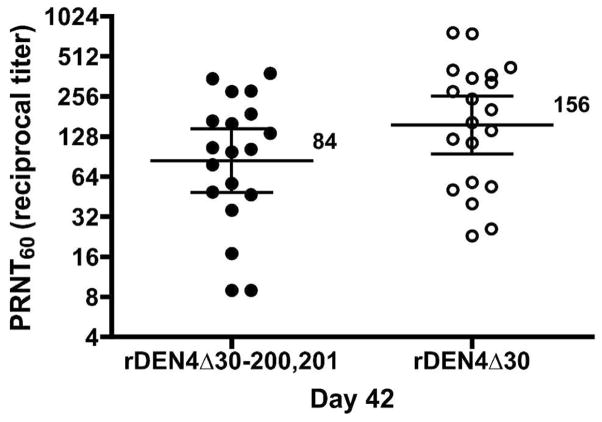
One vaccine recipient was excluded from the serological analysis because of pregnancy. All of the remaining nineteen vaccinees included in the day 28 and day 42 serological analyses seroconverted to DEN4. The geometric mean PRNT60 (reciprocal titer) at study day 28 was 100 (range: 40–530) and on study day 42 was 79 (range: 19–926) (Table 2). When evaluated in a single PRNT assay, the antibody titer induced by the vaccine candidate rDEN4Δ30-200,201 at study day 42 was only slightly lower than that induced by the rDEN4Δ30 parent virus (Figure 2). Sera from eighteen vaccinees were included in the day 180 serological analysis. Seventeen of the eighteen vaccinees who seroconverted to rDENΔ4-200,201 maintained their antibody titer through study day 180, however the mean titer decreased to 22 (range < 10–52).
Figure 2.
Direct comparison of the PRNT60 at day 42 of volunteers vaccinated with rDEN4Δ30-200,201 or rDEN4Δ30. Vaccinees in each trial received 105 plaque-forming unit (PFU) of vaccine as a single subcutaneous dose. Day 42 serum samples collected from vaccinees enrolled in this rDEN4Δ30-200,201 clinical trial and day 42 serum samples retained from vaccinees enrolled in the previous rDEN4Δ30 clinical trial were evaluated in the same PRNT assay. The geometric mean titer is shown and standard deviation levels are indicated.
DISCUSSION
Because of their ability to induce durable humoral and cellular immune responses, live attenuated DENV vaccines are desirable for the control of dengue disease. However, several factors have complicated the development of effective live DENV vaccines, including the lack of an animal model for dengue disease, the lack of a clearly defined clinical correlate of protection, and the requirement to simultaneously protect against all four serotypes while maintaining the delicate balance between immunogenicity and vaccine reactogenicity. In the face of these obstacles, carefully designed clinical evaluation is paramount in determining the suitability of experimental vaccines for further development. Because live attenuated vaccines contain replicating viruses, it is expected that their use will be accompanied by some clinical signs of infection. Symptoms such as high fever, headache, myalgia, arthralgia, bleeding, or eye pain that are seen in natural dengue infection would certainly not be acceptable after vaccination, whereas asymptomatic or sub-clinical signs of infection such as transient leukopenia, mild liver enzyme elevations, mild rash, and low level viremia may be acceptable. Vaccines that elicit only mild reactogenicity while inducing a protective level of immunity may be considered suitable for further development. Unfortunately, decreased reactogenicity as a result of decreased virus replication is often accompanied by decreased immunogenicity. To some extent, this trade-off can be mitigated by changes in dose potency or administration of a second dose of vaccine. To decrease the reactogenicity we observed after a high dose (105 PFU) of rDEN4Δ30, we sought to develop a further attenuated vaccine candidate, focusing on the selection of mutations that specifically limited replication in liver cells rather than relying solely on modifying the dosage to achieve a proper balance between attenuation and immunogenicity. One promising vaccine candidate resulting from this effort was rDEN4Δ30-200,201.
We compared the frequency and severity of solicited adverse events and level of viremia between recipients of 105 PFU of rDEN4Δ30-200,201 and recipients of 105 PFU of rDEN4Δ30, administered in a previous vaccine trial.11 Most notably, none of the volunteers vaccinated with rDEN4Δ30-200,201 had detectable viremia at any time-point during the study compared with 70% of recipients of rDEN4Δ30, a finding similar to that observed in rhesus monkeys.26 The rDEN4Δ30-200,201 induced a dengue-like rash in fewer volunteers than the parent vaccine rDEN4Δ30. In addition, no volunteer who received rDEN4Δ30-200,201 developed an ALT elevation above the upper limit of the laboratory normal, compared with 25% volunteers who received rDEN4Δ30. The abrogation of mild hepatotoxicity by the 200,201 mutation suggests that this mutation restricts replication of the vaccine in the liver of vaccine recipients. It is also possible that this mutation reduces the replication of the virus at other peripheral sites as well.
Although viremia was not detectable in rDEN4Δ30-200,201 vaccinees, the vaccine virus, like its rDEN4Δ30 parent, infected all vaccinees as evidenced by a 100% seroconversion rate. Neutralizing antibody titers in serum from vaccinees in this study were compared in the same PRNT assay to serum collected from volunteers who received 105 PFU of rDEN4Δ30 in our previous trial.27 The level of neutralizing antibody induced by rDEN4Δ30-200,201 at study day 42 was only slightly lower than that induced by rDEN4Δ30, a surprisingly insignificant trade-off considering the decreased reactogenicity. In addition, the antibody response generated by a single dose of rDEN4Δ30-200,201 was remarkably durable, as 94% of vaccinees maintained detectable antibody levels through study day 180.
On the basis of the lack of detectable viremia, lower reactogenicity, and robust immunogenicity of rDEN4Δ30-200,201, this vaccine candidate can be considered for inclusion in a tetravalent vaccine formulation. However, to date this vaccine candidate has only been studied at a single dose level (105 PFU), and additional studies are in progress to define the 50% human infectious dose of rDEN4Δ30-200,201. Considered together with previous clinical findings of rDEN4Δ30, we have identified two suitable vaccine candidates for DENV-4: rDEN4Δ30-200,201 and rDEN4Δ30. We currently consider the rDEN4Δ30 vaccine our lead candidate for inclusion in the tetravalent vaccine for two reasons. First, at a proposed dose of 103 PFU, it is economical to produce and has an acceptable safety profile. Second, rDEN4Δ30 appears slightly more immunogenic in humans than rDEN4Δ30-200,201, and this greater immunogenicity likely would be translated into more durable immunity. However, the present results with rDEN4Δ30-200,201 are very encouraging, not only because they identify a valuable backup vaccine candidate if rDEN4Δ30 has unanticipated adverse reaction upon further testing in humans, but also because the results demonstrate that it is feasible to use genetic techniques to introduce defined mutations into a dengue virus genome to abrogate a specific adverse effect, in this instance, hepatotoxicity.
Acknowledgments
This research was supported by the Intramural Research Program of the NIAID, National Institutes of Health.
Footnotes
Note: This study was registered at clinicaltrials.gov NCT00270699.
Authors’ addresses: Julie H. McArthur, Anna P. Durbin, and Jennifer Marron, Center for Immunization Research, 624 N. Broadway, Room 251, Baltimore, MD 21205, Tel: 410-614-4736, Fax: 410-502-6898, E-mails: jmcarthu@jhsph.edu, adurbin@jhsph.edu, and jmarron@jhsph.edu. Kimberli A. Wanionek and Bhavin Thumar, Center for Immunization Research, 615 North Wolfe Street, Room E5601, Baltimore, MD 21205, Tel: 410-955-7230, Fax: 443-287-3167, E-mails: kwanione@jhsph.edu and bthumar@jhsph.edu. Dennis J. Pierro, Alexander C. Schmidt, and Brian R. Murphy, Laboratory of Infectious Diseases, NIAID, NIH, 50 South Drive, Bethesda, MD 20892, Tel: 301-594-1616, Fax: 301-480-5033, E-mails: pierrod@mail.nih.gov, as337y@nih.gov, and bmurphy@niaid.nih.gov. Joseph E. Blaney Jr and Stephen S. Whitehead, Laboratory of Infectious Diseases, NIAID, NIH, 33 North Drive, Bethesda, MD 20892, Tel: 301-496-7692, Fax: 301-480-4873, Emails: jblaney@niaid.nih.gov and swhitehead@niaid.nih.gov.
References
- 1.Suaya JA, Shepard DS, Beatty ME. Scientific Working Group on Dengue—2006 Report. Geneva: WHO; 2006. Dengue: Burden of Disease and Costs of Illness; pp. 35–49. [Google Scholar]
- 2.Mathers CD, Ezzati M, Lopez AD. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl Trop Dis. 2007;1:e114. doi: 10.1371/journal.pntd.0000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. Geneva: WHO; 1997. [Google Scholar]
- 4.Sabin A. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 5.Okuno Y, Fukunaga T, Tadano M, Fukai K, Ikeda T, Sekii K, Ariyoshi H. Serological studies on volunteers inoculated experimentally with a dengue virus strain in 1943. Biken J. 1983;26:161–163. [PubMed] [Google Scholar]
- 6.Papaevangelou G, Halstead SB. Infections with two dengue viruses in Greece in the 20th century. Did dengue hemorrhagic fever occur in the 1928 epidemic? Am J Trop Med Hyg. 1977;80:46–51. [PubMed] [Google Scholar]
- 7.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 8.Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg. 1989;40:444–451. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- 9.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol. 2007;5:518–528. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- 10.Blaney JE, Jr, Durbin AP, Murphy BR, Whitehead SS. Development of a live attenuated dengue virus vaccine using reverse genetics. Viral Immunol. 2006;19:10–32. doi: 10.1089/vim.2006.19.10. [DOI] [PubMed] [Google Scholar]
- 11.Durbin AP, Karron RA, Sun W, Vaughn DW, Reynolds MJ, Perreault JR, Thumar B, Men R, Lai CJ, Elkins WR, Chanock RM, Murphy BR, Whitehead SS. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am J Trop Med Hyg. 2001;65:405–413. doi: 10.4269/ajtmh.2001.65.405. [DOI] [PubMed] [Google Scholar]
- 12.Durbin AP, McArthur J, Marron JA, Blaney JE, Jr, Thumar B, Wanionek K, Murphy BR, Whitehead SS. The live attenuated dengue serotype 1 vaccine rDEN1Delta30 is safe and highly immunogenic in healthy adult volunteers. Hum Vaccin. 2006;2:167–173. doi: 10.4161/hv.2.4.2944. [DOI] [PubMed] [Google Scholar]
- 13.Durbin AP, McArthur JH, Marron JA, Blaney JE, Thumar B, Wanionek K, Murphy BR, Whitehead SS. rDEN2/4Delta30(ME), a live attenuated chimeric dengue serotype 2 vaccine is safe and highly immunogenic in healthy dengue-naive adults. Hum Vaccin. 2006;2:255–260. doi: 10.4161/hv.2.6.3494. [DOI] [PubMed] [Google Scholar]
- 14.Durbin AP, Whitehead SS, McArthur J, Perreault JR, Blaney JE, Jr, Thumar B, Murphy BR, Karron RA. rDEN4 Delta 30, a live attenuated dengue virus type 4 vaccine candidate, is safe, immunogenic, and highly infectious in healthy adult volunteers. J Infect Dis. 2005;191:710–718. doi: 10.1086/427780. [DOI] [PubMed] [Google Scholar]
- 15.Kanesa-thasan N, Sun W, Kim-Ahn G, Van Albert S, Putnak JR, King A, Raengsakulsrach B, Christ-Schmidt H, Gilson K, Zahradnik JM, Vaughn DW, Innis BL, Saluzzo JF, Hoke CH., Jr Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers. Vaccine. 2001;19:3179–3188. doi: 10.1016/s0264-410x(01)00020-2. [DOI] [PubMed] [Google Scholar]
- 16.Vaughn DW, Hoke CH, Jr, Yoksan S, LaChance R, Innis BL, Rice RM, Bhamarapravati N. Testing of a dengue 2 live-attenuated vaccine (strain 16681 PDK 53) in ten American volunteers. Vaccine. 1996;14:329–336. doi: 10.1016/0264-410x(95)00167-y. [DOI] [PubMed] [Google Scholar]
- 17.Eckels KH, Scott RM, Bancroft WH, Brown J, Dubois DR, Summers PL, Russell PK, Halstead SB. Selection of attenuated dengue 4 viruses by serial passage in primary kidney cells. V. Human response to immunization with a candidate vaccine prepared in fetal rhesus lung cells. Am J Trop Med Hyg. 1984;33:684–689. doi: 10.4269/ajtmh.1984.33.684. [DOI] [PubMed] [Google Scholar]
- 18.Edelman R, Tacket CO, Wasserman SS, Vaughn DW, Eckels KH, Dubois DR, Summers PL, Hoke CH. A live attenuated dengue-1 vaccine candidate (45AZ5) passaged in primary dog kidney cell culture is attenuated and immunogenic for humans. J Infect Dis. 1994;170:1448–1455. doi: 10.1093/infdis/170.6.1448. [DOI] [PubMed] [Google Scholar]
- 19.Kalayanarooj S, Vaughn DW, Nimmannitya S, Green S, Suntayakorn S, Kunentrasai N, Viramitrachai W, Ratanachueke S, Kiatpolpoj S, Innis BL, Rothman AL, Nisalak A, Ennis FA. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176:313–321. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- 20.Kuo CH, Tai DI, Chang Chien CS, Lan CK, Chiou SS, Liaw YF. Liver biochemical tests and dengue fever. Am J Trop Med Hyg. 1992;47:265–270. doi: 10.4269/ajtmh.1992.47.265. [DOI] [PubMed] [Google Scholar]
- 21.Mohan B, Patwari AK, Anand VK. Hepatic dysfunction in childhood dengue infection. J Trop Pediatr. 2000;46:40–43. doi: 10.1093/tropej/46.1.40. [DOI] [PubMed] [Google Scholar]
- 22.Wahid SF, Sanusi S, Zawawi MM, Ali RA. A comparison of the pattern of liver involvement in dengue hemorrhagic fever with classic dengue fever. Southeast Asian J Trop Med Public Health. 2000;31:259–263. [PubMed] [Google Scholar]
- 23.Liu HW, Ho TL, Hwang CS, Liao YH. Clinical observations of virologically confirmed dengue fever in the 1987 outbreak in southern Taiwan. Kao Hsiung I Hsueh Ko Hsueh Tsa Chih. 1989;5:42–49. [PubMed] [Google Scholar]
- 24.Lin YL, Liu CC, Lei HY, Yeh TM, Lin YS, Chen RM, Liu HS. Infection of five human liver cell lines by dengue-2 virus. J Med Virol. 2000;60:425–431. doi: 10.1002/(sici)1096-9071(200004)60:4<425::aid-jmv10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 25.Hanley KA, Lee JJ, Blaney JE, Jr, Murphy BR, Whitehead SS. Paired charge-to-alanine mutagenesis of dengue virus type 4 NS5 generates mutants with temperature-sensitive, host range, and mouse attenuation phenotypes. J Virol. 2002;76:525–531. doi: 10.1128/JVI.76.2.525-531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanley KA, Manlucu LR, Manipon GG, Hanson CT, Whitehead SS, Murphy BR, Blaney JE., Jr Introduction of mutations into the non-structural genes or 3′ untranslated region of an attenuated dengue virus type 4 vaccine candidate further decreases replication in rhesus monkeys while retaining protective immunity. Vaccine. 2004;22:3440–3448. doi: 10.1016/j.vaccine.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 27.Troyer JM, Hanley KA, Whitehead SS, Strickman D, Karron RA, Durbin AP, Murphy BR. A live attenuated recombinant dengue-4 virus vaccine candidate with restricted capacity for dissemination in mosquitoes and lack of transmission from vaccinees to mosquitoes. Am J Trop Med Hyg. 2001;65:414–419. doi: 10.4269/ajtmh.2001.65.414. [DOI] [PubMed] [Google Scholar]