Abstract
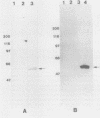
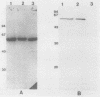
Chlamydia pneumoniae has emerged as an important human respiratory pathogen. From a lambda gt11 gene bank constructed from C. pneumoniae isolate AR-39 DNA, an immunoreactive plaque containing a 3.0-kb insert was purified. In immunoblots, a 60-kDa protein was recognized by anti-C. pneumoniae rabbit immune serum. The recombinant protein was reactive with a Chlamydia genus-specific monoclonal antibody recognizing a 60-kDa protein found in the Sarkosyl-soluble fraction and with rabbit immune serum prepared against the Chlamydia trachomatis 60-kDa GroEL homolog associated with the delayed-type hypersensitivity response. DNA sequence analysis confirmed that the C. pneumoniae gene product is an analog of the C. trachomatis delayed-type hypersensitivity antigen and the Escherichia coli GroEL heat shock protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. E., Cerrone M. C., Beatty P. R., Stephens R. S. Cysteine-rich outer membrane proteins of Chlamydia trachomatis display compensatory sequence changes between biovariants. Mol Microbiol. 1990 Sep;4(9):1543–1550. doi: 10.1111/j.1365-2958.1990.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Bavoil P., Stephens R. S., Falkow S. A soluble 60 kiloDalton antigen of Chlamydia spp. is a homologue of Escherichia coli GroEL. Mol Microbiol. 1990 Mar;4(3):461–469. doi: 10.1111/j.1365-2958.1990.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Brunham R. C., Maclean I. W., Binns B., Peeling R. W. Chlamydia trachomatis: its role in tubal infertility. J Infect Dis. 1985 Dec;152(6):1275–1282. doi: 10.1093/infdis/152.6.1275. [DOI] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. A., Kuo C. C., Grayston J. T. Structural and antigenic analysis of Chlamydia pneumoniae. Infect Immun. 1990 Jan;58(1):93–97. doi: 10.1128/iai.58.1.93-97.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. A., Kuo C. C., Thissen R. W., Grayston J. T. Isolation of a gene encoding a Chlamydia sp. strain TWAR protein that is recognized during infection of humans. Infect Immun. 1989 Jan;57(1):71–75. doi: 10.1128/iai.57.1.71-75.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. A., Kuo C. C., Wang S. P., Grayston J. T. Serological response to Chlamydia pneumoniae infection. J Clin Microbiol. 1990 Jun;28(6):1261–1264. doi: 10.1128/jcm.28.6.1261-1264.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrone M. C., Ma J. J., Stephens R. S. Cloning and sequence of the gene for heat shock protein 60 from Chlamydia trachomatis and immunological reactivity of the protein. Infect Immun. 1991 Jan;59(1):79–90. doi: 10.1128/iai.59.1.79-90.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly R. J., Torian B. E., Stibbs H. H. Identification of a surface antigen of Trichomonas vaginalis. Infect Immun. 1985 Aug;49(2):270–274. doi: 10.1128/iai.49.2.270-274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graeff-Meeder E. R., van der Zee R., Rijkers G. T., Schuurman H. J., Kuis W., Bijlsma J. W., Zegers B. J., van Eden W. Recognition of human 60 kD heat shock protein by mononuclear cells from patients with juvenile chronic arthritis. Lancet. 1991 Jun 8;337(8754):1368–1372. doi: 10.1016/0140-6736(91)93057-g. [DOI] [PubMed] [Google Scholar]
- Erntell M., Ljunggren K., Gadd T., Persson K. Erythema nodosum--a manifestation of Chlamydia pneumoniae (strain TWAR) infection. Scand J Infect Dis. 1989;21(6):693–696. doi: 10.3109/00365548909021699. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Kuo C. C., Wang S. P., Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986 Jul 17;315(3):161–168. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. P., Kuo C. C., Campbell L. A. Current knowledge on Chlamydia pneumoniae, strain TWAR, an important cause of pneumonia and other acute respiratory diseases. Eur J Clin Microbiol Infect Dis. 1989 Mar;8(3):191–202. doi: 10.1007/BF01965260. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. P., Yeh L. J., Kuo C. C. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985 Nov-Dec;7(6):717–725. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- Howard L., Orenstein N. S., King N. W. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974 Jan;27(1):102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman R. D., Johnston M. E., Chiu B., Falk J., Petric M. Immunochemical analysis of immune response to Chlamydia trachomatis in Reiter's syndrome and nonspecific urethritis. Clin Exp Immunol. 1987 Aug;69(2):246–254. [PMC free article] [PubMed] [Google Scholar]
- Kihlström E., Grönberg A., Bengtsson A. Immunoblot analysis of antibody response to Chlamydia trachomatis in patients with reactive arthritis and ankylosing spondylitis. Scand J Rheumatol. 1989;18(6):377–383. doi: 10.3109/03009748909102099. [DOI] [PubMed] [Google Scholar]
- Kornak J. M., Kuo C. C., Campbell L. A. Sequence analysis of the gene encoding the Chlamydia pneumoniae DnaK protein homolog. Infect Immun. 1991 Feb;59(2):721–725. doi: 10.1128/iai.59.2.721-725.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mierendorf R. C., Percy C., Young R. A. Gene isolation by screening lambda gt11 libraries with antibodies. Methods Enzymol. 1987;152:458–469. doi: 10.1016/0076-6879(87)52054-7. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Melgosa M., Kuo C. C., Campbell L. A. Sequence analysis of the major outer membrane protein gene of Chlamydia pneumoniae. Infect Immun. 1991 Jun;59(6):2195–2199. doi: 10.1128/iai.59.6.2195-2199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikku P., Leinonen M., Mattila K., Ekman M. R., Nieminen M. S., Mäkelä P. H., Huttunen J. K., Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988 Oct 29;2(8618):983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson A. F., Kuo C. C. Evidence that the major outer membrane protein of Chlamydia trachomatis is glycosylated. Infect Immun. 1991 Jun;59(6):2120–2125. doi: 10.1128/iai.59.6.2120-2125.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagar E. A., Schachter J., Bavoil P., Stephens R. S. Differential human serologic response to two 60,000 molecular weight Chlamydia trachomatis antigens. J Infect Dis. 1990 Oct;162(4):922–927. doi: 10.1093/infdis/162.4.922. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]