SUMMARY
Cells' ability to detect and orient themselves in chemoattractant gradients has been the subject of numerous studies, but the underlying molecular mechanisms remain largely unknown [1]. Ras activation is the earliest polarized response to chemoattractant gradients downstream from heterotrimeric G proteins in Dictyostelium and inhibition of Ras signaling results in directional migration defects [2]. Activated Ras is enriched at the leading edge, promoting the localized activation of key chemotactic effectors, such as PI3K and TORC2 [2-5]. To investigate the role of Ras in directional sensing, we studied the effect of its mis-regulation using cells with disrupted RasGAP activity. We identified an orthologue of mammalian NF1, DdNF1, as a major regulator of Ras activity in Dictyostelium. We show that disruption of nfaA leads to spatially and temporally unregulated Ras activity, causing cytokinesis and chemotaxis defects. Using unpolarized, latrunculin-treated cells, we show that tight regulation of Ras is important for gradient sensing. Together, our findings suggest that Ras is part of the cell's compass, and that the RasGAP-mediated regulation of Ras activity affects directional sensing.
Keywords: chemotaxis, Ras, GAP, Dictyostelium, gradient sensing
RESULTS
The RasGAP DdNF1 regulates chemotaxis in Dictyostelium cells
To investigate the potential role of Ras in directional sensing during Dictyostelium chemotaxis, we sought to disrupt the regulation of Ras by targeting RasGAP (GTPase activating protein for Ras) function. RasGAPs are negative regulators of Ras proteins, promoting their deactivation by stimulating their intrinsic GTPase activity. We found that, of the seven putative Dictyostelium RasGAP-encoding genes, disruption of one in particular, nfaA (dictybase DDB0233763; Figures S1A-C), results in severe chemotaxis defects (Figure 1). nfaA encodes DdNF1, a putative orthologue of the human RasGAP NF1 (neurofibromin), which regulates p21-Ras signaling and acts as a tumor suppressor [6]. nfaA- cells display delayed aggregation upon starvation on non-nutrient agar, most likely resulting from their inability to efficiently perform chemotaxis, but their development is otherwise comparable to that of wild-type cells, as shown by the expression profile of the developmentally regulated cAMP receptor cAR1 and their ability to fully respond to uniform chemoattractant stimulation (Figures S1D-E and data described below).
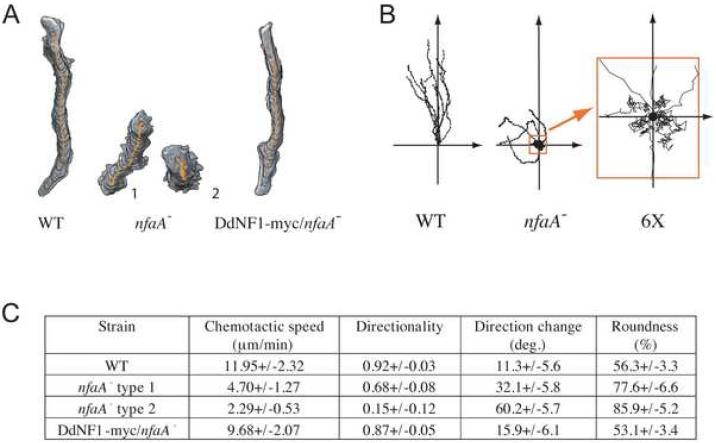
Figure 1. DdNF1 Regulates Chemotaxis.
Chemotaxis assays were performed and analyzed using DIAS as described previously [4, 20, 25-27]. (A) Traces of representative chemotaxing cells in an exponential cAMP gradient delivered by a micropipette. Two types of chemotactic nfaA- cells are shown, type 1 and type 2, representative of cells in the steep and shallow parts of the gradient. (B) Traces of cells chemotaxing in a linear gradient (Dunn chamber). The starting point of each migrating cell was apposed to the axis' origin. A 6X close-up of the nfaA- cells' traces near the origin is shown. (C) DIAS analysis of at least 10 traces from at least 3 independent experiments on cells migrating in an exponential gradient [27]. Speed refers to the speed of the cell's centroid movement along the total path; directionality indicates migration straightness; direction change refers to the number and frequency of turns; and roundness indicates the cell polarity.
Upon exposure to an exponential chemoattractant gradient created by a micropipette containing chemoattractant, wild-type cells rapidly polarize and migrate up the gradient, with >90% of their produced pseudopodia extended forward, towards the chemoattractant source, most of which persist for more than 2 min (Figures 1A and S2; Movie M1). In contrast, nfaA- cells exposed to the exponential chemoattractant gradient display major polarity and chemotaxis defects, as indicated by reduced migration speed and directionality (Figures 1A and 1C; Movie M2). Although nfaA- cells rapidly respond by extending multiple membrane protrusions, most of these are not extended forward, towards the chemoattractant source (Figure S2; Movie M2). Some cells close to the micropipette break their symmetry after a prolonged exposure to the steep chemoattractant gradient and then slowly migrate, but with only ∼50% of the pseudopodia extended forward (nfaA- type 1 cells). Most cells farther from the micropipette (in the shallow and weaker part of the gradient) do not polarize, move very little, and extend pseudopodia randomly relative to the direction of the chemoattractant source that have an average persistence of only ∼40 sec (nfaA- type 2; Figures 1A, 1C, and S2). These chemotaxis defects are even clearer when analyzing the behavior of nfaA- cells placed in a linear chemoattractant gradient using a Dunn chamber (Figure 1B). Whereas wild-type cells become polarized and efficiently migrate up the gradient (Movie M3), the majority of nfaA- cells move randomly relative to the axis of the gradient (Movie M4). Expression of myc-tagged DdNF1 in nfaA- cells rescues these chemotaxis defects (Figures 1A and 1C). These results suggest that DdNF1 regulates one or more Ras signaling pathways that control chemotaxis and, therefore, nfaA- cells provide an ideal cellular context in which to assess the potential role of Ras in directional sensing.
Temporal as well as spatial regulation of Ras activity is crucial to chemotaxis
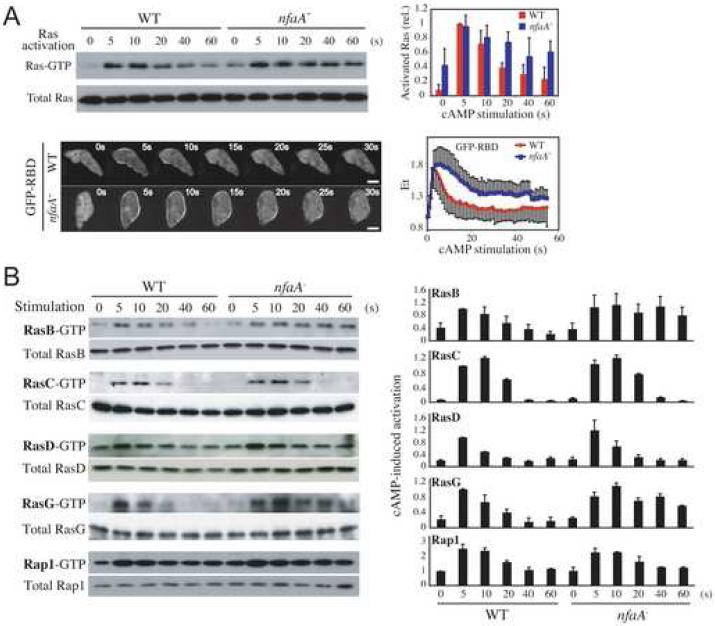
Using a pull-down assay, we show that nfaA- cells display elevated basal levels as well as extended kinetics of cAMP-induced Ras activation compared to those of wild-type cells, which we confirmed by live cell imaging (Figure 2A). In addition, we found that the kinetics of activation of the RasG protein in particular, which have been linked to the regulation of PI3K (phosphatidylinositol-3 kinase) during chemotaxis [2, 7], are delayed and extended considerably in nfaA- cells compared to the RasG activation profile in wild-type cells (Figure 2B). In contrast, chemoattractant-induced activation of RasD, Rap1, and RasC, which also regulates Dictyostelium chemotaxis [7-9], is unaffected. Interestingly, we observed that the kinetics of RasB activation, which was recently suggested to regulate myosin function [10], are extended. However, we observed that cells in which both rasG and nfaA were disrupted display a rasG- chemotaxis phenotype, which suggests that although DdNF1 can regulate RasB, the nfaA- chemotaxis phenotypes mostly result from the mis-regulation of RasG (Figures S3A and S3B).
Figure 2. The DdNF1-Mediated Regulation of RasG Activity Controls Chemotaxis.
(A) cAMP-induced Ras activation detected in a pull-down assay (upper panels) and live cell imaging of GFP-RBD (lower panels) upon uniform cAMP stimulation. Ras-GTP or total Ras proteins were detected in a Western blot. Quantification of the pull-down data and the relative fluorescence intensity of membrane-localized GFP-RBD are shown on the right. Bar = 5 μm. (B) cAMP-induced activation of Rap1 and exogenously expressed FLAG-RasB, -RasC, -RasG, and myc-RasD was assessed in pull-down assays. The Ras proteins were detected by Western blot with anti-Ras (Ab-3), anti-FLAG (M2), anti-myc (9E10), or anti-Rap1 antibodies. Quantification of data is shown on the right. Quantified data represent mean ± SD of at least 3 independent experiments.
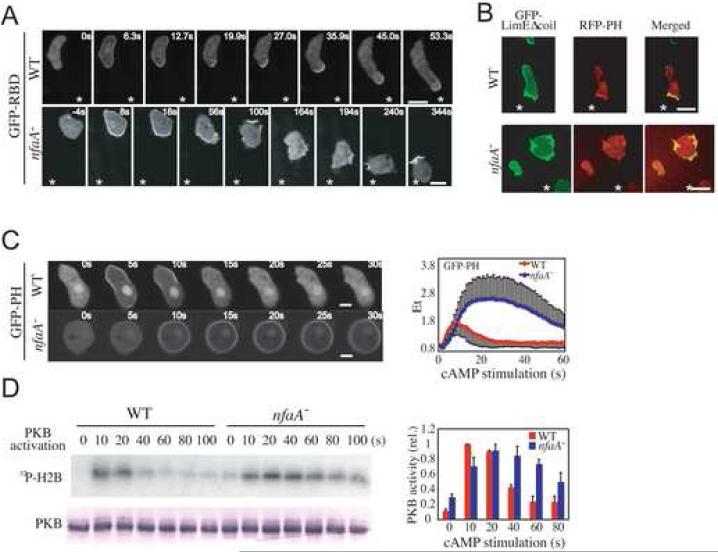
Interestingly, we found that Ras activity is also spatially mis-regulated in chemotaxing nfaA- cells (Figure 3A). Although wild-type and nfaA- cells exhibit a similar uniform Ras activation along the cell cortex upon the initial introduction of the chemoattractant-emitting micropipette, Ras activity in nfaA- cells takes longer to adapt compared to wild-type cells. Then, whereas activated Ras is enriched at the leading edge of chemotaxing wild-type cells (Movie M5), as previously described [2], Ras activity is not spatially restricted relative to the chemoattractant gradient in nfaA- cells, as indicated by the constantly changing localization of the Ras-GTP reporter GFP-RBD (Movie M6). Accumulation of Ras-GTP seems to occur at random sites along the plasma membrane of chemotaxing nfaA- cells, resulting in multiple and sometimes simultaneous lamellipod-like extensions and no defined leading edge.
Figure 3. RasGAP Activity Spatio-Temporally Regulates Ras Signaling.
(A) Imaging of GFP-RBD in cells migrating in an exponential cAMP gradient. *, position of the micropipette. Bar = 10 μm. See Experimental Procedures for details. (B) Imaging of the RFP-PH and GFP-LimEΔcoil in cells migrating in an exponential cAMP gradient. *, position of the micropipette. Bar = 10 μm. (C) Imaging of GFPPH upon uniform cAMP stimulation. Bar = 5 μm. The relative fluorescence intensity of membrane-localized GFP-PH is shown on the right. (D) Activity of immunopurified PKB determined in a kinase assay using the H2B substrate. Quantification of the data is shown on the right. Quantified data represent mean ± SD of at least 3 independent experiments.
PI3K is activated at the leading edge of chemotaxing Dictyostelium cells in a Ras-dependent fashion, resulting in the restricted accumulation of PI(3,4,5)P3 (phosphatidylinositol-3,4,5-triphosphate) and the local recruitment of PI(3,4,5)P3-binding proteins, many of which are modulators of the actin cytoskeleton and coordinate pseudopod protrusion [2, 4, 11-14]. In Figure 3C, we show that PI(3,4,5)P3 production, as detected with a reporter consisting of the PH domain of CRAC (cytosolic regulator of adenylyl cyclase) fused to GFP (GFP-PH), is delayed and considerably prolonged in nfaA- compared to wild-type cells, as is PKB activation (Figure 3D). Although RFP-PH accumulates at the forming and established leading edge in chemotaxing wild-type cells, the PI(3,4,5)P3 reporter localizes to multiple and seemingly random sites along the plasma membrane of nfaA- cells, reminiscent of the localization of active Ras, which also corresponds to sites of F-actin polymerization as shown by the co-localization with the F-actin reporter GFP-LimEΔcoil [15] (Figure 3B; Movies M7 and M8). Basal and cAMP-induced F-actin polymerization was found to be elevated in nfaA- cells compared to wild-type cells (Figure S7A), which is consistent with the observed presence of numerous F-actin-rich membrane protrusions in migrating nfaA- cells. These results suggest that tight RasGAP-mediated regulation of the chemoattractant-induced Ras activity is essential to temporally and spatially restrict the accumulation of Ras-GTP, which directly determines the site of pseudopod protrusion and, therefore, the direction of migration. A similar extended PI(3,4,5)P3 response is observed in rasG- cells expressing constitutively active RasGQ61L (Figures S3C and S3D), which is consistent with RasG and DdNF1 regulating PI3K activity.
Directional sensing requires tightly regulated Ras activity
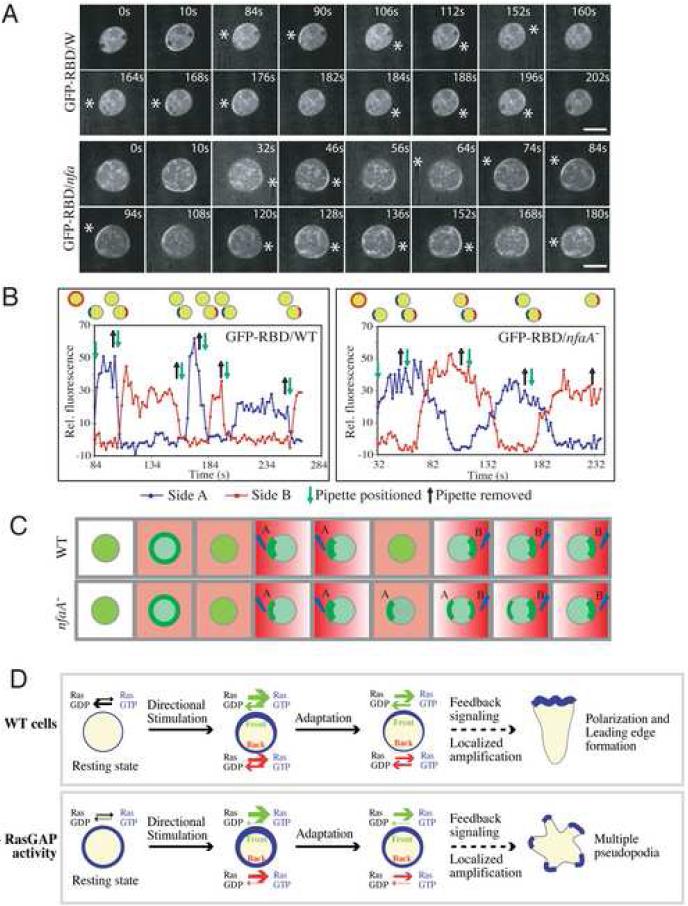
Although evidence suggests that directional sensing involves mechanisms that do not require global cell polarity or an intact cytoskeleton [16], F-actin-dependent positive feedback loops play an important role in the amplification of the PI(3,4,5)P3 signal, in part, through the up-regulation of Ras and PI3K signaling [2, 17]. Therefore, to determine whether the regulation of Ras activity directly affects gradient sensing independently of its role in controlling pseudopod formation, we assessed the spatio-temporal activation of Ras in cells treated with the F-actin polymerization inhibitor Latrunculin B (LatB), which generates motility-paralyzed, symmetrical, and spherical cells without pseudopodia [12]. As previously reported [2], the kinetics and the spatial activation of Ras in wild-type cells exposed to a chemoattractant gradient are unaffected by LatB treatment, as revealed by the localization profile of GFP-RBD (Figure 4A). After the initial uniform activation and adaptation that follow placing the chemoattractant-emitting micropipette in proximity to the cell, GFP-RBD rapidly accumulates in a crescent shape along the plasma membrane closest to the chemoattractant source. Upon repositioning of the micropipette, GFP-RBD is rapidly delocalized from its previous site and rapidly accumulates at the site on the cortex closest to the new position of the micropipette, reflecting the prompt deactivation and activation of Ras at each site, respectively (Movie 9). Interestingly, in LatB-treated nfaA- cells, after repositioning the micropipette, we observed a considerable delay (∼40 sec) before GFP-RBD fully dissociated from its original site on the plasma membrane, as might be expected from a loss of GAP activity. Unexpectedly, however, the chemoattractant-induced Ras activity at the new site closest to the chemoattractant source was also noticeably delayed, as illustrated by the slower rise in Ras-GTP levels, which took ∼30 sec to reach their maximum in nfaA- cells compared to <10 sec in wild-type cells (Figure 4B). As a result, two crescents of plasma membrane-localized GFP-RBD were observed simultaneously, which never occurred in wild-type cells, demonstrating that the mis-regulation of Ras activity affects the ability of cells to sense changes in gradient orientation (Figure 4; Movie 10). These findings provide experimental evidence for Onsum and Rao's prediction in their mathematical model of gradient sensing that cells with impaired RasGAPs would respond sluggishly to changes in the direction of the gradients [18].
Figure 4. Depletion of RasGAP Activity Causes Gradient Sensing Defects.
(A) Imaging of GFP-RBD in LatB-treated cells responding to changes in cAMP gradient orientation. *, position of the micropipette. Data are representative of at least 3 independent experiments. Bar = 10 μm. (B) Quantification of relative fluorescence intensity of membrane-localized GFP-RBD in (A). (C) Illustration of GFP-RBD translocation kinetics upon changes in gradient orientation. (D) RasGAP regulation of Ras helps the cells determine the direction of a chemoattractant gradient. In the resting state, nfaA- cells display elevated levels of Ras-GTP compared to wild-type cells due to the slow intrinsic GTPase activity of Ras. In wild-type cells, upon directional chemoattractant stimulation, there is a global activation of Ras along the cell's plasma membrane, which rapidly adapts. Low levels of polarized Ras activity at the plasma membrane that persist in the direction of the gradient lead to the local polymerization of F-actin and leading edge formation through signal amplification (see text). We speculate that in nfaA- cells, the high levels of Ras-GTP that persist, even after the global adaptation that follows the initial stimulation, trigger feedback signaling and amplification of the signal all around the cell and cause the extension of pseudopodia in every angle relative to the chemoattractant gradient.
DISCUSSION
A growing body of evidence suggests that chemoattractant-mediated PI3K signaling is mostly involved in controlling the motility of chemotaxing cells through modulation of the cytoskeleton, with the cell's compass located upstream of PI3K [1]. Ras is therefore in an ideal position within the chemotactic signaling cascade to be implicated in directional sensing, but substantial evidence has been lacking. The identification of DdNF1 as a major negative regulator of Ras activity, and RasG in particular, in Dictyostelium, provided us with a new tool to further study the role of Ras in chemotaxis and especially in gradient sensing. Using cells with depleted RasGAP activity, we determined that Ras plays a previously unappreciated role in directional sensing, and we uncovered how the RasGAP-mediated spatio-temporal regulation of Ras activity regulates this process.
Our finding that DdNF1 regulates RasB and RasG is consistent with our observation that that nfaA- cells display random cell motility and cytokinesis defects (Figures S4-S6), in addition to chemotaxis defects. Previous studies demonstrated that both Ras proteins regulate cytokinesis, with RasB regulating myosin II function and RasG regulating PI3K activation and F-actin polymerization [2, 7, 10, 19, 20]. We find that growing nfaA- cells display increased levels of activated Ras and PKB, as well as polymerized F-actin compared to wild-type cells (Figures S4A-C). In addition, these cells exhibit considerably enhanced random cell motility (Figures S4D and S5), most likely resulting from the elevated levels of polymerized F-actin. Although DdNF1 also regulates RasB, the fact that the disruption of rasG suppresses the nfaA- chemotaxis phenotypes, and that expression of a “constitutively” active RasGQ61L mutant in rasG- cells result in an increased and prolonged cAMP-induced accumulation of PI(3,4,5)P3 similar to that observed in nfaA- cells (Figure S3D), strongly suggests that the chemotaxis defects result from the mis-regulation of RasG and not RasB. The fact that the kinetics of PI(3,4,5)P3 production in RasGQ61L/rasG- cells are not as extended as in nfaA- cells may account for differences in phenotypes between nfaA- and RasGQ61L/rasG- cells (Figures 2, S3C, [2]). While the RasGQ61L mutant has a higher basal activity and extended activation kinetics compared to wild-type RasG, it is not constitutively in a fully active state.
Consistent with the increase in PI(3,4,5)P3 accumulation underlying most of the nfaA- chemotaxis phenotype is the observation that this phenotype is highly similar to that of cells over-expressing a membrane targeted PI3K (myr-PI3K) as well as cells that lack the PI(3,4,5)P3 phosphatase PTEN, which display elevated PI(3,4,5)P3 accumulation that causes an increase in F-actin polymerization and pseudopod protrusions [4, 21]. However, unlike nfaA- cells, pten- cells or cells expressing myr-PI3K, do chemotax directionally, although with a reduced efficiency compared to wild-type cells. The abnormal accumulation of PI(3,4,5)P3 in RasGAP-deficient cells probably results from direct Ras-dependent mis-regulation of PI3K since the kinetics and profile of chemoattractant-induced translocation of PTEN-GFP upon uniform stimulation, as well as its localization in chemotaxing cells, are unaffected, suggesting that PTEN function is unaltered (Figures S7B-C). In addition, we observed that treatment of nfaA- cells with the PI3K inhibitor LY (LY294002) partially restores chemotaxis, producing cells that migrate as efficiently as LY-treated wild-type cells, which further suggests that Ras-dependent mis-regulation of PI3K is mostly responsible for the nfaA- chemotaxis phenotype (Figures S7D-E).
Our data suggest that RasG is an important regulator of PI3K. As the functions of RasB, RasC and RasD have been shown to overlap with those of RasG and their expression levels are elevated in rasG- cells, we expect that one or more of these Ras proteins most likely regulate PI3K in the absence of RasG [2, 7, 22]. This could explain why rasG- cells do not display severe chemotaxis defects (Figure S3A).
The RasGAP regulation of Ras is a component of the molecular mechanisms of directional sensing
Upon directional sensing, a cell must identify the side of the cell that produces the strongest response to the gradient. This is most likely achieved through differential and sequential activation and inactivation of key responses along the cortex until the cell determines the side with the strongest response, which is closest to the chemoattractant source. Our data indicate that cells depleted in RasGAP activity are unable to do this (Figure 4D). The inability to rapidly down-regulate Ras responses during the initial stages of gradient sensing impairs the ability of cells to efficiently identify the side of the cell closest to the chemoattractant source. We found that the loss of RasGAP activity impairs the ability of cells to rapidly activate Ras in response to a changing gradient. We propose that this process of gradient sensing continues to play a role as the cells migrate up the gradient, allowing the cells to acquire constant positional cues. Thus, although RasGAP-deficient cells are able to respond to chemoattractant stimulation, the failure to spatially control the chemotactic responses prevents the cells from polarizing and efficiently performing directional migration. The severity of the chemotactic phenotype observed when comparing RasGAP-depleted cells migrating within shallow and steep gradients is most likely due to the relative difference in chemoattractant concentration between the cell's anterior and posterior, resulting in a greater difference in relative activation of the signaling responses between the side closest to and that farthest away from the source in a steep opposed to a shallow gradient. Consequently, this increase in the ratio of activation between the presumptive front and back may help the cell decipher the axis of the gradient in the absence of RasGAP function and may explain why some cells perform chemotaxis, albeit inefficiently, in exponential gradients but not in linear gradients.
Together, our data suggest that the regulation of Ras by RasGAPs, including RasG and DdNF1, is a potential regulatory mechanism implicated in directional sensing in Dictyostelium. We suggest that RasGAPs inhibit Ras activity throughout the cell, which is consistent with our finding that DdNF1 is uniformly distributed in chemotaxing cells (Figure S8A), thereby lowering the overall level of active Ras (Ras-GTP) both in the resting state and after stimulation (Figure 4D). We speculate that following adaptation, only the remaining activated Ras at the front is sufficient to trigger feedback signaling through the Ras-PI3K-F-actin positive feedback loop [2]. This could lead to the localized persistence and amplification of the Ras signal, thereby creating a steep gradient of Ras and PI3K activity and promoting leading edge formation [17] (Figures 4D and S8B). We suggest that, in the absence of RasGAP activity, the persisting high levels of Ras-GTP throughout the cell could cause the non-localized (more uniform) activation of the Ras-PI3K-F-actin feedback loop, resulting in signal amplification and extension of multiple pseudopodia all around the cell (Figure 4D). Given that Ras modulates PI3K function in migrating neutrophils [23], and that the signaling pathways regulating chemotaxis in Dictyostelium and leukocytes are surprisingly well conserved [24], we believe that Ras and its regulation by RasGAPs, possibly NF1, are likely to play a similar role in regulating directional sensing in mammalian cells.
ACKNOWLEDGMENTS
We would like to thank members of the Firtel laboratory for their helpful suggestions and constructive discussions. We thank Karen Ong for assistance with FACS analysis. PGC is supported, in part, by a fellowship from the Fonds de la Recherche en Santé du Québec. This work was supported by USPHS grants to RAF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL DATA Supplemental Data include Experimental Procedures, 8 additional figures, and 10 movies.
Supplementary Material
REFERENCES
- 1.Van Haastert PJM, Veltman DM. Chemotaxis: Navigating by Multiple Signaling Pathways. Sci. STKE. 2007;2007:pe40. doi: 10.1126/stke.3962007pe40. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki AT, Chun C, Takeda K, Firtel RA. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J Cell Biol. 2004;167:505–518. doi: 10.1083/jcb.200406177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S, Comer FI, Sasaki A, McLeod IX, Duong Y, Okumura K, Yates JR, 3rd, Parent CA, Firtel RA. TOR complex 2 integrates cell movement during chemotaxis and signal relay in Dictyostelium. Mol Biol Cell. 2005;16:4572–4583. doi: 10.1091/mbc.E05-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 5.Kamimura Y, Xiong Y, Iglesias PA, Hoeller O, Bolourani P, Devreotes PN. PIP(3)-Independent Activation of TorC2 and PKB at the Cell's Leading Edge Mediates Chemotaxis. Curr Biol. 2008;18:1034–1043. doi: 10.1016/j.cub.2008.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trovo-Marqui AB, Tajara EH. Neurofibromin: a general outlook. Clin Genet. 2006;70:1–13. doi: 10.1111/j.1399-0004.2006.00639.x. [DOI] [PubMed] [Google Scholar]
- 7.Bolourani P, Spiegelman GB, Weeks G. Delineation of the roles played by RasG and RasC in cAMP-dependent signal transduction during the early development of Dictyostelium discoideum. Mol Biol Cell. 2006;17:4543–4550. doi: 10.1091/mbc.E05-11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wessels D, Brincks R, Kuhl S, Stepanovic V, Daniels KJ, Weeks G, Lim CJ, Spiegelman G, Fuller D, Iranfar N, et al. RasC plays a role in transduction of temporal gradient information in the cyclic-AMP wave of Dictyostelium discoideum. Eukaryot Cell. 2004;3:646–662. doi: 10.1128/EC.3.3.646-662.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim CJ, Spiegelman GB, Weeks G. RasC is required for optimal activation of adenylyl cyclase and Akt/PKB during aggregation. Embo J. 2001;20:4490–4499. doi: 10.1093/emboj/20.16.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondal S, Bakthavatsalam D, Steimle P, Gassen B, Rivero F, Noegel AA. Linking Ras to myosin function: RasGEF Q, a Dictyostelium exchange factor for RasB, affects myosin II functions. J Cell Biol. 2008;181:747–760. doi: 10.1083/jcb.200710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funamoto S, Milan K, Meili R, Firtel RA. Role of phosphatidylinositol 3′ kinase and a downstream pleckstrin homology domain-containing protein in controlling chemotaxis in Dictyostelium. J Cell Biol. 2001;153:795–810. doi: 10.1083/jcb.153.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 1998;95:81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- 13.Meili R, Ellsworth C, Lee S, Reddy TB, Ma H, Firtel RA. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. Embo J. 1999;18:2092–2105. doi: 10.1093/emboj/18.8.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Affolter M, Weijer CJ. Signaling to cytoskeletal dynamics during chemotaxis. Dev Cell. 2005;9:19–34. doi: 10.1016/j.devcel.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Schneider N, Weber I, Faix J, Prassler J, Muller-Taubenberger A, Kohler J, Burghardt E, Gerisch G, Marriott G. A Lim protein involved in the progression of cytokinesis and regulation of the mitotic spindle. Cell Motil Cytoskeleton. 2003;56:130–139. doi: 10.1002/cm.10139. [DOI] [PubMed] [Google Scholar]
- 16.Devreotes P, Janetopoulos C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J Biol Chem. 2003;278:20445–20448. doi: 10.1074/jbc.R300010200. [DOI] [PubMed] [Google Scholar]
- 17.Charest PG, Firtel RA. Feedback signaling controls leading-edge formation during chemotaxis. Curr Opin Genet Dev. 2006;16:339–347. doi: 10.1016/j.gde.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Onsum M, Rao CV. A mathematical model for neutrophil gradient sensing and polarization. PLoS Comput Biol. 2007;3:e36. doi: 10.1371/journal.pcbi.0030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuxworth RI, Cheetham JL, Machesky LM, Spiegelmann GB, Weeks G, Insall RH. Dictyostelium RasG is required for normal motility and cytokinesis, but not growth. J Cell Biol. 1997;138:605–614. doi: 10.1083/jcb.138.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki AT, Janetopoulos C, Lee S, Charest PG, Takeda K, Sundheimer LW, Meili R, Devreotes PN, Firtel RA. G protein-independent Ras/PI3K/F-actin circuit regulates basic cell motility. J Cell Biol. 2007;178:185–191. doi: 10.1083/jcb.200611138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- 22.Khosla M, Spiegelman GB, Insall R, Weeks G. Functional overlap of the dictyostelium RasG, RasD and RasB proteins. J Cell Sci. 2000;113(Pt 8):1427–1434. doi: 10.1242/jcs.113.8.1427. [DOI] [PubMed] [Google Scholar]
- 23.Suire S, Condliffe AM, Ferguson GJ, Ellson CD, Guillou H, Davidson K, Welch H, Coadwell J, Turner M, Chilvers ER, et al. Gbetagammas and the Ras binding domain of p110gamma are both important regulators of PI(3)Kgamma signalling in neutrophils. Nat Cell Biol. 2006;8:1303–1309. doi: 10.1038/ncb1494. [DOI] [PubMed] [Google Scholar]
- 24.Parent CA. Making all the right moves: chemotaxis in neutrophils and Dictyostelium. Curr Opin Cell Biol. 2004;16:4–13. doi: 10.1016/j.ceb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Chung CY, Firtel RA. PAKa, a putative PAK family member, is required for cytokinesis and the regulation of the cytoskeleton in Dictyostelium discoideum cells during chemotaxis. J Cell Biol. 1999;147:559–576. doi: 10.1083/jcb.147.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeda K, Sasaki AT, Ha H, Seung HA, Firtel RA. Role of PI3 kinases in chemotaxis in dictyostelium. J Biol Chem. 2007;282:11874–11884. doi: 10.1074/jbc.M610984200. [DOI] [PubMed] [Google Scholar]
- 27.Wessels D, Voss E, Von Bergen N, Burns R, Stites J, Soll DR. A computer-assisted system for reconstructing and interpreting the dynamic three-dimensional relationships of the outer surface, nucleus and pseudopods of crawling cells. Cell Motil Cytoskeleton. 1998;41:225–246. doi: 10.1002/(SICI)1097-0169(1998)41:3<225::AID-CM4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 28.Kae H, Lim CJ, Spiegelman GB, Weeks G. Chemoattractant-induced Ras activation during Dictyostelium aggregation. EMBO Rep. 2004;5:602–606. doi: 10.1038/sj.embor.7400151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 30.Jeon TJ, Lee D-J, Merlot S, Weeks G, Firtel RA. Rap1 controls cell adhesion and cell motility through the regulation of myosin II. J. Cell Biol. 2007;176:1021–1033. doi: 10.1083/jcb.200607072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steimle PA, Yumura S, Cote GP, Medley QG, Polyakov MV, Leppert B, Egelhoff TT. Recruitment of a myosin heavy chain kinase to actin-rich protrusions in Dictyostelium. Curr Biol. 2001;11:708–713. doi: 10.1016/s0960-9822(01)00182-8. [DOI] [PubMed] [Google Scholar]
- 32.Graf R, Daunderer C, Schulz I. Molecular and functional analysis of the dictyostelium centrosome. Int Rev Cytol. 2004;241:155–202. doi: 10.1016/S0074-7696(04)41003-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.