Abstract
The herpes simplex virus 1 (HSV-1) infected cell protein 0 (ICP0) is an E3 ubiquitin ligase implicated in cell cycle arrest and DNA repair inhibition. Convection-enhanced delivery (CED) of either the replication-defective, ICP0-producing HSV-1 mutant, d106, or the recombinant d109, devoid of all viral genome expression, was performed to determine the in vivo efficacy of ICP0 in combination with ionizing radiation (IR) or systemic temozolomide (TMZ) in the treatment of glioblastoma multiforme (GBM). Intracranial U87-MG xenografts were established in athymic nude mice. Animal survival was determined after mice underwent intracranial CED of either the replication-defective d106 or d109 viruses, or Hanks' balanced salt solution (HBSS), prior to a single session of whole-brain irradiation or TMZ treatment. Median survival for animals that underwent treatment with HBSS alone, d109 alone, d106 alone, HBSS+IR, HBSS + TMZ, d109+IR, d106+IR and d106 + TMZ, was 28, 35, 41, 39, 44, 39, 68 (P<0.01), and 66 days (P<0.01) respectively. Intracerebral d106 CED resulted in a significant increase in athymic nude mouse survival when combined with IR or TMZ. d106 CED allows for distribution of HSV-1 in human GBM xenografts and persistent viral infection.
INTRODUCTION
Herpes simplex virus 1 (HSV-1) is a double-stranded DNA virus that has been studied for use in the treatment of glioblastoma multiforme (GBM), the most devastating and therapy-resistant central nervous system (CNS) tumor in adults [1-4]. The HSV-1 infected cell protein (ICP) 0 is one of five immediate-early (IE) proteins encoded by the virus which has various effects on cell metabolism [5]. ICP0 is an activator of gene expression and enhances the expression of genes introduced into cells by infection or transfection. ICP0 is thought to relieve a repression mechanism that assembles HSV-1 genomes into a repressed chromatin structure by sequestering or affecting the properties of histone deacetylase (HDAC) enzymes [6, 7]. ICP0 has been shown to function as an E3 ubiquitin ligase [8, 9] conjugating ubiquitin onto proteins, in a RING finger-dependent manner, and targeting them for degradation by the ubiquitin-dependent proteosome degradation pathway [10]. ICP0 is thought to target cellular proteins that repress viral gene expression [8, 11]. Proteins targeted by ICP0 include promyelocytic leukemia protein (PML) and Sp100, which are major constituents of nuclear structures called ND10 bodies [10]. ND10 bodies are discrete nuclear foci where HSV-1 genomes may localize early during infection [12]. Recent studies have suggested that these foci are sites of DNA double-strand break (DSB) repair and inhibition of HSV genome circularization by ICP0 [13, 14]. Other proteins targeted for degradation include centromere proteins, CENP A [15] and CENP-C [16, 17], the E2 ubiquitin-conjugating enzyme cdc34 (UbcH3)[5], ubiquitin specific protease (USP7)[18], and the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) [19, 20]. Disruption of centromeres by ICP0 degradation of CENP-C and CENP-A has been shown to cause cell cycle arrest at both G1/S and G2/M checkpoints [15, 17, 21] independently of p53 [22]. More recently, ICP0 gene expression has been implicated in triggering apoptosis during viral infection of human cells [23].
The combination of ionizing radiation (IR) and temozolomide (TMZ), both DNA-damaging agents, have demonstrated a survival benefit in patients with glioblastoma multiforme (GBM) and is now standard of care [24]. Chemoradiation confers some survival advantage, but resistance of the tumor cells to the effects of chemoradiation limits the success of treatment. The molecular basis of chemo- and radioresistance is DNA repair. Inhibiting DNA repair mechanisms may improve the chemoradiosensitivity of GBM. We have recently shown the replication-defective HSV-1 vector, d106, inhibits DNA repair and enhances the radiosensitivity of human GBM cells in part by apoptosis induction [25]. The mutant virus, d106, is defective in the expression of all of the immediate-early (IE) viral genes except that which encodes ICP0 [26]. Besides ICP0, the only viral protein product readily detected in d106-infected cells is ICP6, which is the product of an early (E) gene encoding the large subunit of viral ribonucleotide reductase, which has been previously shown to have no cytotoxic effect on the host cell [27]. In the present study, intracerebral convection-enhanced delivery (CED) of d106 was performed in the normal mouse brain in comparison to stereotactic manual viral inoculation. d106 CED was then analyzed in a mouse intracranial glioblastoma model in combination with IR to determine virus brain distribution and persistence, xenograft infection, tumoricidal effect, and animal survival. For comparison of in vivo efficacy, the isogenic variant of d106 which does not express any viral proteins was used. This virus, d109, does not express any viral proteins and has no observable cytotoxic effect, even at high multiplicity of infection (MOI) [28]. Animal survival was also analyzed after combination therapy of d106 CED and systemic TMZ.
RESULTS
HSV-1 Manual Stereotactic Injection vs. CED
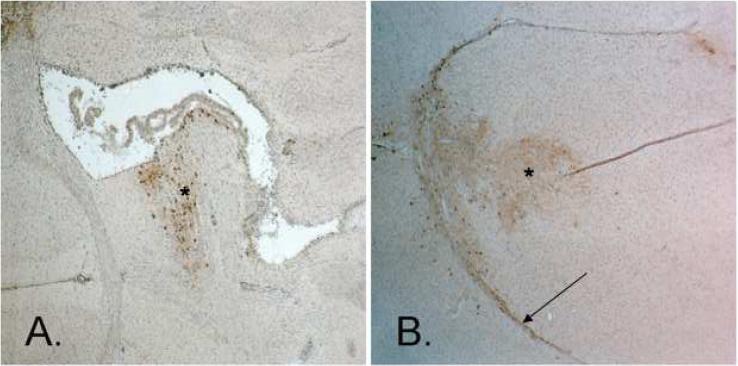
HSV-1 intracerebral cell infection and spatial distribution were determined in the normal mouse brain after stereotactic CED or manual injection. GFP transgene expression was used as a marker for cell infection. Abundant cellular GFP expression has been shown in vitro 24 hours post-infection by the d106 virus [26]. GFP expression was determined 48 hours after stereotactic CED or manual injection of d106. A 10 μl suspension of d106, containing 3 × 106 pfu, was used with each method of delivery. Histologic analysis of tissue sections along the needle tract revealed both greater spatial distribution and intensity of GFP staining after CED in comparison to stereotactic injection (Figure 1). Strong, homogeneous GFP expression after CED suggests greater cell infection by d106. White matter tract spread of HSV-1 was found after CED but not with stereotactic injection.
Figure 1.
Photomicrographs of BALB/c mice brain sections after immunohistochemistry staining for GFP (shown by *). A comparison of intracerebral manual stereotactic injection and convection-enhanced delivery (CED) with 10 μ1 of d106 viral suspension (3 × 106 pfu) revealing greater cell infection and viral spatial distribution after CED. a, Manual stereotactic injection (Magnification, 40X). b, Stereotactic CED. White matter tract spread of HSV-1 was found after CED (shown by arrow) (Magnification, 40X).
Survival Studies after HBSS, d109, or d106 CED and whole-brain IR or TMZ treatment
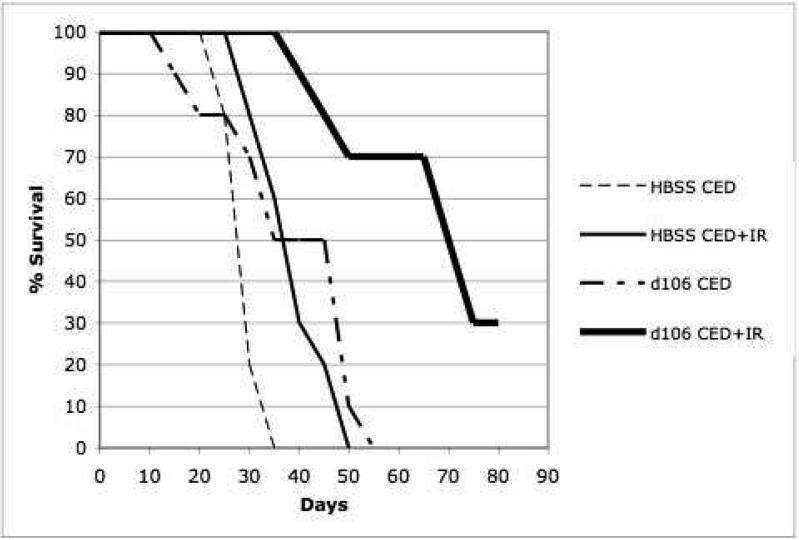
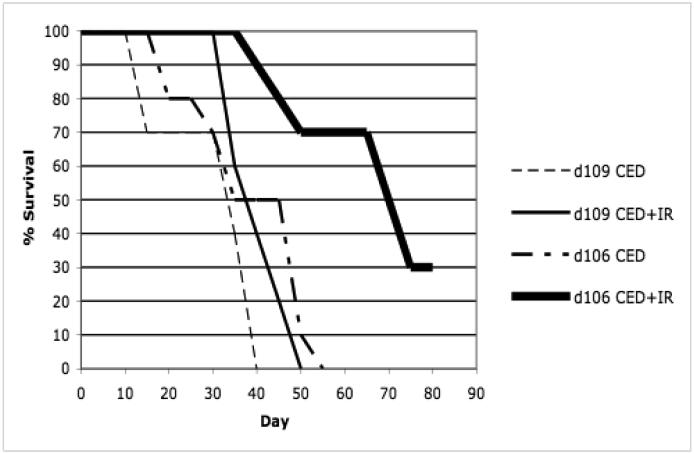
Survival studies were performed on animals that underwent CED of HBSS, d109, d106, and whole-brain irradiation or i.p. TMZ (Figures 2 and 3). A total of 8 groups, consisting of 10 athymic nude mice each, were used for survival analysis 80 days post tumor implantation (Table 1). To determine the possible in vivo effect of ICP0 in combination with IR or TMZ, survival analysis was performed with mice implanted with human glioblastomas which underwent CED of the replication-defective, ICP0-producing mutant, d106, or the control HSV-1 mutant, d109, which does not express any of its viral genome.
Figure 2.
Kaplan-Meier survival curves of athymic nude mice after intracranial implantation of human U87-MG cells and treatment by stereotactic CED of d106, d109, or HBSS and irradiation (0 and 10 Gy). Animals surviving 80 days post-tumor implantation were sacrificed. a, Kaplan-Meier survival curve comparison of athymic nude mice which underwent intracranial implantation of U87-MG cells and treatment by stereotactic CED of d106 or HBSS and irradiation (0 and 10 Gy). Control animals were mice that underwent CED of HBSS and did not receive ionizing radiation (IR). Statistical significance, P < 0.001 by log-rank method of CED of d106 and irradiation compared to CED HBSS and irradiation (n=10 per group). b, Kaplan-Meier survival curve comparison of athymic nude mice which underwent intracranial implantation of U87-MG cells and treatment by stereotactic CED of d106 or d109 and irradiation (0 and 10 Gy). Control animals were mice that underwent CED of d109 and did not receive IR. Statistical significance, P < 0.001 by log-rank method of CED of d106 and irradiation compared to d109 CED and irradiation (n=10 per group).
Figure 3.
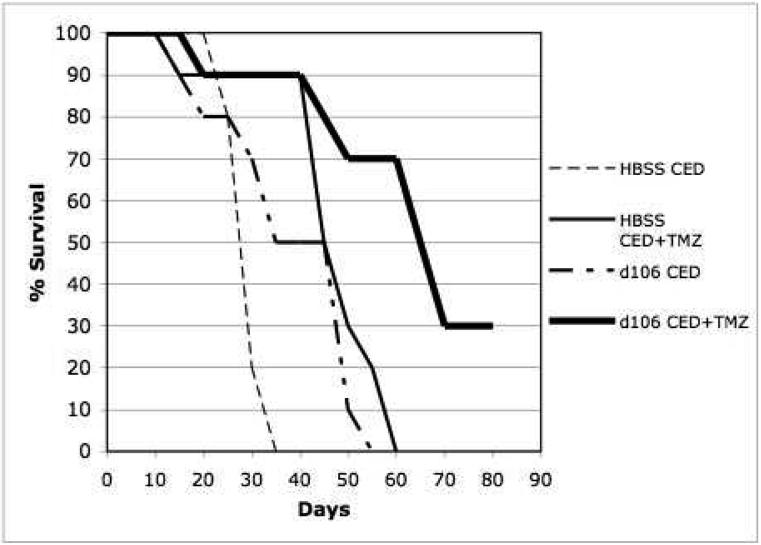
Kaplan-Meier survival curve comparison of athymic nude nice which underwent intracranial implantation of human U87-MG cells and treatment by stereotactic CED of d106 or HBSS with or without systemic TMZ. Statistical significance, P < 0.001 by log-rank method of CED of d106 and TMZ compared to HBSS CED and TMZ treatment.
Table 1.
Summary of treatment group results including number of animals, protocol, median survival, and percent survival at 80 days.
| Treatment groups (n=10) | Protocol | Median survival (days) | Survival at 80 d (%) | |
|---|---|---|---|---|
| Day 5 | Day 7 | |||
| 1. HBSS (control) | CED HBSS | 0 Gy | 23 | 0 |
| 2. HBSS + IR | CED HBSS | 10 Gy | 39 | 0 |
| 3. d106 | CED d106 | 0 Gy | 41 | 0 |
| 4. d106 + IR | CED d106 | 10 Gy | 68 | 30 |
| 5. d109 | CED d109 | 0 Gy | 35 | 0 |
| 6. d109 + IR | CED d109 | 10 Gy | 39 | 0 |
| 7. HBSS + TMZ | CED HBSS + IP TMZ (Days 5-8) | 44 | 0 | |
| 8. d106 + TMZ | CED d106+ IP TMZ (Days 5-8) | 66 | 30 | |
The median survival for control animals that underwent CED of HBSS and no IR or TMZ treatment was 23 days (Figures 2a and 3). The median survival for those animals that underwent CED of HBSS and IR or TMZ treatment was 39 and 44 days, respectively (Figures 2a and 3). The median survival for animals that underwent CED of d109 with or without IR treatment was 35 and 39 days, respectively (Figure 2b). Those animals that underwent CED of d106 and did not undergo IR or TMZ treatment had a median survival of 41 days (Figures 2 and 3). Animals that underwent d106 CED and IR or TMZ treatment had a median survival of 66 and 68 days, respectively (Figures 2 and 3). Median survival was significantly increased (P less than 0.01 by log-rank method) when animals underwent CED of d106 and subsequent whole-brain irradiation or TMZ in comparison to animals that underwent d109 CED with IR treatment or HBSS CED and irradiation or TMZ treatment (Figures 2 and 3).
HSV-1 Xenograft Infection and Cell Transduction After CED
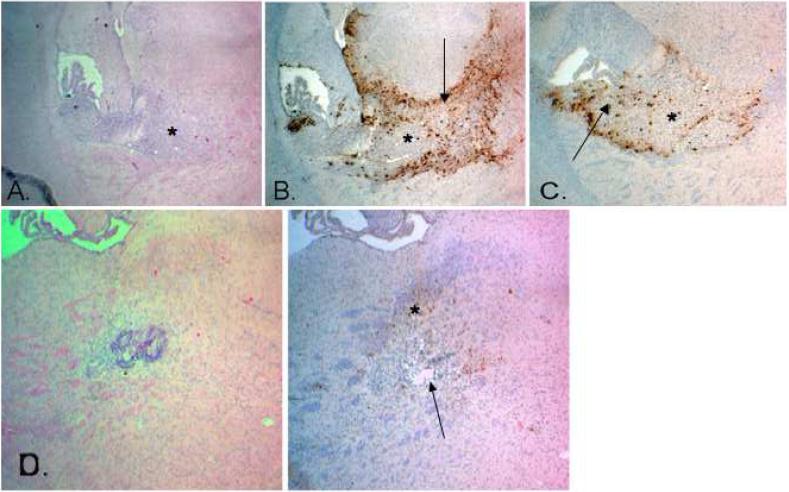
After inoculation of mice with U87-MG tumor cells, CED of HBSS or d106 was performed 5 days later. GFP transgene expression was used as a marker for cell infection. Hematoxylin/eosin staining and intracerebral GFP expression was determined at 7, 9, 12, 15, 17, and 21 days after xenograft implantation (Figure 4). Intratumoral expression of GFP was found in 75% (9 of 12 animals) of the animals that underwent CED of d106 and were randomly sacrificed at 7 and 9 days post-tumor implantation (2 and 4 days after d106 CED) (Table 2 and Figure 4c). All animals that did not have intratumoral expression of GFP had peri-tumoral cell expression of GFP indicating inadequate penetration of the xenograft by the d106 virus. Persistent cellular GFP transgene expression was found at 12 and 16 days (17 and 21 days post-tumor implantation) after d106 CED in cells within and surrounding xenograft sites (Figure 4d).
Figure 4.
Photomicrographs of athymic nude mice brain sections containing human U87-MG xenografts after stereotactic intracerebral CED of d106. Magnification, 40X. a, Hematoxylin/eosin-stained section day 7 after tumor implantation and 2 days after d106 CED revealing boundaries of xenograft (shown by *). b, Corresponding GFP-stained section revealing marked xenograft (*) infection and viral spatial distribution into the surrounding brain (shown by arrow). c, GFP-stained section day 7 showing strong xenograft (*) infection and extensive viral spatial distribution within the tumor (shown by arrow). d, Hematoxylin/eosin-stained section day 17 after tumor implantation (left) and corresponding GFP-stained section (right) revealing complete tumor regression (shown by arrow) and continued GFP expression at the xenograft site suggestive of persistent viral infection (shown by *).
Table 2.
Summary of U87-MG xenograft infection rate and treatment response in animals that underwent d106 CED and whole-brain irradiation that were randomly sacrificed. GFP xenograft infection was determined in animals from treatment groups 3 and 4 on days 7 and 9 post tumor implantation. Treatment response was determined in treatment group 4 on days 12 and up to 21 days post tumor implantation.
| Intra-tumoral GFP expression days 2/4 after d106 CED (%) | Partial tumor regression 7 days after d106 CED + IR (%) | Complete tumor regression up to 16 days after d106 CED + IR (%) |
|---|---|---|
| 9/12 (75) | 3/3 (100) | 10/17 (58) |
Tumoricidal Effect of d106, ICP0, and IR
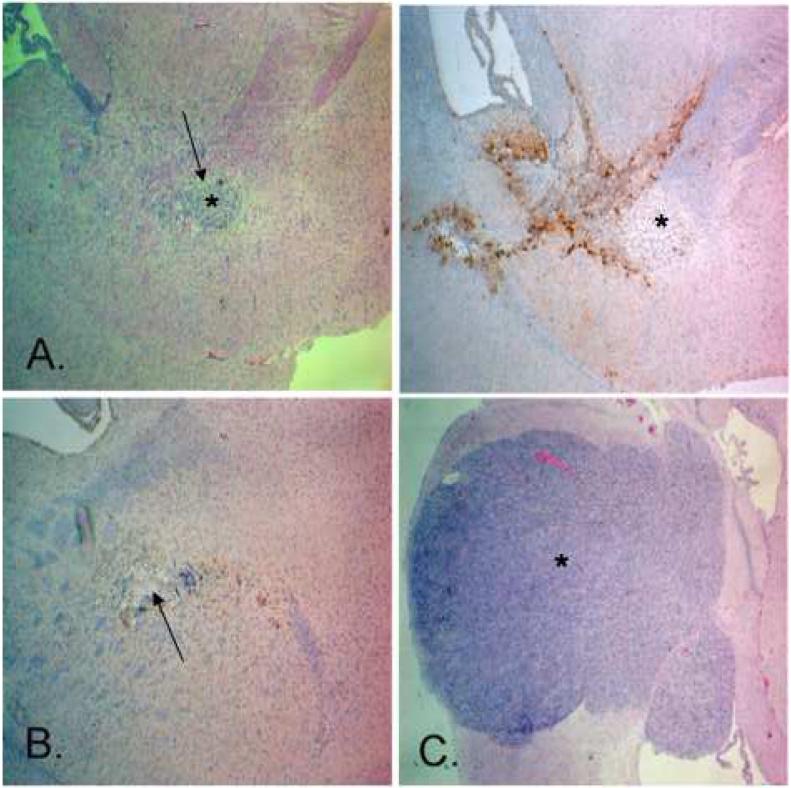
Partial regression of xenograft was found in all three animals randomly sacrificed day 12 post tumor implantation after undergoing d106 CED and irradiation (Table 2; Figure 5a). Complete tumor regression was found in ten of seventeen (10/17) animals which underwent d106 CED and irradiation and were sacrificed by 21 days post tumor induction (Table 2; Figures 4d and 5b). Partial regression of tumor was found in the remaining animals. Animals that underwent whole-brain irradiation after CED of the control vehicle, HBSS, showed tumor enlargement at 17 day post tumor implantation (Figure 5c).
Figure 5.
Photomicrographs of brain sections revealing antitumor efficacy of stereotactic CED of d106 in combination with ionizing radiation (10 Gy) in athymic nude mice implanted with human U87-MG xenografts. Magnification, 40X. a, Hematoxylin/eosin- (left) and GFP-stained (right) sections day 12 after tumor implantation (shown by *) and 7 days after d106 CED showing regression of xenograft (shown by arrow). b, GFP-stained section revealing complete tumor regression on day 17 after tumor implantation (shown by arrow). c, Hematoxylin/eosin-stained section day 17 after tumor implantation in animal that underwent intracerebral CED of HBSS and irradiation showing a large tumor (shown by *).
DISCUSSION
Combined modality therapy with radiation and TMZ, known as chemoradiation, represents a new standard of care for patients with newly diagnosed GBM [1, 24, 29, 30]. Combining the effects of IR and HSV-1 in the therapy of GBM has been reported [31-33]. Replication and tumor kill is increased with HSV-1 oncolytic viruses utilizing large fractions of IR (20 and 25 Gy) [31, 32]. In the case of replication-defective HSV-1 constructs, greater animal survival has been shown with the addition of radiosurgery (margin dose of 15 Gy; center dose of 21.4 Gy) [34, 35]. The use of TMZ and HSV-1 therapy of GBM has also been described and demonstrated a synergistic interaction [36].
HSV-1 recombinant vectors have been tested in multiple human clinical trials for the treatment of GBM [1, 3, 4]. The less than optimal tissue distribution of HSV-1 vectors in the brain remains a limiting factor when considering the efficacy of various HSV constructs for the treatment of malignant gliomas. Delivery of HSV-1 vectors in all clinical trials has been by multiple manual stereotactic intratumoral or peritumoral injections after surgical resection [2, 4]. Viral particles accumulate adjacent to the needle tract, and limited dispersal of particles occurs by diffusion. The binding of viral particles to the heparan sulfate proteoglycans found abundantly in the extracellular matrix and glycocalyx in the brain may contribute to limited dispersal [37].
One method of increasing distribution of HSV-1 in the brain may be convection-enhanced delivery (CED). CED is an approach developed to overcome the obstacles associated with current CNS agent delivery [38, 39]. Multiple clinical trials have involved CED for the treatment of recurrent GBM [40-44]. In CED, a small hydrostatic pressure differential, imposed by a syringe pump to distribute infusate directly to small or large regions of the CNS, is used in a safe, reliable, targeted, and homogeneous manner [45]. CED relies on bulk flow that is driven by a small gradient to distribute molecules within the interstitial spaces of the CNS. Convection is not limited by the infusate's molecular weight, concentration, or diffusivity [38, 39, 46].
Limited use of CED for viral vector delivery to the brain has been reported. CED has been shown to distribute adenovirus [47] and adeno-associated virus type 2 (AAV-2) [48] in the rat striatum. CED of HSV-1 into the brain has not been reported prior to this study.
Our group has recently shown that the HSV-1 IE protein, ICP0, naturally inhibits the repair of DNA double-strand breaks after IR treatment of human GBM cells leading to decreased cell survival and induction of apoptosis in vitro [25]. The ICP0 protein has other effects on cell metabolism that have been described. We present evidence showing intracerebral CED of the replication-defective, ICP0-producing mutant, d106, in combination with whole-brain irradiation or TMZ, enhances the survival of animals implanted with human GBM xenografts. The radiation dose used in our study (10 Gy) is approximately half the dose used in other studies [31, 35]. Also, the total dose of TMZ (20 mg/kg) administered was subtherapeutic as compared to another study combining HSV-1 with TMZ [36]. We believe optimal intracerebral d106 delivery, by convection, allows for adequate xenograft infection, transduction, and ultimate tumor cell demise by chemo- or radiosensitivity enhancement. CED of the replication-defective d106 construct is also shown to be safe in the mouse.
Targeting the repair of DNA after IR or chemotherapy insult in dividing tumor cells and maximizing the delivery of HSV-1 to tumor cells and the surrounding brain where tumor-infiltration has occurred may form the basis of future clinical gene therapy strategies against GBM.
Materials and Methods
Animals, Cells, and HSV-1 Vector
Six- to 7-week old female BALB/c or athymic nude (nu/nu) mice were used, and all procedures were performed with approval by the Institutional Animal Care and Use Committee (IACUC)of the University of Pittsburgh. The human glioblastoma cell line, U87-MG, was obtained from the ATCC. The HSV-1 IE mutant viruses, d106 and d109, are both derived from the wild-type strain KOS and have been previously described [26, 28]. In place of the deletion of infected cell polypeptide (ICP) 27, a green fluorescent protein (GFP) transgene under the control of the human cytomegalovirus (HCMV) IE promoter was inserted.
CED Apparatus
The infusion apparatus consisted of a hydraulic drive serially connected to a digital syringe pump controller (UltraMicroPumpII, World Precision Instruments, Inc., Sarasota, Florida)[49]. The digital controller was able to precisely dispense microliter volumes at a set rate (μl/min) from up to three hydraulic drives simultaneously. Each hyrdraulic drive depressed the plunger of a gas-tight 50 microliter Hamilton syringe fitted with 30-gauge removable needle (Hamilton Co., Reno, Nevada). HSV solution or Hanks' Balanced Salt Solution (HBSS; Gibco Invitrogen Life Technologies, Inc., Grand Island, NY) was used to fill each syringe prior to mounting on the hydraulic drive of the infusion apparatus. A mounting bar was then used to mount the hydraulic drive and Hamilton syringe to up to three small animal stereotaxic instruments (David Kopf Instruments, Tujunga, CA).
HSV-1 Stereotactic Injection vs. CED
Anesthetized BALB/c mice (total of 6 animals) were placed in the stereotaxic instrument and underwent stereotactic manual injection or CED of the d106 virus. The same volume (10 microliters) and titer of virus (3 × 106 pfu) were used for each method of delivery. The rate of CED used was 1 microliter/minute for a total of 10 minutes and performed as described above. For manual stereotactic injection, a gas-tight 10 microliter Hamilton syringe fitted with a 30-gauge removable needle (Hamilton Co., Reno, Nevada) fixed to the stereotaxic instrument was used. Stereotactic manual injection was performed within 10 minutes. For both manual injection and CED, stereotactic placement of the delivery needle into the right striatum, 3 mm below the dural surface was performed. Mice were sacrificed 48 hours after stereotactic manual injection or CED of d106.
Tumor Inoculation, HSV-1 Convection Procedure, Chemotherapy, and Irradiation (Table 1)
Anesthetized athymic nude mice were placed in the stereotaxic instrument and U87-MG cells (5 × 105) were stereotactically inoculated into the right striatum, 3 mm below the dural surface on day 0. On day 5 post-tumor implantation, mice were randomized into eight groups: (1) CED of HBSS (untreated control), (2) CED of HBSS and irradiation, (3) CED of d106 alone, (4) CED d106 and irradiation, (5) CED of d109 alone, (6) CED of d109 and irradiation, (7) CED of HBSS and intraperitoneal (i.p.) TMZ, (8) CED d106 and i.p. TMZ.
All animals underwent CED of 10 microliters of d106 or d109 (3 × 106 pfu) suspension or HBSS at a rate of 1.0 microliter/min on day 5 using the same coordinates as used earlier for tumor cell implantation. After infusion completion, the cannula needle was left in place for 5 minutes and then withdrawn at a rate of 1 mm/min to minimize any infusate leakback.
TMZ (Schering Corp., Kenilworth, NJ)) was given by i.p. injection 3 hours after intracerebral CED of d106 or HBSS on day 5. Four consecutive daily i.p. TMZ injections (5 mg/kg) were given to a total dose of 20 mg/kg. The dose of TMZ used was approximately 0.2LD10 and was chosen to have modest antitumor effect but to not be curative based on previous studies [50].
A single dose of IR (10 Gy) was delivered to anesthetized mice on day 7 in a 137Cs animal irradiator at a dose rate of .87 Gy/min. A lower dose of IR was chosen to have modest antitumor effect but to not be curative. Perforated collimator plates were used for selective whole-brain irradiation of animals.
Animal Observation
All athymic nude mice were observed daily to monitor external appearance, feeding behavior, and locomotion for a total of 80 days. Animals were sacrificed at the first sign of an adverse event (paresis, inability to feed) and brains were removed for histological examination.
On days 7, 9, 12, 15, and 17, three animals from each group of animals inoculated with tumor that underwent CED of d106 or HBSS and underwent irradiation (0 or 10 Gy) were randomly sacrificed for histologic analysis of intracranial tumor burden and/or U87-MG xenograft d106 infection.
A separate repeat group of animals (n=11) that underwent d106 CED and subsequent whole-brain irradiation (10 Gy) were sacrificed at 21 days post-tumor implantation (16 days after d106 CED) to determine tumor response to combination therapy.
Histologic Analysis
Mouse brains were harvested and fixed with 10% neutral buffered formalin. Coronal sections were made at the level of the needle tract to mark the center of the tumor. Serial sections of the cerebral hemisphere were examined from each animal. Tissue blocks were embedded in paraffin and sectioned (5 micrometers). All sections were stained with hematoxylin and eosin.
GFP immunohistochemistry was performed as follows after deparaffination of tissue sections. Drying of tissue sections was performed initially. Slides were then rinsed in deionized water and placed in a slide holder and a Tissue-Tek staining dish filled with Antigen retrieval solution (Abcam Inc., Cambridge, MA). Slides were heated in a microwave oven until the antigen retrieval solution came to a boil and then were allowed to cool. Slides were rinsed in phosphate buffered solution (PBS) prior to treatment with proteinase K. Repeat rinsing with PBS was performed before the addition of 0.3% hydrogen peroxide to the slides to block endogenous peroxidase activity. After rinsing with PBS, a permeabilizing/blocking solution consisting of PBS, 2% horse serum, and 0.2% Triton-X was added. The blocking solution was removed and a primary rabbit polyclonal GFP antibody (sc-8334; Santa Cruz Biotechnology, Santa Cruz, CA), diluted 1:100 in PBS, was added in addition to 2% horse serum and 0.05% Tween 20 and incubated overnight at 4° C. Slides were rinsed in PBS prior to addition of a biotinylated anti-rabbit secondary antibody (dilution 1:100) provided in a rabbit ABC staining system (sc-2018; Santa Cruz Biotechnology, Santa Cruz, CA). Incubation was performed for 30 minutes prior to rinsing of slides with PBS. Incubation with a biotinylated horseradish peroxidase reagent was performed for 30 minutes and rinsing of slides was repeated with PBS. Tissue sections were incubated in 1-3 drops of peroxidase substrate for 2-15 minutes until stain intensity was optimal. Counterstaining of tissue sections was performed in Mayer's hematoxylin for 5 minutes prior to washing in running tap water. Permanent mounting medium was added to each slide prior to glass coverslip placement. Tissue sections were examined by light microscopy using a Nikon Diaphot 300 photomicroscope (Melville, NY). Images were captured by a SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI) and imported into the SPOT version 3.5.9 for MacOS image analysis software package (Diagnostic Instruments, Sterling Heights, MI) running on a Macintosh G3 computer (Apple, Cupertino, CA). Analysis of intratumoral GFP staining was performed on all tissues sections.
Statistical Analysis
Survival data were entered into Kaplan-Meier plots and statistical analysis was performed using a log-rank test. A P value of 0.05 was used as the boundary of statistical significance.
Acknowledgements
This was work was supported in part by grants from the NIH/NINDS (K08 NS053454 to CGH), American Brain Tumor Association (CGH), and the Georgia Cancer Coalition (CGH). C. G. Hadjipanayis is recognized as a Georgia Cancer Coalition Distinguished Scholar.
References
- 1.Papanastassiou V, Rampling R, Fraser M, Petty R, Hadley D, Nicoll J, et al. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9:398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 2.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 3.Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 4.Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 5.Hagglund R, Roizman B. Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1. J Virol. 2004;78:2169–2178. doi: 10.1128/JVI.78.5.2169-2178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lomonte P, Thomas J, Texier P, Caron C, Khochbin S, Epstein AL. Functional interaction between class II histone deacetylases and ICP0 of herpes simplex virus type 1. J Virol. 2004;78:6744–6757. doi: 10.1128/JVI.78.13.6744-6757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu H, Liang Y, Mandel G, Roizman B. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc Natl Acad Sci U S A. 2005;102:7571–7576. doi: 10.1073/pnas.0502658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everett RD. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays. 2000;22:761–770. doi: 10.1002/1521-1878(200008)22:8<761::AID-BIES10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 9.Boutell C, Sadis S, Everett RD. Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro. J Virol. 2002;76:841–850. doi: 10.1128/JVI.76.2.841-850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett RD, Freemont P, Saitoh H, Dasso M, Orr A, Kathoria M, et al. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110-and proteasome-dependent loss of several PML isoforms. J Virol. 1998;72:6581–6591. doi: 10.1128/jvi.72.8.6581-6591.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett RD, Rechter S, Papior P, Tavalai N, Stamminger T, Orr A. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J Virol. 2006;80:7995–8005. doi: 10.1128/JVI.00734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maul GG, Ishov AM, Everett RD. Nuclear domain 10 as preexisting potential replication start sites of herpes simplex virus type-1. Virology. 1996;217:67–75. doi: 10.1006/viro.1996.0094. [DOI] [PubMed] [Google Scholar]
- 13.Jackson SA, DeLuca NA. Relationship of herpes simplex virus genome configuration to productive and persistent infections. Proc Natl Acad Sci U S A. 2003;100:7871–7876. doi: 10.1073/pnas.1230643100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbone R, Pearson M, Minucci S, Pelicci PG. PML NBs associate with the hMre11 complex and p53 at sites of irradiation induced DNA damage. Oncogene. 2002;21:1633–1640. doi: 10.1038/sj.onc.1205227. [DOI] [PubMed] [Google Scholar]
- 15.Lomonte P, Sullivan KF, Everett RD. Degradation of nucleosome-associated centromeric histone H3-like protein CENP-A induced by herpes simplex virus type 1 protein ICP0. J Biol Chem. 2001;276:5829–5835. doi: 10.1074/jbc.M008547200. [DOI] [PubMed] [Google Scholar]
- 16.Everett RD, Earnshaw WC, Pluta AF, Sternsdorf T, Ainsztein AM, Carmena M, et al. A dynamic connection between centromeres and ND10 proteins. J Cell Sci. 1999;112(Pt 20):3443–3454. doi: 10.1242/jcs.112.20.3443. [DOI] [PubMed] [Google Scholar]
- 17.Lomonte P, Everett RD. Herpes simplex virus type 1 immediate-early protein Vmw110 inhibits progression of cells through mitosis and from G(1) into S phase of the cell cycle. J Virol. 1999;73:9456–9467. doi: 10.1128/jvi.73.11.9456-9467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutell C, Canning M, Orr A, Everett RD. Reciprocal activities between herpes simplex virus type 1 regulatory protein ICP0, a ubiquitin E3 ligase, and ubiquitin-specific protease USP7. J Virol. 2005;79:12342–12354. doi: 10.1128/JVI.79.19.12342-12354.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lees-Miller SP, Long MC, Kilvert MA, Lam V, Rice SA, Spencer CA. Attenuation of DNA-dependent protein kinase activity and its catalytic subunit by the herpes simplex virus type 1 transactivator ICP0. J Virol. 1996;70:7471–7477. doi: 10.1128/jvi.70.11.7471-7477.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parkinson J, Lees-Miller SP, Everett RD. Herpes simplex virus type 1 immediate-early protein vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J Virol. 1999;73:650–657. doi: 10.1128/jvi.73.1.650-657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everett RD, Earnshaw WC, Findlay J, Lomonte P. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. Embo J. 1999;18:1526–1538. doi: 10.1093/emboj/18.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hobbs WE, DeLuca NA. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J Virol. 1999;73:8245–8255. doi: 10.1128/jvi.73.10.8245-8255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanfilippo CM, Blaho JA. ICP0 gene expression is a herpes simplex virus type 1 apoptotic trigger. J Virol. 2006;80:6810–6821. doi: 10.1128/JVI.00334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 25.Hadjipanayis CG, DeLuca NA. Inhibition of DNA repair by a herpes simplex virus vector enhances the radiosensitivity of human glioblastoma cells. Cancer Res. 2005;65:5310–5316. doi: 10.1158/0008-5472.CAN-04-3793. [DOI] [PubMed] [Google Scholar]
- 26.Samaniego LA, Neiderhiser L, DeLuca NA. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J Virol. 1998;72:3307–3320. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson PA, Wang MJ, Friedmann T. Improved cell survival by the reduction of immediate-early gene expression in replication-defective mutants of herpes simplex virus type 1 but not by mutation of the virion host shutoff function. J Virol. 1994;68:6347–6362. doi: 10.1128/jvi.68.10.6347-6362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samaniego LA, Wu N, DeLuca NA. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol. 1997;71:4614–4625. doi: 10.1128/jvi.71.6.4614-4625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen MH, Johnson JR, Padzur R. Food and Drug Administration Drug Approval Summary: Temozolomide Plus Radiation Therapy for the Treatment of Newly Diagnosed Glioblastoma Multiforme. Clin Cancer Res. 2005;11:6767–6771. doi: 10.1158/1078-0432.CCR-05-0722. [DOI] [PubMed] [Google Scholar]
- 30.DeAngelis LM. Chemotherapy for brain tumors--a new beginning. N Engl J Med. 2005;352:1036–1038. doi: 10.1056/NEJMe058010. [DOI] [PubMed] [Google Scholar]
- 31.Advani SJ, Sibley GS, Song PY, Hallahan DE, Kataoka Y, Roizman B, et al. Enhancement of replication of genetically engineered herpes simplex viruses by ionizing radiation: a new paradigm for destruction of therapeutically intractable tumors. Gene Ther. 1998;5:160–165. doi: 10.1038/sj.gt.3300546. [DOI] [PubMed] [Google Scholar]
- 32.Bradley JD, Kataoka Y, Advani S, Chung SM, Arani RB, Gillespie GY, et al. Ionizing radiation improves survival in mice bearing intracranial high-grade gliomas injected with genetically modified herpes simplex virus. Clin Cancer Res. 1999;5:1517–1522. [PubMed] [Google Scholar]
- 33.Markert JM, Gillespie GY, Weichselbaum RR, Roizman B, Whitley RJ. Genetically engineered HSV in the treatment of glioma: a review. Rev Med Virol. 2000;10:17–30. doi: 10.1002/(sici)1099-1654(200001/02)10:1<17::aid-rmv258>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 34.Niranjan A, Wolfe D, Tamura M, Soares MK, Krisky DM, Lunsford LD, et al. Treatment of rat gliosarcoma brain tumors by HSV-based multigene therapy combined with radiosurgery. Mol Ther. 2003;8:530–542. doi: 10.1016/s1525-0016(03)00232-6. [DOI] [PubMed] [Google Scholar]
- 35.Niranjan A, Moriuchi S, Lunsford LD, Kondziolka D, Flickinger JC, Fellows W, et al. Effective treatment of experimental glioblastoma by HSV vector-mediated TNF alpha and HSV-tk gene transfer in combination with radiosurgery and ganciclovir administration. Mol Ther. 2000;2:114–120. doi: 10.1006/mthe.2000.0101. [DOI] [PubMed] [Google Scholar]
- 36.Aghi M, Rabkin S, Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J Natl Cancer Inst. 2006;98:38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- 37.WuDunn D, Spear PG. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison PF, Laske DW, Bobo H, Oldfield EH, Dedrick RL. High-flow microinfusion: tissue penetration and pharmacodynamics. Am J Physiol. 1994;266:R292–305. doi: 10.1152/ajpregu.1994.266.1.R292. [DOI] [PubMed] [Google Scholar]
- 40.Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25:837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 41.Weaver M, Laske DW. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J Neurooncol. 2003;65:3–13. doi: 10.1023/a:1026246500788. [DOI] [PubMed] [Google Scholar]
- 42.Weingart J, Strauss LC, Grossman SA. Phase I/II study: intra-tumoral infusion of IL13-pE38QQR cytotoxin for recurrent supratentorial malignant glioma. Neuro-oncol. 2002;4:379. (Abstract) [Google Scholar]
- 43.Kunwar S, Prados MD, Lang FF. Pre and post-operative infusion of IL13-pE38QQR cytotoxin by convection-enhanced delivery (CED) in recurrent malignant glioma: a phase I study. Proc Am Soc Clin Oncol. 2003;22:119. (Abstract) [Google Scholar]
- 44.Voges J, Reszka R, Gossmann A, Dittmar C, Richter R, Garlip G, et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol. 2003;54:479–487. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 45.Croteau D, Walbridge S, Morrison PF, Butman JA, Vortmeyer AO, Johnson D, et al. Real-time in vivo imaging of the convective distribution of a low-molecular-weight tracer. J Neurosurg. 2005;102:90–97. doi: 10.3171/jns.2005.102.1.0090. [DOI] [PubMed] [Google Scholar]
- 46.Strasser JF, Fung LK, Eller S, Grossman SA, Saltzman WM. Distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea and tracers in the rabbit brain after interstitial delivery by biodegradable polymer implants. J Pharmacol Exp Ther. 1995;275:1647–1655. [PubMed] [Google Scholar]
- 47.Chen MY, Hoffer A, Morrison PF, Hamilton JF, Hughes J, Schlageter KS, et al. Surface properties, more than size, limiting convective distribution of virus-sized particles and viruses in the central nervous system. J Neurosurg. 2005;103:311–319. doi: 10.3171/jns.2005.103.2.0311. [DOI] [PubMed] [Google Scholar]
- 48.Cunningham J, Oiwa Y, Nagy D, Podsakoff G, Colosi P, Bankiewicz KS. Distribution of AAV-TK following intracranial convection-enhanced delivery into rats. Cell Transplant. 2000;9:585–594. doi: 10.1177/096368970000900504. [DOI] [PubMed] [Google Scholar]
- 49.Brooks AI, Halterman MW, Chadwick CA, Davidson BL, Haak-Frendscho M, Radel C, et al. Reproducible and efficient murine CNS gene delivery using a microprocessor-controlled injector. J Neurosci Methods. 1998;80:137–147. doi: 10.1016/s0165-0270(97)00207-0. [DOI] [PubMed] [Google Scholar]
- 50.Friedman HS, Dolan ME, Pegg AE, Marcelli S, Keir S, Catino JJ, et al. Activity of temozolomide in the treatment of central nervous system tumor xenografts. Cancer Res. 1995;55:2853–2857. [PubMed] [Google Scholar]