Abstract
unc-3 encodes the C. elegans ortholog of the Olf-1/Early B cell factor family of transcription factors, which in vertebrates regulate development and differentiation of B lymphocytes, adipocytes, and cells of the nervous system. unc-3 mutants are uncoordinated in locomotion. Here we show that unc-3 represses a VC-like motor neuron program in the VA and VB motor neurons, which in wild-type animals control backwards and forwards locomotion, respectively. We identify a physical interaction between UNC-3 and the C2H2 zinc finger transcription factor PAG-3, the mammalian homologs of which are coexpressed in olfactory epithelium and hematopoietic cells. Our data explain the locomotory defects of unc-3 mutants and suggest that interactions between unc-3 and pag-3 orthologs in other species may be functionally important.
Keywords: motor neuron fate, unc-3, pag-3, olf-1, Ebf-1, Gfi-1
Introduction
The known and essentially invariant cell lineage of Caenorhabditis elegans provides a basis for examining the mechanisms that establish cell fates with single cell resolution (Sternberg and Horvitz, 1984). The characterization of C. elegans mutants that move in an uncoordinated fashion has revealed genes that together generate the proper numbers and types of motor neurons, including genes that encode transcription factors. Among these genes is unc-3, originally identified by Brenner in the initial description of C. elegans as an organism with tractable genetics (Brenner, 1974).
unc-3 encodes a 511 amino acid variant HLH protein that is highly conserved in other species (Dubois and Vincent, 2001; Prasad et al., 1998). Vertebrate homologs of unc-3 include four Olf-1/Early B cell factor genes of mice (Garel et al., 1997; Hagman et al., 1993; Wang and Reed, 1993; Wang et al., 2002; Wang et al., 1997). Genetic analyses of O/E genes indicates that they have overlapping expression patterns and functions in the central nervous system, including directing projection of neuronal axons to the olfactory bulb and through the thalamus (Garel et al., 1999; Garel et al., 2002; Wang et al., 2004) (Davis and Reed, 1996; Wang et al., 1997).
In C. elegans, unc-3 is expressed in the two ASI chemosensory neurons and in motor neurons of the ventral nerve cord (Prasad et al., 1998). In the ASI neurons, unc-3 prevents transcription of genes characteristic of other types of neurons; in some cases these genes are direct targets of the UNC-3 protein (Kim et al., 2005).
unc-3 mutants are also profoundly defective in locomotion (Brenner, 1974). The ventral nerve cord motor neurons of unc-3 mutants have axon fasciculation defects, neuromuscular junctions at ectopic sites and make inappropriate synapses with interneurons (Durbin, 1987; Prasad et al., 1998).
In wild-type newly hatched larvae, the ventral nerve cord and associated ganglia contain 22 motor neurons of three classes (White et al., 1986). Ablation of individual classes of motor neurons in newly hatched animals resulted in a model for locomotion in which the A and B class motor neurons mediate muscle contraction for backward and forward movement, respectively (Chalfie et al., 1985). An additional 53 neurons are added to the ventral nerve cord during postembryonic development. With specific exceptions, the fates adopted by these neurons are strikingly correlated with their lineage histories (Sulston, 1976; Sulston and Horvitz, 1977). For example, the motor neurons generated as the most anterior descendant in the P cell lineages adopt a VA motor neuron fate (Pflugrad et al., 1997; Sulston and Horvitz, 1977; White et al., 1976). The embryonic DA and postembryonic VA motor neurons share features, including anteriorly-directed axonal processes, similar synaptic inputs from interneurons, and mediation of backwards movement (Miller and Niemeyer, 1995; Pflugrad et al., 1997; White et al., 1986). Comparable similarities exist for the embryonic and postembryonic B and D class motor neurons (White et al., 1986).
Here we establish a role for unc-3 in specifying a portion of this precise pattern of motor neuron development. We describe the expression pattern of unc-3 in the postembryonic motor neurons and changes in the pattern of motor neuron fates in unc-3 mutants. Using a laser to ablate specific cells in the P cell lineages of unc-3 mutants, we demonstrate that unc-3 represses a VC-like motor neuron fate in the VA and VB motor neurons.
Materials and Methods
Isolation of unc-3 mutations
Plin-11gfp hermaphrodites were mutagenized with EMS (Brenner, 1974), and mutants with an abnormal number of fluorescent nuclei were identified. From a screen of approximately 20,000 haploid genomes using a stereomicroscope equipped with epifluorescence, six mutants with an increased number of fluorescent nuclei were recovered. All six mutants exhibited an uncoordinated movement defect that cosegregated with an increased number of fluorescent nuclei and mapped to LGX. Three of these six failed to complement mutations in pag-3, which has an increased number of VC neurons (Cameron et al., 2002). The remaining three mutants, n3412, n3413 and n3366 carried new alleles of unc-3 and are described here. The unc-3(n3435) deletion allele was isolated by screening a library of chemically-mutagenized C. elegans as described (Ceol and Horvitz, 2001; Jansen et al., 1997). n3435 was backcrossed five times to the wild-type strain N2 before use.
The n3412, n3413 and n3366 mutants carried recessive X-linked mutations that also conferred an uncoordinated (Unc) movement defect like that of unc-3 mutants (Brenner, 1974). We found that unc-3(e151) mutants have an increased number of fluorescent Plin-11gfp-expressing nuclei, like n3412, n3413 and n3366 mutants. n3412, n3413 and n3366 failed to complement unc-3(e151) for the Unc phenotype. Analysis of the unc-3 coding sequence from n3412 mutants revealed a premature stop mutation identical to that of unc-3(e151). n3413 and n3366 mutants also have mutations in the unc-3 coding sequence. unc-3(n3413) is an R166W missense mutation, and unc-3(n3366) is a P180L missense mutation.
Strains and alleles
Strains were maintained at 20° C as described by Brenner (Brenner, 1974). Unless otherwise indicated, strains were obtained from the MIT/Horvitz laboratory collection or were provided by the Caenorhabditis Genetic Center, which is funded by the NIH National Center for Research Resources.
Mutations used were:
LGII. wdIs4, an integrated Punc-4gfp transgene; wdIs6, an integrated Pdel-1gfp transgene (both provided by David Miller); juIs76, an integrated Punc-25gfp transgene; inIs179, an integrated Pida-1gfp transgene.
LGX. oxIs12, an integrated Punc-47gfp transgene; nIs106, an integrated Plin-11gfp transgene (Cameron et al., 2002); pag-3(n3098) (Cameron et al., 2002); wdEx75, an extrachromosomal Pacr-5gfp array (provided by David Miller).
Analysis of unc-3 alleles
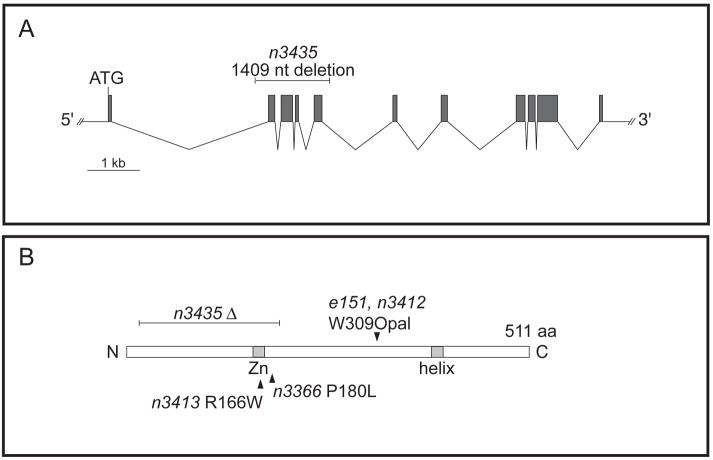
Using the Plin-11gfp reporter as a marker for the VC class of motor neurons, we compared the number of neurons that express the reporter in unc-3(e151), unc-3(n3412), and unc-3(n3435). The unc-3(n3435) allele is a deletion of all of exons 2, 3, 4 and 5 (Figure 6). This mutation results in an in-frame deletion of amino acids 30–188, which encode most of the evolutionarily conserved amino acid sequence in UNC-3, including those regions required for binding to DNA by the UNC-3 homologue O/E-1 in the mouse (Hagman et al., 1993; Hagman et al., 1995). Mutants homozygous for the unc-3(n3435) mutation are viable with an uncoordinated movement defect indistinguishable from that of unc-3(e151) and unc-3(n3412) mutants. All three alleles of unc-3 have similar numbers of Plin-11gfp-expressing neurons (Table 2). Together these data suggest that e151, n3412 and n3435 are strong loss-of-function or null alleles.
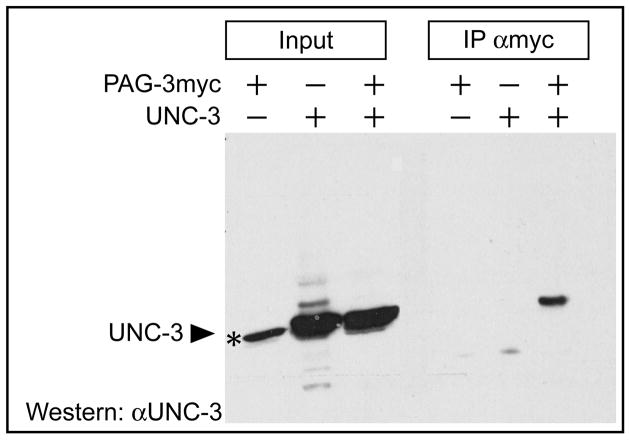
Figure 6. Coimmunoprecipitation of PAG-3 and UNC-3.
HEK293 cells were transfected with the indicated constructs, myc-epitope-tagged PAG-3 was immunoprecipitated with anti-myc antiserum, proteins were resolved using SDS-PAGE, and UNC-3 was detected on a western blot with UNC-3 antiserum (αUNC-3). Filled arrowheads indicate UNC-3 protein. The asterisk indicates an irrelevant band detected by the UNC-3 antiserum running slightly below UNC-3.
Table 2.
Expression of motor neuron markers in unc-3 mutants
| VA and/or DA* |
VB and/or DB
|
VD and/or DD
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stage | Punc-4gfp | Punc-4gfp; unc-3 | #Pacr-5gfp | Pacr-5gfp; unc-3 | † Pdel-1gfp | † Pdel-1gfp; unc-3 | Punc-25gfp | Punc-25gfp; unc-3 | Punc-47gfp | Punc-47gfp; unc-3 |
| L3 | 10.9 ± 0.3 | 15.1 ± 0.4 | 13.3 ± 0.3 | 4.1 ± 0.4 | 9.0 ± 0.0 | 2.5 ± 0.3 | 17.6 ± 0.4 | 18.4 ± 0.2 | 18.7 ± 0.1 | 18.6 ± 0.1 |
| L1 | 2.8 ± 0.3 | 1.3 ± 0.2 | 5.4 ± 0.1 | 1.5 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.0 |
| L3-L1 | 8.1 ± 0.6 | 14.0 ± 0.6 | 7.9 ± 0.4 | 2.6 ± 0.5 | 9.0 ± 0.0 | 2.5 ± 0.3 | 11.6 ± 0.4 | 12.4 ± 0.0 | 12.7 ± 0.1 | 12.6 ± 0.1 |
| VC
|
||||||||||
| § Plin-11gfp | Plin-11gfp; unc-3 | § Pida-1gfp | Pida-1gfp; unc-3 | |||||||
|
|
||||||||||
| Adult | 4.2 ± 0.1 | 8.5 ± 0.3 | 6.0 ± 0.0 | 9.0 ± 0.3 | ||||||
| L3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||||
| L1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||||
The numbers of fluorescent nuclei in the ventral nerve cords of otherwise wild-type and unc-3 mutant transgenic animals are shown. n=30–50 in all cases. Numbers represent mean ± s.e.m. For Punc-4gfp we scored the P3-P11 region; for Pacr-5gfp and Pdel-1gfp we scored the P2-P10 region; these regions were chosen to avoid scoring additional fluorescent nuclei present in the ganglia in the anterior and posterior nerve cord.
The Punc-4gfp reporter detects DA and VA neurons in L3 animals (1, 2). The VC neurons of wild-type animals do not express Punc-4gfp until late L3 or L4 (3).
The Pacr-5gfp reporter is an extrachromosomal array and can be lost mitotically in some cells, which may account for the small difference in the estimate of VB motor neuron number when compared to the integrated Pdel-1gfp reporter.
The Pdel-1gfp reporter is expressed in the VA neurons in the anterior portion of the ventral nerve cords of late L3 animals (4). Initial VA expression is dimmer than in the VB neurons. The numbers for L3 animals do not include these VA nuclei.
The Plin-11gfp and Pida-1gfp reporters are first expressed in L4 animals; young adults were scored. Plin-11gfp is expressed in vulval muscle and hypodermal cells, typically obscuring the VC4 and VC5 nuclei, which flank the vulva. Pida-1gfp is not expressed in the vulva. The VC4 and VC5 neurons are visible in animals carrying Pida-1gfp, accounting for the two additional VC neurons in otherwise wild-type animals.
Cell-lineage and laser-ablation studies
Cell lineages were observed as described (Sulston and Horvitz, 1977). For laser ablation studies, P cell lineages of L1 Plin-11gfp unc-3(n3435) mutants were followed to identify unambiguously the cellular targets for ablation. Mutants were then anesthetized in 30 mM sodium azide, transferred for laser ablation as described (Sulston and White, 1980), and allowed to recover and develop to young adulthood on an NGM agar plate with OP50 bacteria. Fluorescence optics were used to determine the number of Plin-11gfp-expressing neurons.
Antibody staining
Animals carrying the indicated gfp reporter transgenes were fixed as described for 30 minutes in 4% paraformaldehyde (Finney and Ruvkun, 1990) and stained with UNC-3 antibody (Prasad et al., 1998). DAPI was added at 1 μg/mL. FMRFamide staining was performed as described, using collagenase for permeabilization (Li and Chalfie, 1990). Antiserum against FMRFamide was kindly provided by Chris Li.
Dimerization assay and coimmunoprecipitation experiments
To construct the full-length unc-3 expression vector, full-length unc-3 cDNA (SalI/NotI from unc3-pCMV-GST) was cloned into a CMV promoter-driven mammalian expression vector (pRK5). To construct the truncated unc-3 expression vector, the SalI/PvuII fragment from unc3-pCMV-GST, which deletes the nucleotides that encode the C-terminal 31 amino acids against which the UNC-3 antibody was generated (Prasad et al., 1998), was cloned into myc-pRK5. 500 μl of whole-cell extracts prepared from cells transiently transfected with these constructs were incubated with 60 μl of Protein G beads for 2 hrs at 4° C, followed by six washes with PTP buffer (PBS/1 % Triton/protease inhibitors). Protein G beads were prepared by incubating 100 μl beads with antibodies (1 μl αUNC-3 (Prasad et al., 1998) or a c-myc monoclonal antibody) for 1 hr, followed by three washes with PTP. Antibody-protein complex was eluted from the beads by boiling in 150 μl 2× sample buffer and analyzed on a western blot (myc-HRP or αUNC-3).
For coimmunoprecipitations of PAG-3 and UNC-3, full-length pag-3 cDNA (Jia et al., 1997) was cloned into pRK5 containing a C-terminal myc tag using NotI and HindIII sites, and the full-length unc-3 cDNA was cloned into pRK5 as above. Both constructs were expressed in HEK293 cells after transient transfection using calcium phosphate. Whole-cell extracts were prepared by lysis of one 10 cm plate of cells in 1 ml of PTP Buffer at 4° C for 30 min. The extracts were centrifuged at maximum speed for 15 min to clear the lysate. αmyc ascites antibody was incubated with the cleared lysates overnight in 0.1% SDS and 500 mM NaCl. The protein-antibody mixtures were subsequently incubated with 100 μl protein G beads (Ultralink protein G beads, Pierce) for 1 hr at room temperature. The beads were washed six times with PTP/0.1% SDS/500 mM NaCl followed by a single wash with ddH2O. 150 μl 2× sample buffer was added to the beads, the samples were boiled and analyzed using a western blot with the αUNC-3 antibody and an HRP-conjugated secondary antibody.
Yeast two-hybrid assay and plasmid construction
Two-hybrid experiments were performed with the yeast strain Y190 (MATa gal4 gal 80 his3 trp1-901 ade2-101 ura3-52 leu2-3, -112 + URA3::GAL->lacZ, LYS2::GAL->HIS3 cyhr) (Harper et al., 1993). Yeast was grown at 30° C in 1 % yeast extract, 2% peptone, and 2% dextrose (YPD), synthetic complete (SC), or CSM dropout medium (Bio101, Carlsbad, CA). A BsaHI fragment containing the C-terminal third of unc-3 cDNA was subcloned into pAS1-CYH2 (bait) encoding the GAL4 DNA-binding domain and a leu-2 selection marker. The resulting plasmid, pASI-uncHLH, was transformed into Y190, and Leu+ colonies were selected. An individual isolate was transformed with a construct containing the same unc-3 fragment fused to the GAL4 transactivation domain in pACTII. Double transformants were plated on SC-Leu,-Trp,-His minimal plates containing 50 mM 3-aminotriazole (Sigma) to reduce leaky expression of HIS3. His+ colonies from the primary selection were subsequently screened for both the His phenotype and β-galactosidase activity.
Results
unc-3 mutants have extra VC-like neurons
The gene lin-11 is expressed in the six VC motor neurons of the ventral nerve cord. In a genetic screen of descendants of mutagenized hermaphrodites carrying a Plin-11gfp reporter (Cameron et al., 2002) (see Materials and Methods), we identified n3412, n3413 and n3366 as mutants with approximately four extra fluorescent neurons in the ventral nerve cord of adults as compared to wild-type animals (Figure 1, Table 1, and see below). We determined that all three mutants carried recessive alleles of unc-3 (see Materials and Methods). Based on the uncoordinated movement defect, the number of Plin-11gfp-expressing neurons and DNA sequence analysis, the unc-3 reference allele e151, n3412 and a deletion allele n3435 that we isolated are strong loss-of-function or null alleles (Table 1 and Figure 2). Except where indicated, we used the unc-3(n3435) allele for the analyses described here. Images of unc-3(n3435) are shown in Figure 1.
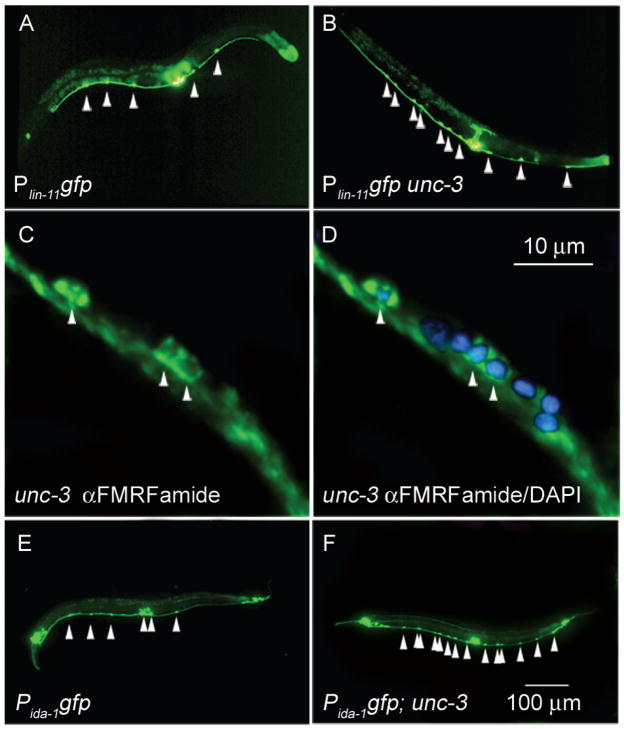
Figure 1. unc-3 mutants have extra VC-like neurons.
(A) Wild-type hermaphrodite expressing the Plin-11gfp reporter. Wild-type animals have six VC neurons (arrowheads), two of which are variably obscured by vulval fluorescence. In this animal, five VC neurons were visible. (B) unc-3 hermaphrodite expressing the Plin-11gfp reporter. (C) unc-3 mutant stained with antiserum against the neuropeptide FMRFamide, with three staining nuclei in a small region of the ventral nerve cord, including two cell bodies that are adjacent. Wild-type animals do not have adjacent FMRF-amide positive neurons (data not shown). (D) unc-3 hermaphrodite stained with antiserum against the neuropeptide FMRFamide and with DAPI. (E) Wild-type hermaphrodite expressing the Pida-1gfp reporter. (F) unc-3 mutant expressing the Pida-1gfp reporter. All mutant animals carried the unc-3(n3435) allele and are adults. Scale bar in panel A applies to images A, B, E and F. Anterior, leftwards, ventral, downwards.
Table 1.
Numbers of VC and VC-like cells in unc-3 mutants
| Genotype | No. fluorescent nuclei | n |
|---|---|---|
| nIs106 (Plin-11gfp) | 4.3 ± 0.4 | 50 |
| nIs106 unc-3(e151) | 8.1 ± 0.1 | 36 |
| nIs106 unc-3(n3412) | 8.3 ± 0.3 | 35 |
| nIs106 unc-3(n3435) | 8.5 ± 0.3 | 46 |
The number of Plin-11gfp-expressing nuclei in the ventral nerve cords of adult hermaphrodites is shown. Values are means ± s.e.m. Two VC neurons, VC4 and VC5, are often obscured by vulval fluorescence and were not scored.
Figure 2. Diagram of the unc-3 locus and alleles.
(A) unc-3 exons are indicated as shaded boxes. The n3435 deletion is indicated above exons 2, 3, 4 and 5. (B) The UNC-3 protein is represented with an indication of the location of the protein sequences altered in the e151, n3412, n3413, n3366 and n3435 alleles. Shaded boxes indicate the zinc finger and helix domains.
We examined the expression in unc-3 mutants of two other VC markers, ida-1 (Zahn et al., 2001) and the neuropeptide FMRFamide (Schinkmann and Li, 1992). Using a Pida-1gfp reporter construct, unc-3 mutants had about three extra neurons that express the reporter (Table 2). Using an antiserum against FMRFamide, we identified extra FMRFamide-expressing neurons in the ventral cord (Figure 1), although it was not possible to count precisely the number of neurons in stained animals because they were fragmented by the staining protocol. The ventral nerve cord of unc-3 mutants therefore contains approximately three to four extra neurons that express three different markers of VC fate. We refer to these extra neurons as “VC-like.”
The A and B class motor neurons are abnormal or absent in unc-3 mutants
unc-3 mutants are severely defective in forwards and backwards movement, suggesting that the class A (DA and VA) and B (DB and VB) neurons, which mediate locomotion (Chalfie et al., 1985), could be defective. At hatching, L1 animals have nine DA, seven DB and six DD neurons in the ventral nerve cord and associated ganglia (Sulston and Horvitz, 1977). Beginning late during L1, a series of divisions by the W and P blast cells generates postembryonic neurons, adding 12 VA, 11 VB, six VC and 13 VD neurons as well as additional cells to the ventral nerve cord; these divisions are completed during the early L2 (Sulston and Horvitz, 1977).
To assess the role of unc-3 in establishing this precise pattern of motor neuron types, we surveyed specific classes of motor neurons using gfp reporters. For most neuronal classes we counted the number of neurons expressing each reporter in L3 larvae, which have differentiated embryonic and postembryonic class A, B and D motor neurons. To estimate the number of postembryonic neurons, we subtracted the number of neurons expressing each reporter in L1 animals, which have only the embryonic motor neurons, from the number in L3 animals. The VC neuron reporters are not expressed until the late L3 or L4 (Table 2 and data not shown), and these reporters were scored in young adults. Using these data we estimated the number of reporter-expressing embryonic and postembryonic neurons in unc-3 mutants. Our results are presented in Table 2.
The embryonic DA and postembryonic VA and VC motor neurons express the Punc-4gfp reporter (Lickteig et al., 2001; Miller and Niemeyer, 1995). In L1 unc-3 mutants, the number of embryonic DA motor neurons is reduced to 1.3 ± 0.2 Punc-4gfp-expressing neurons compared to 2.8 ± 0.3 in otherwise wild-type animals (P<0.0001, unpaired two-tailed t-test) (Table 1). The ventral nerve cord of unc-3 mutants has a normal number of neuronal nuclei (data not shown) suggesting that the DA neurons are not missing and instead develop abnormally in unc-3 mutants. In contrast to the decreased number of embryonic Punc-4gfp-expressing neurons, L3 unc-3 mutants have an increased number of Punc-4gfp-expressing neurons compared to wild-type animals; 15.1 ± 0.4 neurons express Punc-4gfp in unc-3 mutants compared to 10.9 ± 0.3 in the wild-type (P<0.0001, unpaired two-tailed t-test) (Table 2). Previous work had suggested that the levels of Punc-4gfp expression in unc-3 mutants are not markedly different from wild-type (Prasad et al., 1998). On re-examining the effects of unc-3 on the numbers of neurons that express unc-4, we discovered stage-dependent and opposite effects in the embryonic and postembryonic ventral nerve cord, likely accounting for why this difference had not been observed earlier.
We observed that expression of Punc-4gfp in L3 unc-3 mutants occurred in pairs of adjacent nuclei along the ventral nerve cord with the exception of the posterior nerve cord, where single fluorescent nuclei were present. This observation suggested that the additional Punc-4gfp-expressing neurons might normally be the VB motor neurons, which are generally immediately posterior to the VA motor neuron nuclei and absent from the P11 and P12 lineages in the posterior, where the lineal homologs of the VB neurons undergo programmed cell death (Sulston and Horvitz, 1977). We confirmed that the VB neurons of unc-3 mutants express Punc-4gfp by directly observing partial cell lineages of the P9 and P10 blast cells of six unc-3 mutants carrying the Punc-4gfp transgene. Each P9 and P10 cell lineage of wild-type animals generates one VA and one VB neuron as daughter cells of the neuroblasts P9.aaa and P10.aaa (Pn.aaa, the anterior daughter of the anterior daughter of the anterior daughter of any P blast cell) (Sulston and Horvitz, 1977). We observed that all of the VA neurons of unc-3 mutants expressed gfp within an hour after the division that generated them; expression in the VB neurons became apparent two to three hours later in seven of 12 VB neurons generated by the two P cell lineages in the six animals. Most but not all VB neurons of unc-3 mutants express Punc-4gfp. 33 of 50 VB neurons scored at random from the P9 and P10 descendants of 25 L3 unc-3 mutants expressed gfp, compared to none of 50 wild-type animals (data not shown). We considered the possibility that the unc-4 gene might mediate essential aspects of the unc-3 mutant phenotype; if so, the movement defects of an unc-4; unc-3 double mutant might be more similar to unc-4 than to unc-3. We constructed unc-4; unc-3 double mutants and confirmed the genotype by DNA sequencing. The movement defect of the double mutant was indistinguishable from an unc-3 mutant, suggesting that unc-4 is one of multiple target genes of unc-3 in ventral nerve cord motor neurons.
Consistent with the VB motor neurons being abnormal in unc-3 mutants, B class motor neuron markers were often not expressed in unc-3 mutants. As assessed using the Pacr-5gfp reporter, which is expressed in DB and VB motor neurons (Winnier et al., 1999), the number of embryonic DB motor neurons of L1 unc-3 mutants was reduced from 5.4 ± 0.1 in the wild type to 1.5 ± 0.1 in unc-3 mutants (P<0.0001, unpaired two-tailed t-test) (Table 2). As determined by expression of Pacr-5gfp and a second marker, Pdel-1gfp, which is expressed specifically by VB motor neurons (Winnier et al., 1999), two-thirds of the VB motor neurons of unc-3 mutants also failed to develop normally (Table 2). These defects in the class B motor neurons, which are required for forward movement (Chalfie et al., 1985), likely account for the severe abnormality in forward movement of unc-3 mutants.
By contrast, expression of two class D-specific markers, Punc-47gfp and Punc-25gfp, was normal in the embryonic DD and postembryonic VD motor neurons of unc-3 mutants (Table 2).
In summary, unc-3 is essential for normal differentiation of DA, DB and VB motor neurons but apparently dispensable for differentiation of class D neurons. unc-3 represses the VA and VC-specific gene unc-4 in the VB motor neurons, suggesting that these neurons are transformed to a VA or VC-like fate. We provide further support for this suggestion below.
unc-3 represses the VC fate in the VA and VB motor neurons
We previously described the gene pag-3, which when mutant results in a phenotype with similarities to that of unc-3 mutants. pag-3 mutants are uncoordinated and have an increased number of VC-like motor neurons, a reduced number of VA neurons and a marked deficiency in the number of VB motor neurons (Cameron et al., 2002). pag-3 determines the fates of the neuroblasts that generate the VA, VB and VC motor neurons during postembryonic development. In wild-type animals the Pn.aa neuroblasts divide to generate a posterior VC neuron and Pn.aaa, a neuroblast that is the mother of the VA and VB motor neurons (the wild-type P cell lineage is diagrammed in Figure 3) (Sulston and Horvitz, 1977). In pag-3 mutants the Pn.aaa neuroblast instead reiterates the fate of its mother, Pn.aa, generating additional VC-like neurons and a neuroblast that again reiterates the pattern of division of Pn.aa (Cameron et al., 2002). Together these defects result in extra VC-like neurons and fewer neurons that express VA and VB markers.
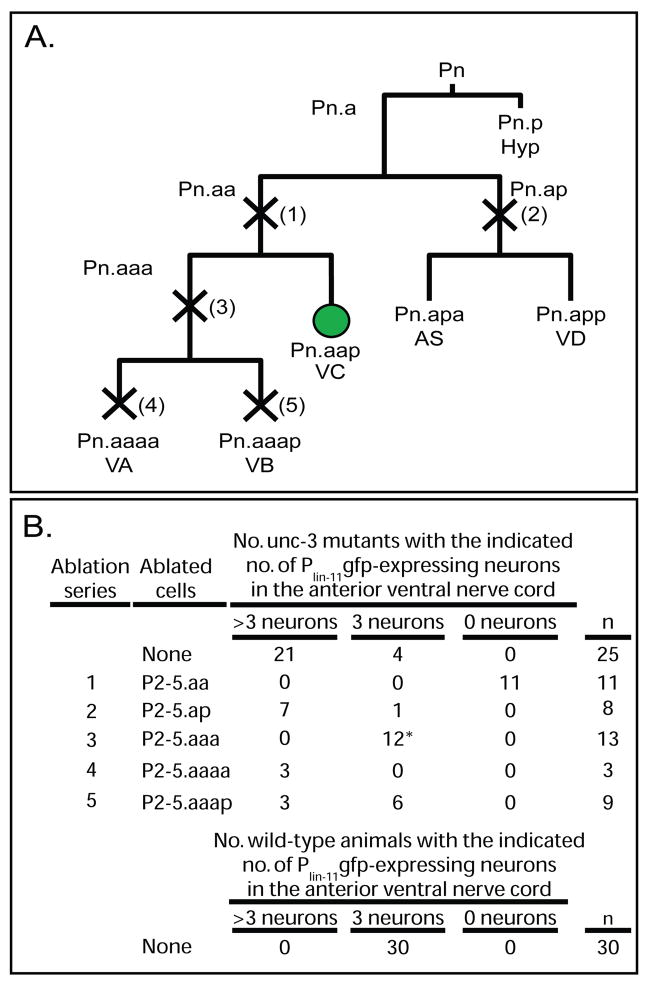
Figure 3. Identification of the source of the extra VC-like neurons in unc-3 mutants.
(A) Diagram of a wild-type P cell lineage, with normal motor neuron fates indicated below the terminal cells generated by the wild-type lineage (11). Five series of laser ablation experiments were performed. An “X” across a dividing cell (vertical line) and a number in parentheses adjacent to the “X” indicates each of five cell ablation studies. P cell lineages were observed in late L1 unc-3(n3435) mutant larvae, and the indicated cells were ablated shortly after their generation. Recovered animals were then allowed to grow until young adulthood, at which point they begin to express Plin-11gfp, and were then scored. Pn.aap, the posterior daughter of the anterior daughter of the anterior daughter of any P blast cell. The Pn.aap cells, which in P3-P8 lineages of wild-type animals differentiate to become VC neurons and express Plin-11gfp, are indicated with a green circle. To avoid possible confusion between gfp-expressing descendants of P2 and P3 we chose to ablate P2-5 progeny. (B) Summary of laser-ablation data. Numbers in the “Ablation series” correspond to cells killed as indicated in (A). The number of animals with the indicated number of fluorescent nuclei are shown; animals lacking fluorescent nuclei in the operated region are indicated as having “0 neurons.” n, number of successfully operated animals.
To determine directly whether unc-3 mutants might have a lineage defect like that of pag-3 mutants, we observed P cell lineages in unc-3 mutants. We found that the pattern and timing of divisions were normal; specifically, we did not observe a reiterative pattern of divisions that might lead to additional VC-like motor neurons or other cells (data not shown, and see below). To explore further which neurons might be transformed to a VC-like fate in unc-3 mutants as defined by expression of Plin-11gfp, we followed P cell lineages in unc-3 mutants carrying the VC-specific Plin-11gfp reporter and used a laser microbeam to ablate specific cells. We sought to identify those cell(s) that when ablated restored a wild-type pattern of Plin-11gfp reporter expression; such cells would be either directly or indirectly responsible for generation of the extra Plin-11gfp -expressing cells.
Whereas wild-type animals have three VC neurons (P3.aap, P4.aap and P5.aap) in the anterior ventral nerve cord (Sulston and Horvitz, 1977), most Plin-11gfp unc-3 mutants have more than three fluorescent VC-like nuclei in this region (Figure 2). Ablations of the P2-P5.ap neuroblasts, which in wild-type animals generate the AS and VD neurons, did not alter the number or pattern of fluorescent nuclei. Ablations of the P2-5.aa neuroblasts, which in wild-type animals generate the VA, VB and VC neurons, removed all fluorescent nuclei. These data suggest that extra VC-like neurons arise as descendants of Pn.aa. Ablations of P2-5.aaa, which in wild-type animals divide once to generate the VA and VB motor neurons, restored the pattern of fluorescence to that of the wild type. Ablations of P2-5.aaaa, which in wild-type animals are the VA motor neurons, resulted in at least some animals with extra fluorescent VC-like nuclei, as did ablations of P2-5.aaap, which normally are the VB motor neurons (Figure 3). Together these data suggest that unc-3 represses a VC-like program of development in some VA and VB motor neurons. unc-3 could perform this role in the precursors of the VA and VB neurons or alternatively in differentiating cells.
unc-3 is expressed in differentiating VA and VB neurons and not in the VC, VD or DD motor neurons
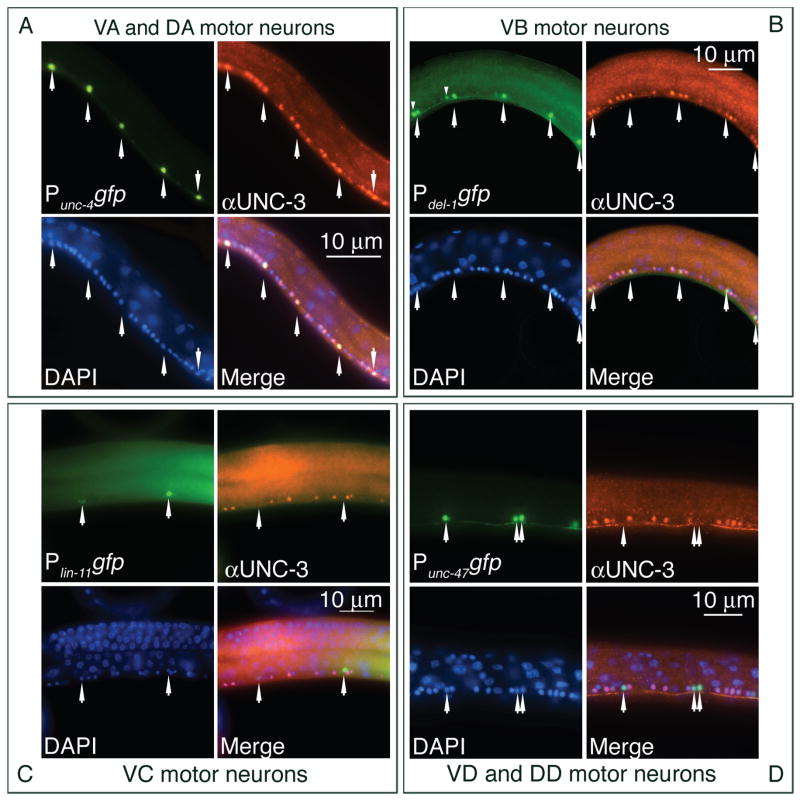
We extended the previously published analysis of the unc-3 expression pattern by using UNC-3 antiserum (Prasad et al., 1998) to stain transgenic animals carrying gfp reporters expressed in specific classes of motor neurons. We confirmed that L1 animals shortly after hatching showed expression of unc-3 in the embryonic DA and DB motor neurons; no staining of the DD neurons was detected (data not shown). L2 and L3 animals showed bright staining of the VA and VB motor neurons but not of the precursor cell that generated them (Figure 4 and data not shown). We never observed staining of the VC, VD or DD motor neurons. Staining remained readily detectable in the motor neurons of adult animals, although at lower levels than in L2 animals (Figure 4 and data not shown). In summary, we detected UNC-3 in the class A and B motor neurons, which are defective in unc-3 mutants, but not in the class D or VC neurons, which appear normal.
Figure 4. unc-3 is expressed postembryonically in the VA and VB but not the VC or VD motor neurons.
Each set of four panels displays images of neurons showing (i) gfp from a motor neuron type-specific promoter, (ii) UNC-3 protein detected with UNC-3 antiserum (αUNC-3), (iii) DAPI, (iv) a merged image. Each set of four panels assesses expression of unc-3 in the indicated class(es) of motor neurons. Residual fluorescence from GFP after fixation was used to detect specific classes of motor neurons. (A) unc-3 is expressed in the class A motor neurons. Images are from animals carrying the Punc-4gfp reporter, which is expressed in DA, VA and VC motor neurons (17, 39). unc-3 is not expressed in the VC neurons (see panel C). (B) unc-3 is expressed in the class B motor neurons. Images are from animals carrying the Pdel-1gfp reporter, which is expressed in the VB motor neurons. In L3 animals the Pdel-1gfp reporter is visible in some VA neurons at the anterior end of the ventral nerve cord (19). The more anterior nucleus of each pair of fluorescent nuclei in the anterior half of this image is a VA motor neuron and is marked by a small arrowhead above the nucleus. (C) unc-3 is not expressed in the VC motor neurons. Images are from animals carrying the Plin-11gfp reporter, which is expressed in the VC motor neurons (13). (D) unc-3 is not expressed in the VD motor neurons. Images are from animals carrying the Punc-47gfp reporter, which is expressed in the DD and VD motor neurons (40). Anterior, leftwards; ventral, downwards. Arrowheads indicate specific motor neurons identified by gfp expression. L3 animals were examined except for Plin-11gfp, for which young adults were examined.
UNC-3 forms homodimers
The O/E proteins of vertebrates include a variant HLH domain that is essential for homodimerization and for heterodimerization with other O/E family members (Hagman et al., 1995; Wang et al., 1997). The invertebrate homologs do not contain a canonical HLH motif, as they lack the second helix present in the vertebrate proteins. An alternative HLH structure has been proposed, consisting of an evolutionarily conserved helix and an additional, more N-terminal helix, but the functionality of this organization in UNC-3 or the Drosophila counterpart Collier has not been demonstrated (Crozatier et al., 1996).
Biochemical studies of the UNC-3 ortholog EBF1 of mice revealed two distinct dimerization domains, the HLH domain and a domain that mediated interaction between DNA-bound EBF1 proteins (Hagman et al., 1993; Hagman et al., 1995). O/E-1 can bind weakly to an Olf1 binding site as a monomer, and an isoform of O/E-4 lacking the second helix can bind DNA, suggesting that proteins lacking the second dimerization domain might be functional as monomers (Wang et al., 1997). The completely sequenced C. elegans genome contains only one O/E homolog, UNC-3, and it is therefore unresolved whether UNC-3 heterodimerizes with an unrecognized O/E-like protein, homodimerizes using interactions that do not require the conserved second -helix that is absent from the invertebrate proteins, or acts as a monomer.
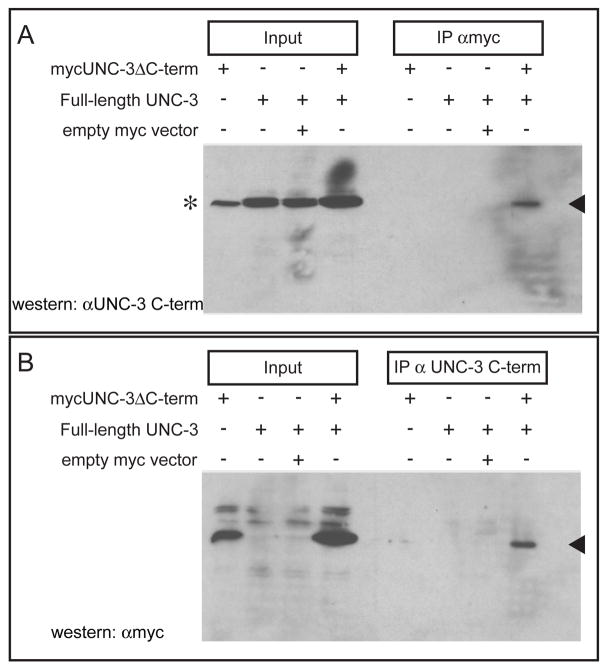
We expressed in HEK293 cells a full-length UNC-3 together with myc epitope-tagged UNC-3 in which the C-terminal 31 amino acids had been deleted and performed immunoprecipitation experiments. An antibody that recognized either the myc epitope or the C-terminus of UNC-3 was used for immunoprecipitation, and the other antibody was used for western blots. In both sets of experiments the full-length and C-terminally truncated forms of UNC-3 coimmunoprecipitated, suggesting that UNC-3 can homodimerize (Figure 5).
Figure 5. UNC-3 can form homodimers.
HEK293 cells were transfected with the indicated constructs, immunoprecipitations were performed using the indicated antisera, proteins were resolved using SDS-PAGE, and UNC-3 was detected on western blots with UNC-3 antisera that distinguished full length and truncated UNC-3. (A) mycUNC-3ΔC-term, a myc-epitope-tagged construct that encodes an UNC-3 protein lacking the C-terminal 31 amino acids to which the UNC-3 antibody (αUNC-3 C-term) binds; the protein generated by this construct is not recognized by the αUNC-3 C-term antiserum, which was raised against this C-terminal peptide (2). Filled arrowhead indicates full-length UNC-3. The asterisk indicates an irrelevant band detected by the UNC-3 antiserum running slightly below UNC-3. (B) Filled arrowhead indicates mycUNC-3ΔC-term.
We independently confirmed homomeric interactions of UNC-3 protein using a yeast 2-hybrid assay. When the C-terminal UNC-3 region including amino acids 336–492 containing the α-helical loop was fused to the GAL4 DNA-binding domain and the same region of UNC-3 was fused to a transcriptional activation domain, we observed β-galactosidase and HIS3-reporter expression that was dependent on the presence of both plasmids (data not shown).
UNC-3 and PAG-3 are coexpressed in the VA and VB motor neurons and can physically interact
pag-3 encodes a predicted 337 amino acid transcription factor with five C2H2 zinc fingers (Jia et al., 1997). PAG-3 antiserum detects protein in the Pn.aa neuroblasts and their descendants, the VA, VB and VC neurons (Cameron et al., 2002); that protein becomes undetectable very shortly after the VA, VB and VC neurons are generated. PAG-3 is not present in the ASI neurons (data not shown), which express UNC-3 (Prasad et al., 1998). PAG-3 and UNC-3 are therefore coexpressed in the VA and VB motor neurons during a very brief period immediately after these neurons are generated. As unc-3 is expressed in only the ASI neurons and ventral nerve cord motor neurons, the VA and VB motor neurons are likely to be the only cells of the postembryonic larva or adult in which pag-3 and unc-3 are coexpressed.
The mammalian orthologs of UNC-3, the O/E proteins, physically interact with OAZ, a protein with 30 predicted C2H2 zinc fingers (Tsai and Reed, 1998). We therefore tested the possibility that UNC-3 and PAG-3, a C2H2 zinc finger protein, might physically interact. We constructed epitope-tagged proteins, expressed them in HEK293 cells and tested their interaction using a coimmunoprecipitation assay. Immunoprecipitation of either UNC-3 or PAG-3 from cell lysates expressing both proteins resulted in coimmunoprecipitation of both proteins (Figure 6), suggesting that UNC-3 and PAG-3 might physically interact in differentiating VA and VB motor neurons. If unc-3 and pag-3 act together to repress the VC fate in the VA and VB neurons, unc-3 pag-3 double mutants might be expected to have a number of VC neurons similar to that of single mutants. Indeed, unc-3 pag-3 mutants have the same number of VC-like neurons as pag-3 mutants (Table 3). To address the possibility that unc-3 expression may depend upon pag-3 function in a manner analogous to that of unc-86 and mec-3 (Xue et al., 1992; Xue et al., 1993), we examined the expression pattern of unc-3 in pag-3 mutants by whole-mount staining with UNC-3 antiserum. Many motor neurons in the ventral nerve cord of pag-3 mutants expressed UNC-3 protein, ruling out the possibility that pag-3 is required for unc-3 expression by all motor neurons (data not shown).
Table 3.
Numbers of VC and VC-like neurons in unc-3 and pag-3 double mutants
| Genotype | No. fluorescent nuclei |
|---|---|
| ced-3; Plin-11gfp | 8.7 ± 0.1 |
| ced-3; Plin-11gfp unc-3(e151) | 12.6 ± 0.2 |
| ced-3; Plin-11gfp unc-3(n3412) | 13.3 ± 0.2 |
| ced-3; Plin-11gfp unc-3(n3435) | 12.2 ± 0.2 |
| ced-3; Plin-11gfp pag-3 | 19.9 ± 0.3 |
| ced-3; Plin-11gfp unc-3(e151) pag-3 | 19.6 ± 0.4 |
| ced-3; Plin-11gfp unc-3(n3435) pag-3 | 19.5 ± 0.3 |
The number of fluorescent nuclei seen in the ventral nerve cords of adult hermaphrodites is shown, scoring all fluorescent descendants of P2 through P12, inclusive. The ced-3(n717) mutation was included in these strains to ensure that all VC-like cells generated would survive and be counted, as some VC-like cells generated by the P2 and P9-P12 lineages of pag-3 mutants undergo programmed cell death (Cameron et al., 2002). The pag-3(n3098) allele used is a strong loss-of-function or null allele (Cameron et al., 2002).
Discussion
Prior studies of unc-3 established its role in regulating transcription in the ASI chemosensory neurons (Kim et al., 2005). In the ASI neurons, unc-3 directly represses the transcription of genes normally expressed in other neuron types and is required for transcription of daf-7, a gene that regulates entry in the dauer larva stage, an alternative developmental program adapted for survival under unfavorable environmental conditions. Together, these findings suggest that unc-3 mutants have defects in dauer entry at least in part because the transcriptional program associated with the ASI fate is defective. Our findings concerning the role of unc-3 in the motor neurons of the ventral nerve cord suggest a similar explanation for the defects in locomotion of unc-3 mutants. Using motor neuron-specific markers, the A and B class motor neurons are abnormal in unc-3 mutants. Our laser-ablation data indicate that some VA and VB motor neurons of unc-3 mutants express Plin-11gfp, a marker that is normally restricted to VC neurons. Some VB neurons of unc-3 mutants also express the VA and VC-specific marker Punc-4gfp. The timing of this expression, which begins shortly after the cells are generated, is characteristic of VA motor neurons and our data suggest that more VB neurons express Punc-4gfp than Plin-11gfp or Pida-1gfp, specific markers of the VC fate. These data suggest that in unc-3 mutants the VB motor neurons are transformed to a mixed VA and VC-like fate similar to that of the VA neurons of unc-3 mutants. We therefore propose that unc-3 represses a VC-like program in the VA and VB neurons, and a VA-like program in the VB neurons. As the A and B class motor neurons are required for backwards and forwards locomotion (Chalfie et al., 1985), respectively, these abnormalities are probably responsible for the profound defect in locomotion of unc-3 mutants. Previous studies of unc-3 mutants had described inappropriate synaptic neural connections and a disorganized ventral nerve cord with defasciculated axons (Durbin, 1987; Prasad et al., 1998), including FMRFamide-positive axons on the wrong side of the nerve cord (Wightman et al., 1997). Some of these defects might reflect on the abnormal motor neuron fates we observed rather than a primary role for unc-3 in controlling fasciculation or connectivity.
The vertebrate O/E genes are expressed in postmitotic neurons of the developing CNS (Davis and Reed, 1996). Electroporation into chick embryos of a dominant-negative form of the O/E protein Ebf1 blocks neuronal differentiation without interfering with cell cycle exit (Garcia-Dominguez et al., 2003). Our analyses of mutant C. elegans completely lacking unc-3 function revealed that the timing of neuroblast cell divisions was similar to that in wild-type animals, and we did not observe additional rounds of neuroblast divisions, as might be expected if unc-3 were required for cell cycle exit.
In mice, mutations in members of the O/E gene family result in abnormal neuronal migration and axon pathfinding in several systems (Garel et al., 2000; Garel et al., 1999; Wang et al., 2004). Migrating facial branchiomotor neurons respond to their changing environment by altering gene expression; this process is defective in O/E-1 mutants (Garel et al., 2000). In the olfactory system, disruption of the O/E-2 or O/E-3 genes leads to defects in axonal projection to the olfactory bulb (Wang et al., 2004). In spite of the overlapping expression of all four O/E family members in these sensory neurons, loss of both alleles of a single member (or even single alleles of O/E-2 and O/E-3 in double heterozygous animals) is sufficient to produce widespread defects in neuronal organization. These experiments further suggested that defects in axon pathfinding might be a consequence of reduced odorant receptor expression in migrating axons, suggesting that they are cell autonomous defects rather than a result of an inappropriate response to environmental cues during migration. Our data from C. elegans, in which we have precisely described defects in cell fates established by differentiating neurons in their normal locations, strongly support the proposal that the neurons are intrinsically defective (Garel et al., 1997; Wang et al., 2004).
unc-3 is expressed in chemosensory neurons and in several different classes of motor neurons, suggesting that the UNC-3 protein cooperates with other factors to determine cell fates. We report here that UNC-3 and PAG-3 can physically interact when coexpressed in mammalian cells, and our expression studies using antibodies that recognize UNC-3 and PAG-3 suggest that the two proteins are present together transiently in the VA and VB motor neurons as they are generated by the Pn.aaa divisions. To test the idea that PAG-3 and UNC-3 act together to specify some aspect of VA or VB fate, we constructed unc-3 pag-3 double mutants and found that they had a phenotype similar to that of pag-3 single mutants in the ventral nerve cord. However, the VA and VB motor neurons are missing or abnormal in pag-3 mutants at least in part because of a cell lineage defect in Pn.aaa, the mother of the VA and VB motor neurons (Cameron et al., 2002) and we are therefore unable to directly test the functional importance of the interaction between PAG-3 and UNC-3 in a convincing manner in these neurons. Nonetheless, it is interesting to note the extent to which the expression patterns overlap of the vertebrate orthologs of pag-3 and unc-3, the Gfi-1 and the O/E genes, in olfactory epithelium (Wallis et al., 2003) and hematopoietic cells. We have previously described the expression and role of the Gfi-1 ortholog pag-3 in the P neuroblast lineages (Cameron et al., 2002) and of the Gfi-1 orthologs in hematopoietic lineages (Hock et al., 2003; Saleque et al., 2002). We describe here that pag-3 expression immediately precedes expression of unc-3, that pag-3 and unc-3 are briefly coexpressed, and that pag-3 is repressed as unc-3 expression is established. A similar pattern exists for pag-3 and unc-3 orthologs as hematopoietic stem cells (HSCs) commit to lymphoid lineages. Gfi-1 is expressed in HSCs and controls their proliferation, then is partially repressed as the HSCs generate committed progenitors including common lymphoid progenitors, in which O/E-1 expression is first detected (Akashi et al., 2003; Dias et al., 2005; Hock et al., 2003; Krivtsov et al., 2006). These data suggest that Gfi and O/E proteins might physically interact to specify aspects of cell lineage and cell fate in vertebrates, as we have found that such proteins do in C. elegans.
Acknowledgments
We thank Bob Horvitz, in whose laboratory the genetic screen and initial analysis of unc-3 was conducted, for his support and for his much appreciated critical reading of the manuscript, and Malia Potts for comments on the science and the manuscript. This work was supported by NIH GM069667 to S.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akashi K, He X, Chen J, Iwasaki H, Niu C, Steenhard B, Zhang J, Haug J, Li L. Transcriptional accessibility for genes of multiple tissues and hematopoietic lineages is hierarchically controlled during early hematopoiesis. Blood. 2003;101:383–9. doi: 10.1182/blood-2002-06-1780. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron S, Clark SG, McDermott JB, Aamodt E, Horvitz HR. PAG-3, a Zn-finger transcription factor, determines neuroblast fate in C. elegans. Development. 2002;129:1763–74. doi: 10.1242/dev.129.7.1763. [DOI] [PubMed] [Google Scholar]
- Ceol CJ, Horvitz HR. dpl-1 DP and efl-1 E2F act with lin-35 Rb to antagonize Ras signaling in C. elegans vulval development. Mol Cell. 2001;7:461–73. doi: 10.1016/s1097-2765(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci. 1985;5:956–64. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozatier M, Valle D, Dubois L, Ibnsouda S, Vincent A. Collier, a novel regulator of Drosophila head development, is expressed in a single mitotic domain. Curr Biol. 1996;6:707–18. doi: 10.1016/s0960-9822(09)00452-7. [DOI] [PubMed] [Google Scholar]
- Davis JA, Reed RR. Role of Olf-1 and Pax-6 transcription factors in neurodevelopment. J Neurosci. 1996;16:5082–94. doi: 10.1523/JNEUROSCI.16-16-05082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias S, Silva H, Jr, Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201:971–9. doi: 10.1084/jem.20042393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois L, Vincent A. The COE--Collier/Olf1/EBF--transcription factors: structural conservation and diversity of developmental functions. Mech Dev. 2001;108:3–12. doi: 10.1016/s0925-4773(01)00486-5. [DOI] [PubMed] [Google Scholar]
- Durbin R. Studies on the development and organization of the nervous system of Caenorhabditis elegans. Cambridge University; Cambridge, UK.: 1987. [Google Scholar]
- Finney M, Ruvkun G. The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell. 1990;63:895–905. doi: 10.1016/0092-8674(90)90493-x. [DOI] [PubMed] [Google Scholar]
- Garcia-Dominguez M, Poquet C, Garel S, Charnay P. Ebf gene function is required for coupling neuronal differentiation and cell cycle exit. Development. 2003;130:6013–25. doi: 10.1242/dev.00840. [DOI] [PubMed] [Google Scholar]
- Garel S, Garcia-Dominguez M, Charnay P. Control of the migratory pathway of facial branchiomotor neurones. Development. 2000;127:5297–307. doi: 10.1242/dev.127.24.5297. [DOI] [PubMed] [Google Scholar]
- Garel S, Marin F, Grosschedl R, Charnay P. Ebf1 controls early cell differentiation in the embryonic striatum. Development. 1999;126:5285–94. doi: 10.1242/dev.126.23.5285. [DOI] [PubMed] [Google Scholar]
- Garel S, Marin F, Mattei MG, Vesque C, Vincent A, Charnay P. Family of Ebf/Olf-1-related genes potentially involved in neuronal differentiation and regional specification in the central nervous system. Dev Dyn. 1997;210:191–205. doi: 10.1002/(SICI)1097-0177(199711)210:3<191::AID-AJA1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Garel S, Yun K, Grosschedl R, Rubenstein JL. The early topography of thalamocortical projections is shifted in Ebf1 and Dlx1/2 mutant mice. Development. 2002;129:5621–34. doi: 10.1242/dev.00166. [DOI] [PubMed] [Google Scholar]
- Hagman J, Belanger C, Travis A, Turck CW, Grosschedl R. Cloning and functional characterization of early B-cell factor, a regulator of lymphocyte-specific gene expression. Genes Dev. 1993;7:760–73. doi: 10.1101/gad.7.5.760. [DOI] [PubMed] [Google Scholar]
- Hagman J, Gutch MJ, Lin H, Grosschedl R. EBF contains a novel zinc coordination motif and multiple dimerization and transcriptional activation domains. Embo J. 1995;14:2907–16. doi: 10.1002/j.1460-2075.1995.tb07290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hock H, Hamblen MJ, Rooke HM, Traver D, Bronson RT, Cameron S, Orkin SH. Intrinsic requirement for zinc finger transcription factor gfi-1 in neutrophil differentiation. Immunity. 2003;18:109–20. doi: 10.1016/s1074-7613(02)00501-0. [DOI] [PubMed] [Google Scholar]
- Jansen G, Hazendonk E, Thijssen KL, Plasterk RH. Reverse genetics by chemical mutagenesis in Caenorhabditis elegans. Nat Genet. 1997;17:119–21. doi: 10.1038/ng0997-119. [DOI] [PubMed] [Google Scholar]
- Jia Y, Xie G, McDermott JB, Aamodt E. The C. elegans gene pag-3 is homologous to the zinc finger proto- oncogene gfi-1. Development. 1997;124:2063–73. doi: 10.1242/dev.124.10.2063. [DOI] [PubMed] [Google Scholar]
- Kim K, Colosimo ME, Yeung H, Sengupta P. The UNC-3 Olf/EBF protein represses alternate neuronal programs to specify chemosensory neuron identity. Dev Biol. 2005;286:136–48. doi: 10.1016/j.ydbio.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, Golub TR, Armstrong SA. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–22. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- Li C, Chalfie M. Organogenesis in C. elegans: positioning of neurons and muscles in the egg-laying system. Neuron. 1990;4:681–95. doi: 10.1016/0896-6273(90)90195-l. [DOI] [PubMed] [Google Scholar]
- Lickteig KM, Duerr JS, Frisby DL, Hall DH, Rand JB, Miller DM., 3rd Regulation of neurotransmitter vesicles by the homeodomain protein UNC-4 and its transcriptional corepressor UNC-37/groucho in Caenorhabditis elegans cholinergic motor neurons. J Neurosci. 2001;21:2001–14. doi: 10.1523/JNEUROSCI.21-06-02001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DM, 3rd, Niemeyer CJ. Expression of the unc-4 homeoprotein in Caenorhabditis elegans motor neurons specifies presynaptic input. Development. 1995;121:2877–86. doi: 10.1242/dev.121.9.2877. [DOI] [PubMed] [Google Scholar]
- Pflugrad A, Meir JY, Barnes TM, Miller DM., 3rd The Groucho-like transcription factor UNC-37 functions with the neural specificity gene unc-4 to govern motor neuron identity in C. elegans. Development. 1997;124:1699–709. doi: 10.1242/dev.124.9.1699. [DOI] [PubMed] [Google Scholar]
- Prasad BC, Ye B, Zackhary R, Schrader K, Seydoux G, Reed RR. unc-3, a gene required for axonal guidance in Caenorhabditis elegans, encodes a member of the O/E family of transcription factors. Development. 1998;125:1561–8. doi: 10.1242/dev.125.8.1561. [DOI] [PubMed] [Google Scholar]
- Saleque S, Cameron S, Orkin SH. The zinc-finger proto-oncogene Gfi-1b is essential for development of the erythroid and megakaryocytic lineages. Genes Dev. 2002;16:301–6. doi: 10.1101/gad.959102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkmann K, Li C. Localization of FMRFamide-like peptides in Caenorhabditis elegans. J Comp Neurol. 1992;316:251–60. doi: 10.1002/cne.903160209. [DOI] [PubMed] [Google Scholar]
- Sternberg PW, Horvitz HR. The genetic control of cell lineage during nematode development. Annu Rev Genet. 1984;18:489–524. doi: 10.1146/annurev.ge.18.120184.002421. [DOI] [PubMed] [Google Scholar]
- Sulston JE. Post-embryonic development in the ventral cord of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:287–97. doi: 10.1098/rstb.1976.0084. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–56. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Sulston JE, White JG. Regulation and cell autonomy during postembryonic development of Caenorhabditis elegans. Dev Biol. 1980;78:577–597. doi: 10.1016/0012-1606(80)90353-x. [DOI] [PubMed] [Google Scholar]
- Tsai RY, Reed RR. Identification of DNA recognition sequences and protein interaction domains of the multiple-Zn-finger protein Roaz. Mol Cell Biol. 1998;18:6447–56. doi: 10.1128/mcb.18.11.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis D, Hamblen M, Zhou Y, Venken KJ, Schumacher A, Grimes HL, Zoghbi HY, Orkin SH, Bellen HJ. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development. 2003;130:221–32. doi: 10.1242/dev.00190. [DOI] [PubMed] [Google Scholar]
- Wang MM, Reed RR. Molecular cloning of the olfactory neuronal transcription factor Olf-1 by genetic selection in yeast. Nature. 1993;364:121–6. doi: 10.1038/364121a0. [DOI] [PubMed] [Google Scholar]
- Wang SS, Betz AG, Reed RR. Cloning of a novel Olf-1/EBF-like gene, O/E-4, by degenerate oligo-based direct selection. Mol Cell Neurosci. 2002;20:404–14. doi: 10.1006/mcne.2002.1138. [DOI] [PubMed] [Google Scholar]
- Wang SS, Lewcock JW, Feinstein P, Mombaerts P, Reed RR. Genetic disruptions of O/E2 and O/E3 genes reveal involvement in olfactory receptor neuron projection. Development. 2004;131:1377–88. doi: 10.1242/dev.01009. [DOI] [PubMed] [Google Scholar]
- Wang SS, Tsai RYL, Reed RR. The characterization of the Olf-1/EBF-like HLH transcription factor family: implications in olfactory gene regulation and neuronal development. J Neurosci. 1997;17:4149–58. doi: 10.1523/JNEUROSCI.17-11-04149.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the ventral nerve cord of Caenorhabditis elegans. Philos Trans R Soc Lond [Biol] 1976;275:327–48. doi: 10.1098/rstb.1976.0086. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B: Biol Sci. 1986;311:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wightman B, Baran R, Garriga G. Genes that guide growth cones along the C. elegans ventral nerve cord. Development. 1997;124:2571–80. doi: 10.1242/dev.124.13.2571. [DOI] [PubMed] [Google Scholar]
- Winnier AR, Meir JY, Ross JM, Tavernarakis N, Driscoll M, Ishihara T, Katsura I, Miller DM., 3rd UNC-4/UNC-37-dependent repression of motor neuron-specific genes controls synaptic choice in Caenorhabditis elegans. Genes Dev. 1999;13:2774–86. doi: 10.1101/gad.13.21.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue D, Finney M, Ruvkun G, Chalfie M. Regulation of the mec-3 gene by the C. elegans homeoproteins UNC-86 and MEC-3. Embo J. 1992;11:4969–79. doi: 10.1002/j.1460-2075.1992.tb05604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue D, Tu Y, Chalfie M. Cooperative interactions between the Caenorhabditis elegans homeoproteins UNC-86 and MEC-3. Science. 1993;261:1324–8. doi: 10.1126/science.8103239. [DOI] [PubMed] [Google Scholar]
- Zahn TR, Macmorris MA, Dong W, Day R, Hutton JC. IDA-1, a Caenorhabditis elegans homolog of the diabetic autoantigens IA-2 and phogrin, is expressed in peptidergic neurons in the worm. J Comp Neurol. 2001;429:127–43. doi: 10.1002/1096-9861(20000101)429:1<127::aid-cne10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]