Abstract
The properties of dorsal root ganglion (DRG) neurons have been mostly investigated in culture of dissociated cells, and it is uncertain whether these cells maintain the electrophysiological properties of the intact DRG neurons. Few attempts have been made to record from DRG neurons in the intact ganglion using the patch clamp technique. In this study, rat DRGs were dissected and incubated for at least 1 hour at 37°C in collagenase (10 mg/ml). We used oblique epi-illumination to visualize DRG neurons and perform patch clamp recordings. All DRG neurons exhibited strong delayed rectifier potassium current and a high threshold for spike generation (−15 mV) that rendered the cells very weakly excitable, generating only one action potential upon strong current injection (>300 pA). It is therefore possible that cultured DRG neurons, commonly used in studies of pain processing, may be hyperexcitable because they acquired "neuropathic" properties due to the injury induced by their dissociation. Electrical stimulation of the attached root produced an antidromic spike in the soma that could be blocked by intracellular hyperpolarization or high frequency stimulation. Imaging intracellular calcium concentration with Oregon Green BAPTA-1 indicates that antidromic stimulation caused a long-lasting increase in intracellular calcium concentration mostly near the cell membrane. This study describes a simple approach to examine the electrophysiological and pharmacological properties and intracellular calcium signaling in DRG neurons in the intact ganglion where the effects of somatic spike invasion can be studied as well.
Keywords: dorsal root ganglion, patch clamp, calcium imaging, collagenase, epi-illumination, antidromic stimulation
1. Introduction
The electrophysiological and pharmacological properties of the somata of primary afferent neurons have often been examined, not only because they are simple and accessible model neurons, but also because of the presumed similarities between the properties of the cell bodies and those of their central and peripheral terminals. However, since there are virtually no synapses on DRG neurons, the role of the electrical excitability of DRG somata in afferent signaling remains a mystery. Nevertheless, DRG neurons can be specifically targeted by intravenous injection of therapeutic substances which because of their size are excluded by blood-nerve barriers from peripheral nerves, and blood-brain barriers from spinal cord and brain, but readily penetrate DRGs (Abram et al., 2006).
One fundamental question in sensory physiology is to elucidate the functional significance of the pseudounipolar morphology of the DRG neurons and whether they can modulate afferent sensory input via their T-shaped connection with the afferent axon. While Devor and Obermayer (1984) have suggested that somatic excitability may ensure reliable afferent spike propagation past the DRG, a recent computational modeling study has indicated that the reliability of propagation past the T-junction was essentially unaffected by sodium ions permeability in the soma and initial segment (Amir and Devor, 2003). Therefore additional experimental data in the intact DRG is warranted to explain whether the excitability of the somata can influence afferent sensory input (Ma and LaMotte, 2007). In order to address this question, it is important to develop a reliable method to record from DRG somata in the intact ganglion where DRGs are preserved together with their peripheral and central axons.
Sensory ganglion cells were among the first vertebrate neurons from which intracellular recordings were made. They have remained popular choices for basic neurophysiological investigations because of their relatively large size and simple geometry. Numerous studies recorded from DRG neurons in the intact ganglion using sharp intracellular electrodes in vivo (ex. Sato and Austin, 1961; Harper and Lawson, 1985a, b; Ritter and Mendell, 1992; Ma and LaMotte, 2007) and in vitro (ex. Desarmenien et al.,1981; Urban and Somjen, 1990; Waddell and Lawson, 1990;Song et al., 2003; Huang and Hanini, 2005; Ma and LaMotte, 2007). However, there have been few attempts to perform patch clamp recordings in the intact DRG (Yagi and Sumino, 1998; Zheng et al., 2007) or the nodose ganglion (Li and Schild, 2002). The advantage of the patch clamp technique over sharp electrode recordings is the ability to better voltage clamp the membrane potential in order to analyze the electrophysiological and pharmacological properties of the various voltage-dependent currents. Moreover, with the patch-clamp technique, it is easier to load neurons with calcium-sensitive dyes and monitor changes in intracellular calcium concentration in response to electrical stimulation or drug application.
The main obstacle to perform patch clamp recordings from DRG neurons is the presence of tight connective tissue between DRG neurons, and the wrapping of individual DRG neurons by satellite cells (Gartner and Hiatt, 2001). As the patch pipette must have unimpeded access to the cell membrane to form a high resistance seal (Hamill et al., 1981), the tissue overlying the somata must be treated by collagenase to facilitate access of the patch pipette to the membrane and establish giga-ohm seal before rupturing the membrane and going into whole-cell configuration. In this study, we used an improved method of enzyme treatment to better dissolve the connective tissue surrounding superficial DRG neurons of adult rats. The treatment is relatively simple and less time consuming compared to previous studies. It consists of incubation of the DRGs with only one enzyme, collagenase, at relatively high concentration (10 mg/ml), and does not require any surgical procedure such as slicing or peeling which may cause traumatic injury and alter the functional properties of the neurons (Li and Schild, 2002; Zheng et al., 2007). This method allowed us to perform patch clamp recordings and calcium imaging from DRG neurons in the intact ganglion with a satisfactory yield (up to 70% success, comparable to patching from CNS neurons). Our method is useful to study the properties of DRG neurons in normal and pathological conditions and to test whether previous pharmacological responses obtained in cultured DRG neurons may have resulted from an abnormal expression of various types of receptors during the dissociation and culture procedures.
2. Materials and Methods
2.1. DRG preparation
Sprague-Dawley rats (4–8 weeks old), of either sex, were decapitated in accordance with Institutional Animal Care and Use Committee and NIH guidelines. DRGs from segments L4-S2 with central and peripheral roots attached were removed and incubated for at least one hour in 1x F12 medium (Nutrient mixture Ham, GIBCO) containing collagenase (Worthington)10 mg/ml at 37°C then later at room temperature until used.
2.2. Electrophysiological recordings
Patch pipettes were pulled from borosilicate glass capillaries with an inner filament (1.5 mm outer diameter, 0.84 mm inner diameter, WPI, Sarasota, FL) on a pipette puller (P-97, Sutter Instrument Company, Novato, CA). For patch clamp recordings, electrodes were filled with a solution of the following composition, in mM: 124 K-gluconate, 10 phosphocreatine di tris salt, 10 HEPES, 0.2 EGTA, 4 Mg2ATP, and 0.3 Na2GTP (pH 7.3 adjusted with KOH). In calcium imaging experiments, 0.2 mM EGTA was replaced by 0.2 mM Oregon Green BAPTA-1 hexapotassium salt (Invitrogen Corp., Carlsbad, CA). The pipette resistance was 7 – 10 MΩ. In other experiments, 0.02% Lucifer Yellow was added to the intracellular solution for in situ labeling. The extracellular solution was made of artificial cerebrospinal fluid (ACSF), which had the following composition in mM: 124 NaCl, 26 NaHCO3, 4 KCl, 2 MgCl2, 2 CaCl2, 0.4 ascorbic acid, 2 sodium pyruvate, and 20 glucose. The osmolarity of the solutions was measured by the freezing-point method using an Advanced Model 3320 Micro-Osmometer (Advanced Instruments, Inc., Norwood, MA). The intracellular solution used in the patch pipette had lower osmolarity (266 mOsm) than the extracellular solution (307 mOsm) in order to improve seal formation (Hamill et al., 1981) and to prevent cell swelling which may occur during long time recordings.
A single DRG was placed in a recording chamber and continuously perfused at the rate of 1.5 ml/min with normal ACSF equilibrated with 95% O2 - 5% CO2. All recordings were performed at 30 °C. To better visualize the neurons, especially in thick DRGs, and monitor the adequacy of cell surface cleaning before gigaohm seal formation, we used a reflected oblique epi-illumination technique (see below). We used the “blow and seal” method (Sakmann and Stuart, 1995) for the patch clamp recordings. Positive pressure was continuously applied using 1 ml syringe connected via a tubing (Saint-Gobain Tygon R-3603 Clear Laboratory Tubing; SGPPL No.: AAC00001; wall thickness: 1/32 in.; I.D.: 1/32 in.; Fischer Scientific, Pittsburgh, PA) to the holder of the patch pipette which was advanced under visual control towards DRG neurons. In most cases, it was necessary to rupture the satellite cells around the target DRG neuron by the patch pipette with application of positive pressure (Yagi and Sumino, 1998). After rupturing the satellite cells that surround the DRG neurons, we monitored the pipette resistance in current clamp mode which was evaluated by the membrane potential change in response to repetitive positive current pulses (10 pA, 3 ms, 2 Hz). An increase in the voltage response indicated an increase in the resistance of the pipette as it touched the membrane of the selected neuron. Then the pressure was immediately released and slight negative pressure was applied by mouth suction to establish a high resistance seal (3–10 GΩ). Finally, the membrane was ruptured by further suction in order to record in whole-cell configuration.
Recordings were made using Muticlamp-700B amplifier (Molecular Devices, Sunnyvale, CA). Analog signals were low-pass filtered at 2 kHz, and digitized at 5 kHz using a Digidata- 1440A interface and pClamp10 software (Molecular Devices, Sunnyvale, CA). The junction potential was 9 – 10 mV, and all reported voltage measurements were uncorrected for these potentials. Only neurons with access resistance < 30 MΩ were included in this study. No series resistance compensation was performed. Electrical stimulation was performed via a Master-8cp stimulator (AMPI Inst. Ltd, Jerusalem, Israel) using 2 stainless steel wires (50 µm in diameter, A-M Systems, Inc., Everett, WA), insulated except at their tips. The tips of the electrodes were positioned superficially in the rootlet at a distance of 0.5 – 1 mm from the recorded cell in order to exclude the possibility of direct excitation of the T-junction or the initial segment of the recorded DRG neuron. Isolated, constant-current stimulus pulses of 200 – 500 µA and 200 µs duration were applied. Drugs and solutions were applied to the DRG by switching the perfusion with a three-way electronic valve system. High resolution photos were taken using 8 megapixel digital camera (Nikon, Coolpix 8700) connected to the microscope eyepiece via an adapter (MM99-5700, Martin Microscope Company, Easley, SC). Data were imported into Origin 7.0 (Microcal Software Inc., Northampton, MA) for plotting and further analysis. Data were expressed as mean ± SEM.
2.3. Optical recordings
An upright microscope (Olympus BX51WI, Tokyo, Japan) was placed on an X-Y translator (Somapatch-O-XY, Soma Scientific Instruments, Inc., Irvine, CA) installed on an anti-vibration table (30" × 48", TMC 63-543, Peabody, MA). The microscope was equipped with a video adapter for 2 camera ports (Olympus, UV-210), with the option to switch 50% of the light to each port. On one port, a high-resolution 3-CCD color camera (Hitachi HV-D30, Imaging Products Group, Little River, SC) was installed and the S-video output of this camera was connected to the S-video input of a 17" LCD flat panel television (Samsung SyncMaster 710MP, resolution: 1280 × 1024 pixels) to monitor the patch clamping procedure. The other port had a fast data acquisition camera (NeuroCCD-SMQ Imaging System, RedShirtImaging, Fairfield, CT) connected to the video adapter via a 0.38X optical coupler. Light emitted from a mercury lamp house passes through an epifluorescent illuminator containing a brightfield filter cube. A stimulus isolation unit (AMPI Inst. Ltd, Jerusalem, Israel) was used to deliver current pulses via a bipolar stimulation electrode. A fine motorized micromanipulator, 4 axes, (MX7500, Siskiyou Corp., Grants Pass, OR) controlled by remote devices and one manual coarse micromanipulator (MX110L, Siskiyou, and M-3333, Narishige, Japan) were installed around a custom-made plexiglass patch clamp recording chamber mounted above the microscope condenser. The recording chamber was fixed on an aluminum sheet (3" × 6" × 0.63", SMA-063-A, Small Parts, Inc., Miami Lakes, FL) and it was heated to 30 °C using 4 power resistors (MP930-10.0-1%, 10 Ohm, 30 W, connected in parallel, Newark, Chicago, IL) connected in parallel to a Bipolar Temperature Controller (TC2BIP, Cell MicroControls, Norfolk, VA) powered by a 12 V DC battery. The motorized micromanipulator was used to hold the headstage of the patch clamp amplifiers (Multiclamp 700B, Molecular Devices, Sunnyvale, CA). The coarse micromanipulator was used to place the stimulating electrodes that were connected to the stimulus isolation unit of a Master-8cp stimulator (AMPI Inst. Ltd). The NeuroCCD-SMQ camera has a resolution of 80 × 80 pixels, is back-illuminated and has a cooled CCD characterized by 2.7 kHz full-frame rate, 14 bit A-to-D resolution, well depth of 300,000 electrons/msec. This camera has a very low read noise (9 electrons rms at 1 kHz frame rate), 2 kHz full frame rate, and faster frame shift time (7 µsec). Analysis and display of data are made using the NeuroPlex program (RedShirtImaging, Fairfield, CT) written in IDL (Interactive Data Language, Research Systems, Boulder, CO). The fluorescent images of the stained cell were projected by a water-immersion objective (40X, 0.8 NA or 10X, 0.3 NA, Olympus) via an optical coupler (0.38X) onto the CCD chip. For calcium imaging we used a wide blue filter set (Chroma set# 11012v2, with an excitation filter of 420–490 nm, a dichroic mirror of 500 nm and a long-pass emitter of >515 nm. Slow changes in light intensity caused by bleaching of the dye (exponentially decaying baseline) were usually much slower than the rising phase of the spike-induced optical signals, and had no effect on the timing information used to draw conclusions in this work. In some instances, however, we corrected for the bleaching effect by subtracting an appropriate exponential function using the Neuroplex analysis software. To prevent photodynamic damage, the illumination time was kept as short as possible using a computer-controlled mechanical shutter (Vincent Associates, Rochester, NY, model# VS25S2ZM1R3, 25 mm aperture, ~3 ms to fully open or close) which was opened only for the recording period. To correct for spatial differences in illumination intensity and light path length, we used Neuroplex software to calculate the fractional change in fluorescence for each detector (80 × 80 pixels) using this formula:
where F is the fluorescence detected at time t, Fopen is the fluorescence detected at the beginning of the acquisition before stimulation and after opening of the shutter, and Fclosed is the intensity of fluorescence detected when the shutter was closed. The raw images were calculated using the resting light intensity for each detector (F = Fopen - Fclosed) which corrects for detector offset and background auto-fluorescence.
2.4. Neuronal visualization
One intact ganglion was transferred each time to a custom-made patch-clamp recording chamber and the ganglion was fixed between 2 nylon meshes. Neurons were visualized using an upright microscope. We first used transmitted oblique illumination which was achieved by slightly offsetting the condenser iris diaphragm which was partially closed. This type of illumination results in an increase in contrast and resolution, and the production of a shadowed, relief-like pseudo three-dimensional appearance in the image of the DRG somata (Filler and Peuker, 2000). However because the quality of transmitted light often deteriorated as a function of DRG thickness, we used reflected oblique epi-illumination, which gave superior quality imaging (Fig. 1,Fig 2A1,A2). The microscope was equipped with an epi-illuminator which consists of a mercury lamphouse coupled to a vertical illuminator that is positioned above the main frame of the microscope. Light emitted from the mercury lamp passes through a collector lens and then through the field and aperture diaphragms. Then, a cube mirror unit containing a brightfield filter set (Chroma, set # 33002; exciter KG1, dichroic mirror 50/50bs, emitter E420LPv2) was used to direct the light through the objective and onto the specimen. The objective in this case serves as the condenser, assuring perfect alignment and maximal illumination and collection efficiencies. The dichroic mirror, which reflects 50% and transmits 50% of the light, permitted visualization of the DRG using white light from top. The reflected light passes through a UV barrier filter into the microscope eyepieces or camera system. Light intensity was reduced to 25% or 5% using neutral density filters. Reflected oblique epi-illumination was achieved by reducing the aperture diaphragm to a minimum and by offsetting light by slight turning the illuminator revolving turret which contains the brightfield mirror unit cube (Fig. 1).
Figure 1. Schematic diagram of the light path for oblique epi-illumination.
An upright microscope (Olympus BX51WI) was equipped with a video adapter for 2 camera ports, with the option to switch 50% of the light to each port. On one port, a high-resolution 3-CCD color camera was installed. The other port had a fast data acquisition camera connected to the video adapter via a 0.38X optical coupler. Light emitted from a mercury lamp house passes through an epifluorescent illuminator containing a brightfield filter cube. Note that light entering the objective was off-centered by slightly turning the illuminator revolving turret in order to achieve oblique epi-illumination.
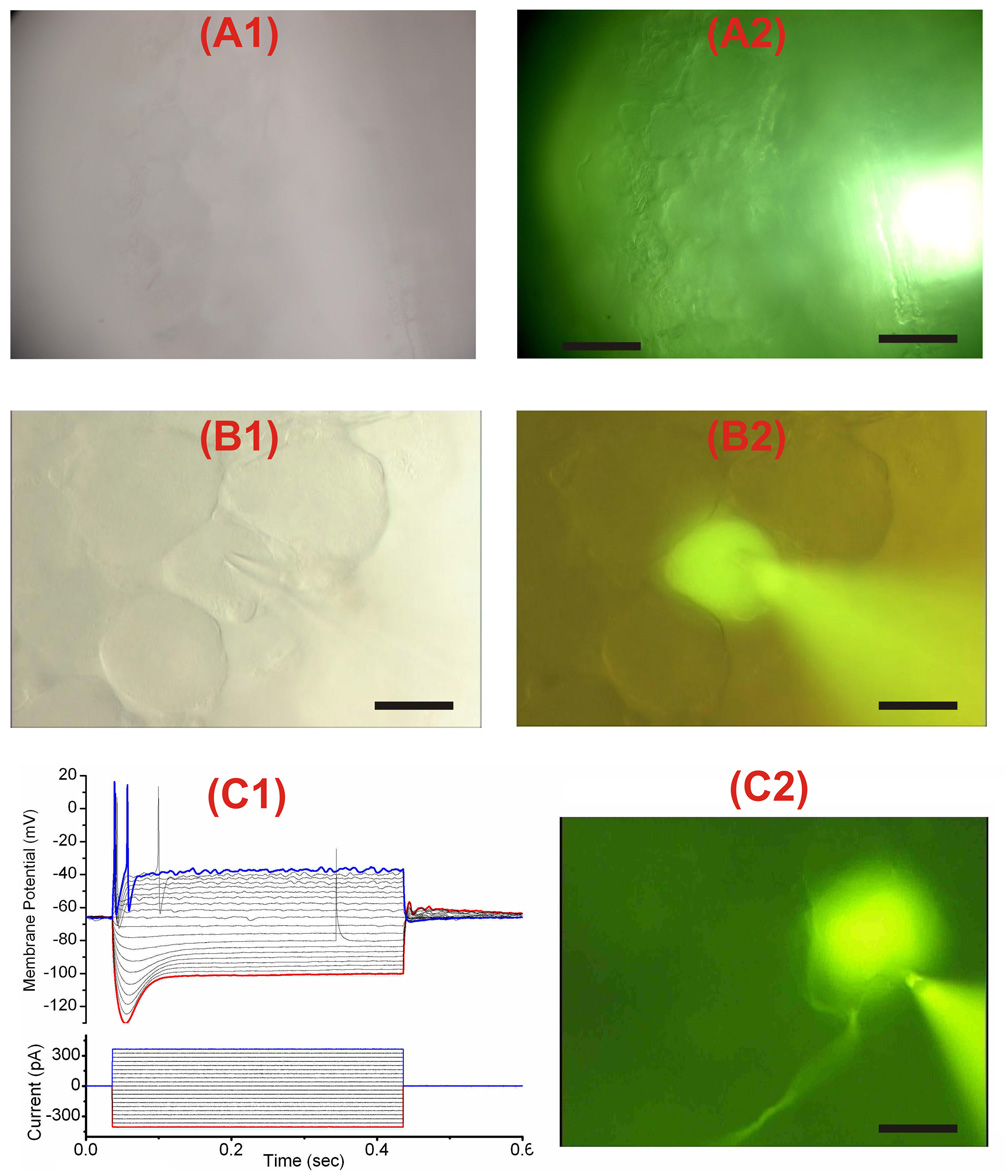
Figure 2. Visualization and recording from DRG neurons.
(A1–A2) Images of DRG neurons, visualized with 40X objective, were taken using transillumination (A1) and oblique epi-illumination (A2). This latter type of illumination enhanced the contrast and resolution of the image of neurons especially of thick DRGs where transmitted light was not effective for clear visualization. Epi-illumination was achieved by reducing the aperture diaphragm to a minimum and by slightly offsetting the light. It results in an increase in contrast and resolution, and the production of a shadowed, relief-like pseudo 3D appearance in the image of the DRG somata. Scale bar is 160 µm in (A1) and (A2). (B1) and (B2) DRG neuron visualized with trans-illumination which works adequately in this instance because the neuron was located superficially at the periphery of the ganglion. (C1) Membrane potential responses to 400 ms current injection of different amplitude from −400 pA to +400 pA in a DRG neuron filled with Lucifer Yellow shown in (C2). Scale bar is 20 µm in (B1), (B2) and (C2).
3. Results
3.1. Incubation of the DRG in collagenase
Dissolving the connective tissue surrounding DRG somata is a necessary step to clean the cell membrane and establish gigaohm seal with the patch pipette. This may be one of the main reasons why there were relatively few studies that attempted patch clamp recording from DRG neurons in the intact ganglion. In some of these previous studies, DRGs were first incubated in a solution that contained collagenase (1 mg/ml) at room temperature then the thick tissue around DRG somata was further loosened by focal application of collagenase (10 mg/ml) and thermolysine (100 U/ml) through a pipette with a tip diameter of 50 µm for 10–30 min at room temperature (Yawo, 1989; Yawo and Chuhma, 1994; Yagi and Sumino, 1998). The first studies that described a patch clamp recording method of DRG neurons that did not require further cleaning was published by LaMotte’s group (Zhang et al., 1997, Zhang et al., 1998) who surgically peeled of the perineurium and epineurium before incubating the DRGs in collagenase (1 mg/ml) for 30 min. However, in these studies, DRG neurons from adult animals could not be recorded and it was reported that “the probability of forming gigaseal was greatly reduced in cells from rats older than 15 days”. In contrast, Li and Schild (2002) were able to successfully patch neurons in the adult nodose ganglion but only after manually trimming the ganglion using a scalpel or cutting the top 50–100 µm layer of the agarose-embedded ganglion using a microtome. These procedures were followed by enzyme treatments (collagenase 1 mg/ml for 40 min and then Trypsin-3X for 15 min). In the latter method, it is possible that sectioning or dissecting the ganglion could cause mechanical trauma which might alter the physiological properties of the neurons.
We reasoned that adult DRG neurons might have relatively thicker layer of collagen compared to young rats and therefore a higher concentration of collagenase and a longer incubation period might be necessary to clean their membrane for successful patch clamping. Instead of focally applying the enzyme, the whole DRGs was incubated for at least one hour in 1x F12 medium (Nutrient mixture Ham, Invitrogen Corp., Carlsbad, CA) containing collagenase (type 3, Worthington Biochemical Corp., Lakewood, NJ) at the relatively high concentration (10 mg/ml) for at least 1 hour at 37°C. Treating the DRGs with just 1 mg/ml collagenase as in a recent study (Zheng et al., 2007) did not yield satisfactory results. However, it is possible that peeling off the perineurium and epineurium as in previous studies (Yawo, 1989; Yawo and Chuhma, 1994; Zhang et al., 1997, 1998; Yagi and Sumino, 1998; Zheng et al., 2007) might have helped the enzyme to better access and clean the membrane of DRG neurons. In all experiments (n = 8 rats), we found that treatment with collagenase (10 mg/ml) was enough to clear the thick tissue around superficial DRG neurons without the need to peel off the perineurium and epineurium which were often loosened by gentle shaking of the incubation medium. Nevertheless, we noticed that there was a higher probability of establishing a successful gigaohm seal in the first hour after transferring the DRG from the incubation medium to the recording chamber. Therefore, it is likely that the neurons progressively re-acquired connective tissue in the absence of collagenase although no neurite outgrowth was observed. However, this phenomenon was not investigated further. For this reason, after the initial incubation period at 37°C, we continued to incubate the DRGs in the presence of collagenase (10 mg/ml) at room temperature until used. In these conditions, the DRGs remain viable for patch clamping for up to 10 hours with no apparent change in the probability of successfully establishing tight seal between the membrane of DRG neurons and the patch pipette.
3.2. Electrophysiological properties of DRG neurons
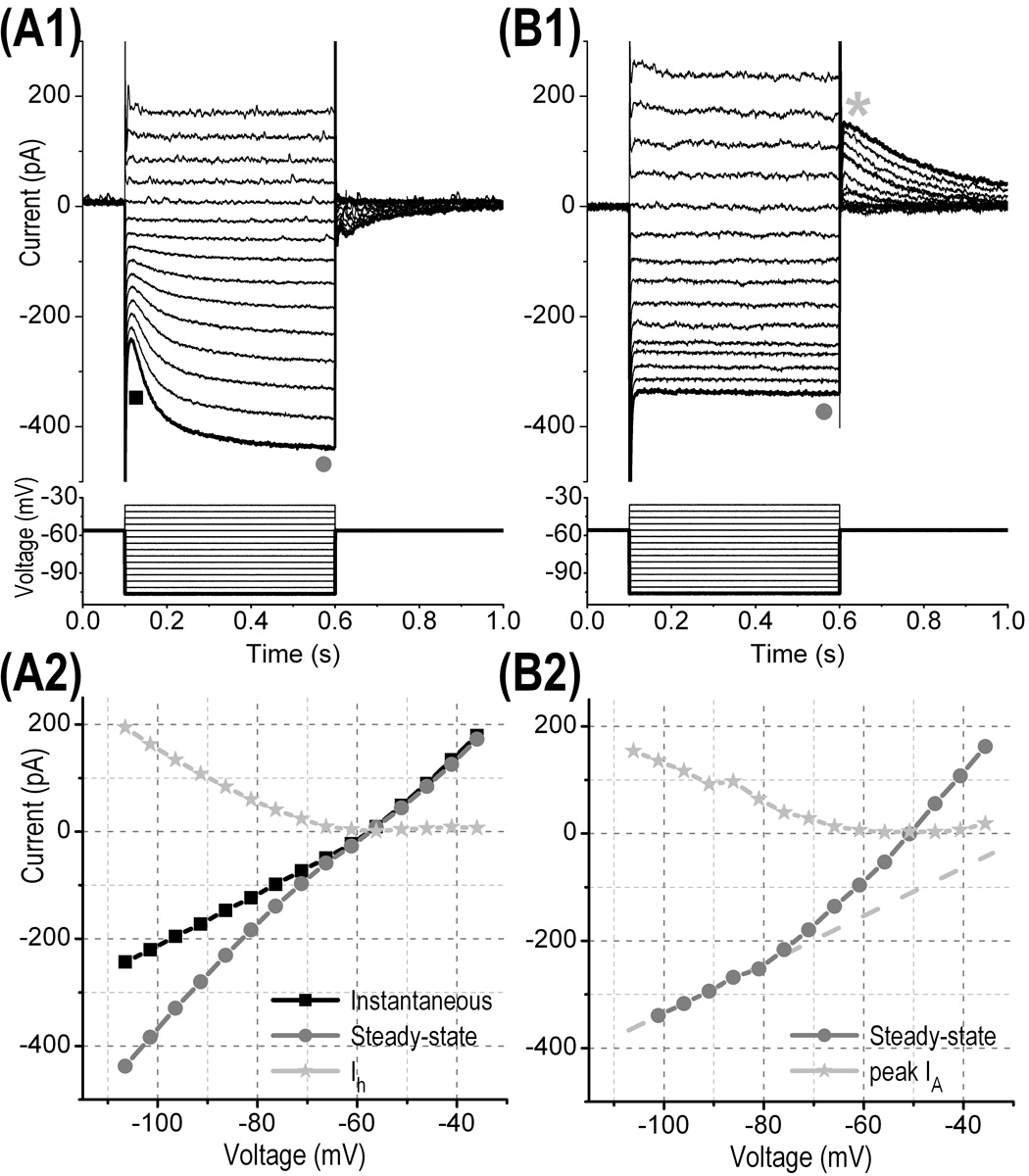
We performed patch clamp recordings from DRG neurons (n = 18, Fig. 2B1,B2,C1,C2) and from satellite cells (n = 2). DRG neurons have a resting potential of −54 ± 2 mV and an input resistance of 345 ± 40 MΩ; nine cells had hyperpolarization-activated delayed current (Ih) (Fig. 3A1,A2) and six had fast transient potassium current (IA) (Fig. 3B1,B2). All DRG neurons exhibited a strong delayed rectifier potassium current that activated at membrane potential positive to −70 mV (Fig. 3B1,B2). This latter current together with a high threshold for spike generation (−15 ± 1 mV) rendered the cells very weakly excitable, generating usually only one action potential upon strong current injection (>300 pA). The membrane properties of the DRG neurons recorded with patch clamp electrodes are therefore very similar to those found when recordings were performed with sharp intracellular electrodes (Villiere and McLachlan, 1996). Unlike DRG neurons, only satellite cells were found to be dye-coupled by Lucifer Yellow (not shown) confirming previous results obtained by injecting Lucifer Yellow via sharp intracellular electrodes (Huang et al., 2005; Huang and Hanani, 2005). These data indicate that DRG neurons recorded in whole-cell mode in the intact ganglion appeared to exhibit functional membrane currents and thus were not damaged by treatment with high concentration of collagenase. An exhaustive investigation of the different categories of DRG neurons (for ex., Fang et al., 2005) was beyond the scope of the current study because our main goal was to describe the advantage of our improved patch clamp methods.
Figure 3. Typical membrane currents of DRG neurons recorded in whole-cell voltage clamp mode in the intact ganglion.
(A1) and (A2) Current responses of 2 different DRG neurons to membrane voltage steps (bottom of each panel) of 500 ms duration from −50 mV below baseline holding potential to +30 mV above baseline holding potential (5 mV increment between consecutive sweeps). (B1) Current-voltage traces of the instantaneous current (squares) and the steady-state current (circles) measured respectively at the position of the square and the circle shown in (A1). The time-dependent inward current (Ih) trace (calculated by subtracting the 2 previous traces) indicates that Ih was produced with hyperpolarizing voltage steps <−65 mV. (B2) The current-voltage trace of the steady-state current measured at the position of the circle shown in B1 exhibits an outward rectification at membrane potential >−70 mV (as compared to the extrapolated dashed straight line) suggesting the activation of a potassium delayed rectifier. The peak amplitude of a potassium IA current (asterisk shown in (A1)) that developed after the cessation of hyperpolarizing voltage steps was also plotted as a function of the hyperpolarizing voltage step. Note that IA developed after hyperpolarizing voltage steps <−65 mV.
3.3. Antidromic stimulation
One intriguing question about DRGs is whether electrical excitability of the soma may be required to insure the reliable propagation of impulses past the DRG T-junction and into the spinal cord as it has been suggested by Devor (1999). It is possible that the membrane potential of DRG neurons may play a role in gating spike propagation along their axon. This hypothesis was tested indirectly by examining whether antidromic spike invasion of the soma is influenced by membrane potential hyperpolarization. However, it is important to note that in these experiments it is not possible to determine the exact location at which the spikes may fail to propagate along the T-junction and it is unknown whether the spikes continue to propagate along the central axon despite failing to invade the soma.
We took advantage of the fact that the soma can be better voltage clamped with a patch pipette than with a sharp intracellular electrode to test the hypothesis that somatic spike invasion can be prevented by clamping the membrane at different holding potentials (HP). DRG neurons (n = 4) were recorded in voltage clamp mode at HP = −55 mV and the root was stimulated with an intensity that is just supra-threshold for evoking an antidromic spike 100% of the time. When the membrane was clamped at more negative potentials (HP = −60 mV and HP = −65 mV), the antidromic spike occasionally failed to invade the soma with a probability that increased with further hyperpolarization (Fig. 4A,B). At HP of −70 mV or −75 mV, the antidromic spike failed to invade the soma 100% of the time.
Figure 4. Blockade of antidromic spikes by repetitive stimulation or by somatic hyperpolarization.

(A) Antidromic spikes were evoked by root stimulation and recorded in voltage clamp (3 superimposed traces were acquired at HP = −55 mV and 3 others were acquired at HP = −70 mV). Note that the action current was produced at consistent latency at HP = −55 mV and that it failed at HP = −70 mV. (B) A DRG neuron was recorded at HP of −55 mV and the root was stimulated every 5 sec (arrows) with an intensity that was just supra-threshold for evoking an antidromic spike 100% of the time. When the membrane was clamped at more negative potentials, the antidromic spike failed to invade the soma with a probability that increased with further hyperpolarization. At HP of −70 mV, the antidromic spike failed to invade the soma 100% of the time. Spikes were all-or-none events of more than 1 nA of amplitude (mostly inward currents) whereas stimulation artifacts induced relatively small upward deflections. (C) Repetitive antidromic stimulation at intervals of 100 ms or shorter often failed to evoke subsequent spikes. Arrows in all panels indicate the time of electrical stimulation of the root of the DRGs.
Next, we used repetitive antidromic stimulation to test the hypothesis that the afterhyperpolarization (AHP) current produced after generation of the first evoked spike could prevent subsequently evoked spikes from invading the soma (Fig. 4C). Indeed, stimulation at intervals of 100 ms or shorter often failed to evoke subsequent action currents suggesting the presence of a refractory period that prevents the invasion of the soma by high frequency repetitive spikes. However, it is possible that failure of evoking closely repetitive spikes occurred in the stimulated axon which during the spike AHP was refractory to additional spike initiation or propagation. These results indicate that the membrane potential as well as the firing frequency might influence spike propagation from the axon to the DRG soma.
3.4. Calcium imaging
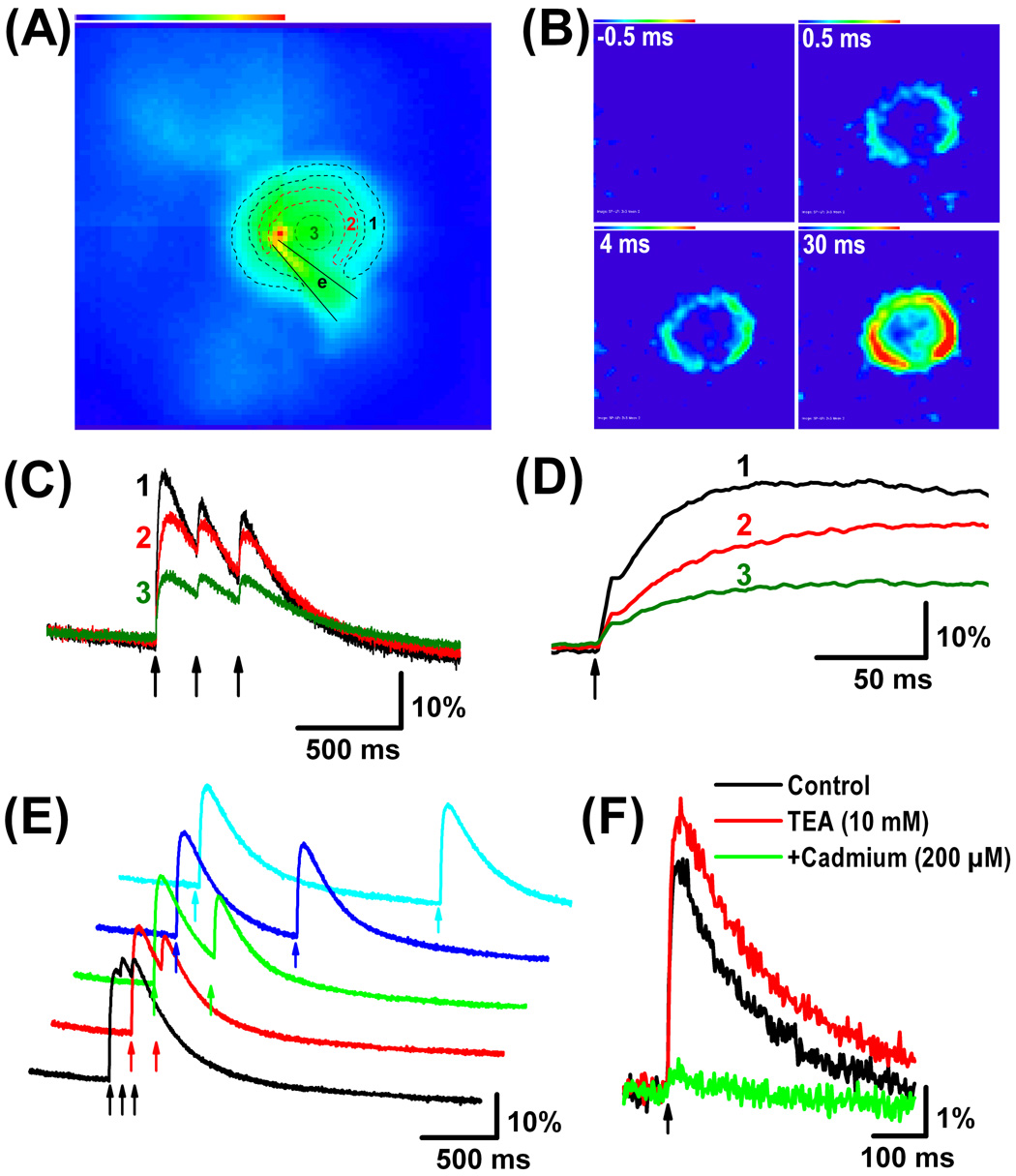
Another advantage of patch clamp technique is that calcium-sensitive dyes can be easily introduced into the cell via simple diffusion by including them in the pipette solution. We therefore recorded DRG neurons (n = 3) with a solution that contained the high affinity calcium-sensitive fluorescent dye Oregon Green BAPTA-1 (OGB-1, 200 µM). We examined changes in intracellular calcium concentration in response to antidromic stimulation by imaging at high temporal resolution (500–2000 frames/s). We normalized the images by calculating the relative change in fluorescence ΔF/F (see Methods). Taking the ratio of the images in this way corrects for spatial differences in cell thickness (light path length), indicator concentration, and accessible cytosolic volume. The focus of the cell was always chosen as the image with the maximum visible diameter, which in a perfectly spherical cell would represent a section through the center of the cell. Therefore, the light emitted from the central region of the cell originated mainly from the nucleus and to a lesser extent from the out-of-focus sub-membrane regions located below and above the nucleus. The resting light intensity frame showed that the intensity of fluorescence (i.e. calcium concentration) was higher in the center of the cell, where the nucleus is located, compared to the sub-membrane region (Fig. 5A). This could be because OGB-1 exhibits significant nuclear compartmentalization (Thomas et al., 2000). Antidromic spike invasion resulted in an increase of the dye fluorescence intensity of up to 30%. The highest relative change in the intensity of the fluorescent dye occurred near the cell membrane (Fig. 5A–D) while the region of the nucleus exhibited relatively lower fluorescence intensity change (about 30% less than the change in the sub-membrane region). A similar rim of calcium signal at the edge of the membrane was obtained in chromaffin cells in response to a depolarizing pulse (50 ms) in voltage clamp (Marengo and Monck, 2000). The latter findings were obtained by taking snapshots of calcium images using a slow camera. In this study, we used the natural stimulus which is the antidromic spike invasion as a mean to increase intracellular calcium concentration.
Figure 5. Changes in intracellular calcium in a DRG neuron in response to somatic spike invasion produced by antidromic stimulation.
(A) Raw image or resting light intensity frame (i.e. before stimulation, 80 × 80 pixels) of a DRG neuron recorded with a solution that contained the calcium-sensitive dye OGB-1 (200 µM). Color linear scale indicates values from 0 mV (blue) to 1900 mV (red). Different regions of interest are indicated by dashed lines with regions 1, 2 and 3 corresponding to sub-membrane, cytosolic and nuclear regions, respectively. Note that in delineating these regions of interest, we avoided the region (e) where the patch pipette came into contact with the cell. (B) The changes in intracellular calcium concentration were examined in response to antidromic stimulation by imaging at high temporal resolution (2000 frames/s). Note that antidromic stimulation resulted in an increase of the dye fluorescence intensity especially in the sub-membrane region. Individual frames (calculated by substracting the resting light intensity frame from the absolute frame) were acquired at different times (shown on top left of frames) relative to the onset of the stimulus and were divided by the resting light intensity frame to better visualize the regions of higher relative changes in fluorescence. Color linear scale indicates values from 4% (blue) to 30% (red). (C) The highest relative change in the intensity of the fluorescent dye in response to 3 repetitive stimuli (arrows, 200 ms interval) occurred in the sub-membrane region (black trace 1) followed by the region of cytosol (red trace 2) whereas the nuclear region (green trace 3) exhibited the lowest fluorescence intensity change. (D) Same traces as in (C) are displayed at an expanded time scale to better show the rising phase of the response to the first stimulus. Note that the fluorescence intensity reached its peak between 30 and 70 ms depending on the region examined. (E) The calcium concentration was not increased significantly further with repetitive stimulation (arrows) when the inter-stimulus interval was decreased progressively from 1500 ms (top trace) to 50 ms (lower trace). (F) The change in fluorescence in response to antidromic stimulation was increased by application of the potassium channel blocker, tetraethylammonium (TEA, 10 mM) and blocked by additional application of the calcium channel blocker, cadmium (200 µM).
Interestingly, at high frame acquisition rate (2000 frames/s) two phases could be distinguished. The first phase was very rapid with a time-to-peak of about 1 ms whereas the second phase had a time-to-peak much slower: about 30 ms in the sub-membrane region and about 70 ms in the cytosolic region (Fig. 5D). These data suggest that the first phase might correlate with somatic spike invasion during activation of voltage-dependent calcium channels, while the second phase occurred during the spike AHP and thus involved probably release of calcium from intracellular stores. The intracellular calcium concentration could not be increased significantly further with repetitive stimulation at intervals ranging from 50 ms to 1500 ms (Fig. 5E). Application of the potassium channel blocker, tetraethylammonium (TEA, 10 mM) increased the stimulation-evoked change in intracellular calcium concentration probably due to spike broadening which would result in a relatively larger calcium influx. In contrast, application of cadmium (200 µM) almost completely blocked the response (Fig. 5F) indicating that the stimulation-evoked increase in fluorescence of the OGB-1 dye resulted solely from an increase in intracellular calcium concentration produced by calcium influx through voltage-dependent calcium channels. This latter experiment ruled out possible imaging artifacts that could be produced by cell movement during stimulation.
4. Discussion
This study describes a practical approach to perform patch clamp recording and calcium-sensitive dye imaging from DRG neurons in the intact ganglion. Unlike in the dissociated culture, this approach is expected to yield results about the electrophysiological and pharmacological properties of these neurons that are more related to physiological conditions. Indeed, many recent studies have provided evidence that DRG neurons in culture exhibit higher excitability and responsiveness to many drugs as compared to neurons in the intact ganglion (Ma and LaMotte, 2005; Ma et al., 2006; Zheng et al., 2007). An additional advantage of recording DRG neurons in the intact ganglion is that it is possible to stimulate these cells antidromically and record somatic spike invasion or failure using both electrophysiological and imaging techniques.
Our results are in excellent agreement with a recent patch recording study on DRG neurons in the intact ganglion (Zheng et al., 2007), where it was shown that these neurons do not usually exhibit any spontaneous activity, and they were much less excitable than those that were dissociated and cultured. When corrected with the junction potential, the resting membrane potential (−64 mV) and the threshold for spike generation (−25 mV) are very close to those obtained with sharp recording of naïve neurons in the intact DRG (Ma and Lamotte, 2007). The observations that DRG cells were excitable (i.e. produce spikes upon current injection), had Ih, IA and delayed potassium current suggest that our incubation method with collagenase did not apparently affect the expression or the function of membrane channels. Therefore, it is unlikely that the function of membrane receptors might have been compromised by the same incubation procedure. However, additional tests are needed to exclude the possibility that the properties of membrane channels and receptors might have been altered by collagenase. It will be interesting in future studies to investigate whether in the intact ganglion, DRG neurons become more excitable and more responsive to various pharmacological agents only after becoming hypersensitive due to nerve or ganglion injury produced by compression, inflammation, axotomy, or dissociation. For example, an "inflammatory soup" of several mediators applied to a previously compressed DRG increases the firing rate and depolarization of DRG neurons and elicits ectopic discharge in some neurons, but these effects fail to occur in uncompressed ganglia from naïve animals (Song et al., 2003).
So far, it is still unknown under what condition the excitability of DRG neurons can affect peripheral sensory transmission to the spinal cord. During hypoxia, it was found that dorsal root-evoked impulse conduction might stop at the bifurcation of the axon and fail to invade the soma (Urban and Somjen, 1990). Results with paired-pulse stimulation indicated that impulses passing the axon bifurcation leave a long-lasting (> 25 ms) post-spike subnormal period (Urban and Somjen, 1990). A computational modeling study (Amir and Devor, 2003) suggested that electrical excitability of the DRG soma and initial segment is not necessary for through-conduction but it promotes spike invasion into the cell soma. However, a recent study (Ma and Lamotte, 2007) conducted in chronically compressed DRGs suggested that a continuous or long-duration bursting pattern of spontaneous activity could totally block the action potentials from peripheral receptors whereas a relatively low frequency of spontaneous activity might just add to the peripheral input. However, it remains unknown how the excitability of the soma can affect impulse transmission from peripheral receptors to the spinal cord. This issue can only be addressed if simultaneous recordings were made from the peripheral and central axons, the T-junction and the somata of a single DRG neuron. However, it is very hard to perform electrophysiological recording from individual axons. In future studies, it may be possible to overcome this problem by an imaging technique, in which a single neurons can be filled with a voltage-sensitive dye and activity at different sites can be monitored by imaging the soma and axons with a high speed camera (for review, see Stuart and Palmer, 2006; Zhou et al., 2007). In this study, we simply tested the feasibility of imaging DRG somata in the intact ganglion with a calcium-sensitive dye introduced via the patch pipette.
In previous studies, intracellular calcium rise was produced by ATP application (Chaban et al., 2003), mechanical stimulation (Chaban et al., 2004) or membrane depolarization (Hagenacker et al., 2005). However, in physiological conditions, rapid change in intracellular calcium concentration may occur only during somatic spike invasion, which activates voltage-dependent calcium channels. Therefore, in order to better mimic the physiological conditions, we monitored the changes in intracellular calcium concentration in response to antidromic stimulation of DRG neurons in the intact ganglion. Our optical recordings using a high speed imaging camera indicate that intracellular calcium concentration continued to increase even during the AHP following antidromic spike invasion of the soma. This long-lasting increase in calcium concentration may have resulted from calcium-induced-calcium release from intracellular stores (Ouyang et al., 2005; Jackson and Thayer, 2006). It is also possible that activation of Ih current during the AHP might have induced further calcium influx since Ih channels in DRG neurons exhibit significant calcium ion permeability (Yu et al., 2004). While it was noted previously (Thayer and Miller, 1990) that eliciting action potentials in cultured DRG neurons produced long-lasting increase in intracellular calcium, the kinetics of this increase could not be closely analyzed because the ratiometric Fura-2 imaging technique did not allow imaging at high temporal resolution.
In conclusion, the weak excitability of DRG neurons raises the question whether the overwhelming results obtained so far on cultured DRG neurons would be reproducible in the intact ganglion. Moreover, it remains to be determined whether the properties of cultured DRG neurons indeed resemble those of their peripheral and central terminals. This study describes an original approach that combines the patch clamp technique with fast calcium imaging of DRG neurons in the intact ganglion. This approach is expected to yield more reliable information regarding the effects of many pharmacological agents on the physiological properties and the intracellular calcium dynamics of intact DRG neurons.
Acknowledgements
This work was supported by National Institutes of Health grants: DC06356, DC07123, RR020146, NS40434, and DK077733.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abram SE, Yi J, Fuchs A, Hogan QH. Permeability of injured and intact peripheral nerves and dorsal root ganglia. Anesthesiology. 2006;105:146–153. doi: 10.1097/00000542-200607000-00024. [DOI] [PubMed] [Google Scholar]
- Amir R, Devor M. Electrical excitability of the soma of sensory neurons is required for spike invasion of the soma, but not for through-conduction. Biophys J. 2003;84:2181–2191. doi: 10.1016/S0006-3495(03)75024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban VV, Li J, Ennes HS, Nie J, Mayer EA, McRoberts JA. N-methyl-D-aspartate receptors enhance mechanical responses and voltage-dependent Ca2+ channels in rat dorsal root ganglia neurons through protein kinase C. Neuroscience. 2004;128:347–357. doi: 10.1016/j.neuroscience.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits ATP-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- Desarmenien M, Santangelo F, Linck G, Headley PM, Feltz P. Physiological study of amino acid uptake and receptor desensitization: the GABA system in dorsal root ganglia. Adv Biochem Psychopharmacol. 1981;29:309–319. [PubMed] [Google Scholar]
- Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain Suppl. 1999;82(6):S27–S35. doi: 10.1016/S0304-3959(99)00135-9. [DOI] [PubMed] [Google Scholar]
- Devor M, Obermayer M-L. Membrane differentiation in rat dorsal root ganglia and possible consequences for back pain. Neurosci Lett. 1984;51:341–346. doi: 10.1016/0304-3940(84)90400-2. [DOI] [PubMed] [Google Scholar]
- Fang X, McMullan S, Lawson SN, Djouhri L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J Physiol. 2005;565:927–943. doi: 10.1113/jphysiol.2005.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler TJ, Peuker ET. Reflection contrast microscopy (RCM): a forgotten technique? J Pathol. 2000;190:635–638. doi: 10.1002/(SICI)1096-9896(200004)190:5<635::AID-PATH571>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Gartner LP, Hiatt JL. Color textbook of histology. second edition. New York: W.B. Saunders Company; 2001. p. 141. [Google Scholar]
- Hagenacker T, Splettstoesser F, Greffrath W, Treede RD, Busselberg D. Capsaicin differentially modulates voltage-activated calcium channel currents in dorsal root ganglion neurones of rats. Brain Res. 2005;1062:74–85. doi: 10.1016/j.brainres.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol. 1985a:35931–35946. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol. 1985b;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TY, Cherkas PS, Rosenthal DW, Hanani M. Dye coupling among satellite glial cells in mammalian dorsal root ganglia. Brain Res. 2005;1036:42–49. doi: 10.1016/j.brainres.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Huang TY, Hanani M. Morphological and electrophysiological changes in mouse dorsal root ganglia after partial colonic obstruction. Am J Physiol Gastrointest Liver Physiol. 2005;289:G670–G678. doi: 10.1152/ajpgi.00028.2005. [DOI] [PubMed] [Google Scholar]
- Jackson JG, Thayer SA. Mitochondrial modulation of Ca2+ -induced Ca2+ -release in rat sensory neurons. J Neurophysiol. 2006;96:1093–1104. doi: 10.1152/jn.00283.2006. [DOI] [PubMed] [Google Scholar]
- Li BY, Schild JH. Patch clamp electrophysiology in nodose ganglia of adult rat. J Neurosci Methods. 2002;115:157–167. doi: 10.1016/s0165-0270(02)00010-9. [DOI] [PubMed] [Google Scholar]
- Ma C, Greenquist KW, Lamotte RH. Inflammatory mediators enhance the excitability of chronically compressed dorsal root ganglion neurons. J Neurophysiol. 2006;95:2098–2107. doi: 10.1152/jn.00748.2005. [DOI] [PubMed] [Google Scholar]
- Ma C, LaMotte RH. Enhanced excitability of dissociated primary sensory neurons after chronic compression of the dorsal root ganglion in the rat. Pain. 2005;113:106–112. doi: 10.1016/j.pain.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Ma C, LaMotte RH. Multiple sites for generation of ectopic spontaneous activity in neurons of the chronically compressed dorsal root ganglion. J Neurosci. 2007;27:14059–14068. doi: 10.1523/JNEUROSCI.3699-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengo FD, Monck JR. Development and dissipation of Ca(2+) gradients in adrenal chromaffin cells. Biophys J. 2000;79:1800–1820. doi: 10.1016/S0006-3495(00)76431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang K, Wu C, Cheng H. Ca(2+)-induced Ca(2+) release in sensory neurons: low gain amplification confers intrinsic stability. J Biol Chem. 2005;280:15898–15902. doi: 10.1074/jbc.C500026200. [DOI] [PubMed] [Google Scholar]
- Ritter AM, Mendell LM. Somal membrane properties of physiologically identified sensory neurons in the rat: effects of nerve growth factor. J Neurophysiol. 1992;68:2033–2041. doi: 10.1152/jn.1992.68.6.2033. [DOI] [PubMed] [Google Scholar]
- Sakmann B, Stuart G. Patch-pipette Recordings from the soma, dendrites, and axon of neurons in brain slices. In: Sakmann B, Neher E, editors. Single-channel recording. New York: Plenum Press; 1995. pp. 199–211. [Google Scholar]
- Sato M, Austin G. Intracellular potentials of mammalian dorsal root ganglion cells. J Neurophysiol. 1961;24:569–582. doi: 10.1152/jn.1961.24.6.569. [DOI] [PubMed] [Google Scholar]
- Song XJ, Vizcarra C, Xu DS, Rupert RL, Wong ZN. Hyperalgesia and neural excitability following injuries to central and peripheral branches of axons and somata of dorsal root ganglion neurons. J Neurophysiol. 2003;89:2185–2193. doi: 10.1152/jn.00802.2002. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Palmer LM. Imaging membrane potential in dendrites and axons of single neurons. Pflugers Arch. 2006;453:403–410. doi: 10.1007/s00424-006-0149-3. [DOI] [PubMed] [Google Scholar]
- Thayer SA, Miller RJ. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Tovey SC, Collins TJ, Bootman MD, Berridge MJ, Lipp P. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium. 2000;28:213–223. doi: 10.1054/ceca.2000.0152. [DOI] [PubMed] [Google Scholar]
- Urban L, Somjen GG. Reversible effects of hypoxia on neurons in mouse dorsal root ganglia in vitro. Brain Res. 1990;520:36–42. doi: 10.1016/0006-8993(90)91689-e. [DOI] [PubMed] [Google Scholar]
- Villiere V, McLachlan EM. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. J Neurophysiol. 1996;76:24–1941. doi: 10.1152/jn.1996.76.3.1924. [DOI] [PubMed] [Google Scholar]
- Waddell PJ, Lawson SN. Electrophysiological properties of subpopulations of rat dorsal root ganglion neurons in vitro. Neuroscience. 1990;36:811–822. doi: 10.1016/0306-4522(90)90024-x. [DOI] [PubMed] [Google Scholar]
- Yagi J, Sumino R. Inhibition of a hyperpolarization-activated current by clonidine in rat dorsal root ganglion neurons. J Neurophysiol. 1998;80:1094–1104. doi: 10.1152/jn.1998.80.3.1094. [DOI] [PubMed] [Google Scholar]
- Yawo H, Chuhma N. Omega-conotoxin-sensitive and -resistant transmitter release from the chick ciliary presynaptic terminal. J Physiol. 1994;477:437–448. doi: 10.1113/jphysiol.1994.sp020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H. Rectification of synaptic and acetylcholine currents in the mouse submandibular ganglion cells. J Physiol. 1989;417:307–322. doi: 10.1113/jphysiol.1989.sp017803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Duan KL, Shang CF, Yu HG, Zhou Z. Calcium influx through hyperpolarization-activated cation channels (I(h) channels) contributes to activity-evoked neuronal secretion. Proc Natl Acad Sci U S A. 2004;101:1051–1056. doi: 10.1073/pnas.0305167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Donnelly DF, Song XJ, Lamotte RH. Axotomy increases the excitability of dorsal root ganglion cells with unmyelinated axons. J Neurophysiol. 1997;78:2790–2794. doi: 10.1152/jn.1997.78.5.2790. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Donnelly DF, LaMotte RH. Patch clamp recording from the intact dorsal root ganglion. J Neurosci Methods. 1998;79:97–103. doi: 10.1016/s0165-0270(97)00164-7. [DOI] [PubMed] [Google Scholar]
- Zheng JH, Walters ET, Song XJ. Dissociation of dorsal root ganglion neurons induces hyperexcitability that is maintained by increased responsiveness to cAMP and cGMP. J Neurophysiol. 2007;97:15–25. doi: 10.1152/jn.00559.2006. [DOI] [PubMed] [Google Scholar]
- Zhou WL, Yan P, Wuskell JP, Loew LM, Antic SD. Intracellular long-wavelength voltagesensitive dyes for studying the dynamics of action potentials in axons and thin dendrites. J Neurosci Methods. 2007;164:225–239. doi: 10.1016/j.jneumeth.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]