Abstract
Epidemiological evidence indicates that prenatal nutritional deprivation may increase the risk of schizophrenia. The goal of these studies was to use an animal model to examine the effects of prenatal protein deprivation on behaviors and receptor binding with relevance to schizophrenia. We report that prenatally protein deprived (PD) female rats showed an increased stereotypic response to apomorphine and an increased locomotor response to amphetamine in adulthood. These differences were not observed during puberty. No changes in haloperidol-induced catalepsy or MK-801-induced locomotion were seen following PD. In addition, PD female rats showed increased3H-MK-801 binding in the striatum and hippocampus, but not in the cortex. PD female rats also showed increased 3H-haloperidol binding and decreased dopamine transporter binding in striatum. No statistically significant changes in behavior or receptor binding changes were found in PD males with the exception of increased 3H-MK-801 binding in cortex. This animal model may be useful to explore the mechanisms by which prenatal nutritional deficiency enhances risk for schizophrenia in humans and may also have implications for developmental processes leading to differential sensitivity to drugs of abuse.
Keywords: prenatal protein deprivation, dopamine, NMDA, stereotypy, locomotor activity, schizophrenia, drug abuse
Introduction
Considerable evidence suggests that schizophrenia may be a neurodevelopmental disorder in which early abnormities in brain development result in brain malfunction and symptomatology that emerges in early adulthood (Ursula et al 2006; St Clair et al 2005; Arnold 1999; Pilowsky et al 1993; Weinberger 1996). Prenatal nutritional deficiency has been hypothesized to be a risk factor for schizophrenia since the 1950s (Pasamanick et al 1956). A series of studies of the Dutch Hunger Winter showed that birth cohorts exposed to famine during early gestation had a two-fold increase in risk of schizophrenia in male and female offspring (Susser et al 1998 Hoek et al 1998; Susser et al 1996; Susser and Lin 1992). These findings have been replicated in a much larger cohort in China (St. Clair et al, 2005). Further, there was an increase in schizoid personality disorder when male offspring from the Dutch Hunger Winter were studied (Hoek et al 1996). A recent seroepidemiologic study from a large birth cohort in northern California revealed an association between elevated maternal homocysteine, a marker of folic acid deficiency, and schizophrenia (Brown et al, 2007). Other epidemiologic and laboratory studies of schizophrenia have found evidence of other potential pre- and perinatal etiologies, including prenatal exposure to infections including influenza, toxoplasmosis and genital/reproductive infection (Romero et al 2008; Smith et al 2007; Brown et al 2006) and obstetric complications (Hultman et al 1999; McNeil et al 2000a). In addition to epidemiological studies, neuroimaging studies have found that ventriculomegaly and decreased hippocampal volume in schizophrenia are related to obstetric complications (McNeil et al 2000b) and neuropathology studies have found cytoarchitectonic abnormalities in temporal lobe and prefrontal cortex in post-mortem brains of patients with schizophrenia indicative of prenatal damage and possibly altered neuronal migration (Akbarian et al 1996; Akbarian et al 1993).
Previous studies show parallels between long-term effects of prenatal protein deprivation in animals and clinical studies of schizophrenia. For instance, clinical studies as well as animal studies show altered dopamine neurochemistry (Almeida et al 1996a; Butler et al 1999; Chen et al 1995; Chen et al 1997; Toda and Abi-Dargham 2007), changes in morphology of the hippocampal formation, including dendritic abnormalities (Cintra et al 1997; Diaz-Cintra et al 1991; Diaz-Cintra et al 1994; Rosoklija et al 2000), deficits in learning and memory (Tonkiss and Galler 1990; Tonkiss et al 1990; Tonkiss et al 1991; Goldman-Rakic 1994; Barch and Smith 2008; Ranganath et al 2008) and changes in anxiety-like behavior and physiological parameters (Watkins et al 2008). The few studies to have examined the effects of prenatal protein deprivation on dopamine and glutamate mediated behaviors have shown increased sensitivity to dopamine agonists (Brioni et al 1986; Shultz et al 1999) and NMDA antagonists (Tonkiss et al 1998). However, these studies examined behaviors only in adulthood. In our previous study, we identified deficits in pre-pulse inhibition and accompanying changes in NMDA receptor binding following prenatal protein deprivation in female, but not male rats (Palmer et al 2004); these changes were present at postnatal day 56 (PDN 56; young adult) but not PND 35 (puberty).
Another example of a developmental model examining long-term effects of a perinatal lesion is the work of Lipska and colleagues (Al-Amin et al 2000; Lipska et al 1993; Lipska et al 1995; Lipska and Weinberger 1993; Tseng et al 2007). These investigators and others found that ventral hippocampal lesions in seven day old rat pups increased dopamine- and glutamate-mediated behaviors (Al-Amin et al 2000; Lipska et al 1993; Lipska and Weinberger 1993), produced deficits in learning and memory (Le Pen et al 2000) and produced alterations in prepulse inhibition of startle (Le Pen et al 2000; Lipska et al 1995) that emerged in post- rather than pre-pubertal rats. However, some cognitive impairment and deficits in social behavior occurred pre- as well as post-pubertally (Chambers et al 1996; Sams-Dodd et al 1997). Ventral hippocampal lesions have also been found to alter dopamine and glutamate receptor binding and to alter prefrontal dopaminergic and glutamatergic systems post- rather than pre-pubertally (Schroeder et al 1999; Tseng et al 2007).
In order to further evaluate the behavioral and neurochemical alterations caused by prenatal protein deprivation, we designed these studies to examine changes that may be relevant to schizophrenia. Dopamine-mediated stereotypy, locomotion, and catalepsy were examined before and after puberty because of dopamine involvement in schizophrenia and post-pubertal onset of schizophrenia (Abi-Dargham et al 2000; Carlsson and Lindqvist 1963; Laruelle and Abi-Dargham 1999; Stone et al 2007). Glutamate-mediated locomotion was also examined pre- and post-puberty because of glutamate involvement in schizophrenia (Javitt and Zukin 1991; Olney et al 1999; Stone et al 2007; Javitt et al 2008). Dopamine and NMDA receptor function were examined because of evidence of alterations in these neurotransmitter systems in schizophrenia (Stone et al 2007; Abi-Dargham 2004; Javitt and Zukin 1991; Laruelle and Abi-Dargham 1999; Meador-Woodruff and Healy 2000; Olney et al 1999; Coyle and Tsai 2004).
Results
Litter size, composition and Body Weight
Table 1 summarizes the number of offspring born, number surviving, and related parameters. Rats were tested before (PND 34-42) and after (PND 56-64) puberty. Rats in Group 1 received apomorphine, amphetamine, and haloperidol; while rats in Group 2 received apomorphine, amphetamine, and MK-801 (Table 2). Each rat in each group received all conditions at each age. PD males and females for Groups 1 and 2 were drawn from a total of ten litters while ND males and females for Groups 1 and 2 were drawn from twelve litters (Table 1). PD female rats had significantly decreased body weights compared to ND females at most time points (Table 3). For male rats, the difference between PD and ND rats was only significant at the earliest time points; nevertheless, the mean body weight of PD male rats showed a non-significant trend towards being lower then ND males at all time points (Table 3).
Table 1.
Offspring born, number surviving, and distribution for testing.
| Group | # Dams Mated |
# Gave Birth |
Mean # Offspring |
# Litters Cross- Fostered |
Culled To |
# Survived | # Tested for Behavior |
|---|---|---|---|---|---|---|---|
| Protein Deprived | |||||||
| Group 1 | 10 | 7 (70%) | 13.4 | 6 | 26M 22F |
19M 68% 13F 59% |
11M 7F 1-3 of each sex from 4 litters |
| Group 2 | 10 | 9 (90%) | 13.7 | 6 | 24M 24F |
19M 79% 20F 83% |
10M 10F 1-2 of each sex from 6 litters |
| PD Total | 20 | 16 (80%) | 13.6 | 12 | 50M 46F |
38M 76% 33F 72% |
21M 17F 1-3 of each sex from 10 litters |
| Non-Deprived | |||||||
| Group 1 | 20 | 13 (65%) | 14.2 | 6 | 24M 24F |
24M 100% 24F 100% |
15M 17F M: 3/litter from 5 litters F: 2-3/litter from 6 litters |
| Group 2 | 20 | 12 (60%) | 15.3 | 6 | 24M 24F |
24M 100% 24F 100% |
10M 10F 1-2 of each sex from 6 litters |
| ND Total | 40 | 25 (63%) | 14.7 | 12 | 48M 48F |
48M 100% 48F 100% |
25M 27F M:1-3/litter from 11 litters F:1-3/litter from 12 litters |
All litters were culled to 4 males and 4 females with the exception of one litter in PD Group 1 which was culled to 6 males and 2 females as it only included 2 females.
Table 2.
Measures Used
| Pre-Pubertal | Post-Pubertal | ||||||
|---|---|---|---|---|---|---|---|
| PND 34 | PND 37-39 | PND 40-42 | PND 56 | PND 59-61 | PND 62-64 | PND 80 | |
| Group 1 | apomorphine- stereotypy1 |
novel-2, and amphetamine1- locomotion |
haloperidol1 -catalepsy |
apomorphine1- stereotypy |
novel2-, saline2- , and amphetamine1- locomotion |
haloperidol1- catalepsy |
3H- Haloperidol4, 3H-MK-8015, 3H-GBR- 129356 binding |
| Group 2 | apomorphine- stereotypy1 |
novel2- and amphetamine1- locomotion |
novel2, and MK-8013- locomotion |
apomorphine1- stereotypy |
novel2- and amphetamine1- locomotion |
novel2 and MK- 8013- locomotion |
|
dopamine-mediated behaviors
behavioral alterations to stress
behaviors mediated by the NMDA
dopamine binding
binding to the NMDA glutamate receptor
binding to the dopamine transporter
Table 3.
Body weight of mice as a function of age and comparisons of male and female body weight between ND and PD groups.
| Female PD N=17 |
Female ND N=27 |
t | df | p | |
|---|---|---|---|---|---|
| PND 1 | 5.4±0.2 | 6.4±0.1 | 4.2 | 42 | <0.001 |
| PND 3 | 7.4±0.5 | 8.8±0.2 (n=25) |
2.9 | 40 | 0.014 |
| PND 34 | 132.9±3.8 (n=14) |
145.7±2.8 | 2.7 | 39 | 0.01 |
| PND 37-39 | 154.0±4.1 | 163.5±2.8 | 1.9 | 42 | 0.06 |
| PND 40-42 | 174.9±4.7 | 183.8±3.1 | 1.6 | 42 | 0.11 |
| PND 56 | 234.8±8.9 | 256.3±5.0 | 2.3 | 42 | 0.03 |
| PND 59-61 | 249.9±9.4 | 277.5±4.7 (n=26) | 2.9 | 41 | 0.006 |
| PND 63-64 | 261.6±9.8 | 288.7±5.4 (n=26) | 2.6 | 41 | 0.01 |
|
Male PD N=21 |
Male ND N=25 |
||||
| PND 1 | 5.9±0.2 | 6.8±0.1 | 4.3 | 44 | <0.001 |
| PND 3 | 8.1±0.3 | 9.3±0.2 (n=24) | 3.1 | 43 | 0.003 |
| PND 34 | 158.8±5.5 | 162.7±2.5 | 0.6 | 44 | 0.5 |
| PND 37-39 | 184.8±5.5 | 192.2±2.6 | 1.2 | 44 | 0.2 |
| PND 40-42 | 218.1±6.4 | 225.0±3.6 | 1.0 | 44 | 0.4 |
| PND 56 | 367.3±9.7 | 378.1±5.3 | 1.0 | 44 | 0.3 |
| PND 59-61 | 396.2±11.7 | 409.2±6.1 | 1.0 | 44 | 0.3 |
| PND 63-64 | 429.0±13.1 | 436.1±7.4 | 0.5 | 44 | 0.6 |
A range for the postnatal day (PND) is given for some days because rats were weighed and tested over that age range. Where some data are missing the number of subjects at that time point are indicated in parentheses.
Apomorphine-Induced Stereotypy
At PND 34, apomorphine-induced stereotypy of PD and ND female rats did not differ significantly (Figure 1A). However, at PND 56, PD female rats showed a significant increase in stereotypy compared to ND females (F[1,39]=7.20, p=0.011), with differences at almost every time point after injection (Figure 1B). PD males did not show significant differences from ND males on apomorphine-induced stereotypy at either PND 34 or 56 (Figure 1C and 1D). Body weight of PD females was not correlated to total stereotypy score for either test age; however, for ND females there was a significant correlation between body weight and total stereotypy score at PND 35 (r=-0.48, n=27, p=0.012), but no significant correlation at PND 56. Body weight of PD males was not significantly correlated with total stereotypy score for either test age; however, weight of ND males was significantly correlated with the total stereotypy score and PND 56 (r=-0.46, n=25, p=0.02), but not at PND 35.
Figure 1.
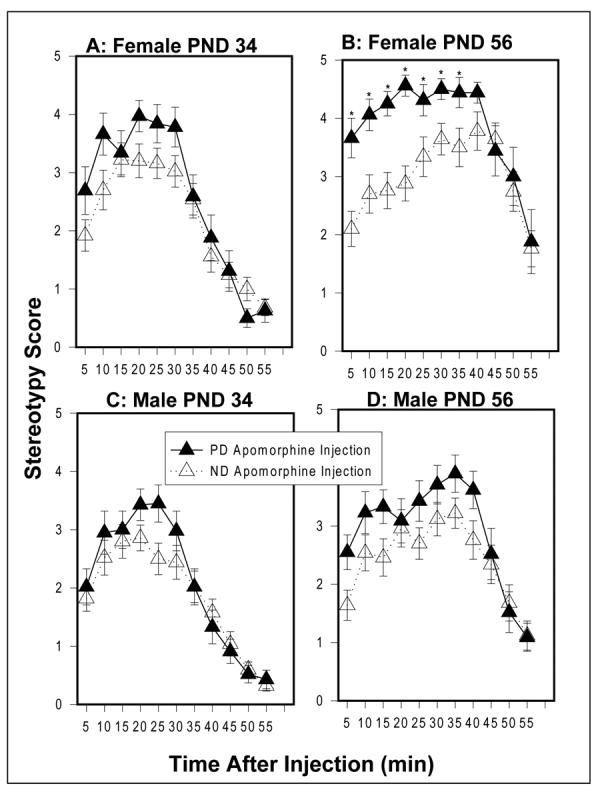
Apomorphine-induced (0.75 mg/kg) stereotypy in females at (A) PND 34 and at (B) PND 56 and in males at (C) PND 34 and at (D) PND 56. Values are mean ± sem. *p<0.05 (post hoc t tests). PD females (n=16); ND females (n=25); PD males (n=21); ND males (n=25).
Amphetamine-Induced Locomotion
At PND 37-39, the ambulatory locomotor activity of PD and ND female rats did not differ significantly following exposure to a novel environment or d-amphetamine injection (Figure 2A). However, at PND 59-61, while the PD and ND females did not differ significantly after exposure to a novel environment, PD female rats showed a significant increase in d-amphetamine-induced locomotion compared to ND rats (main effect for diet F[1,36]=5.47, p=0.025), with differences at several time points after injection (Figure 2B). PD males did not differ from ND males on ambulatory activity following exposure to a novel environment or d-amphetamine injection at either age (Figure 2C and 2D).
Figure 2.
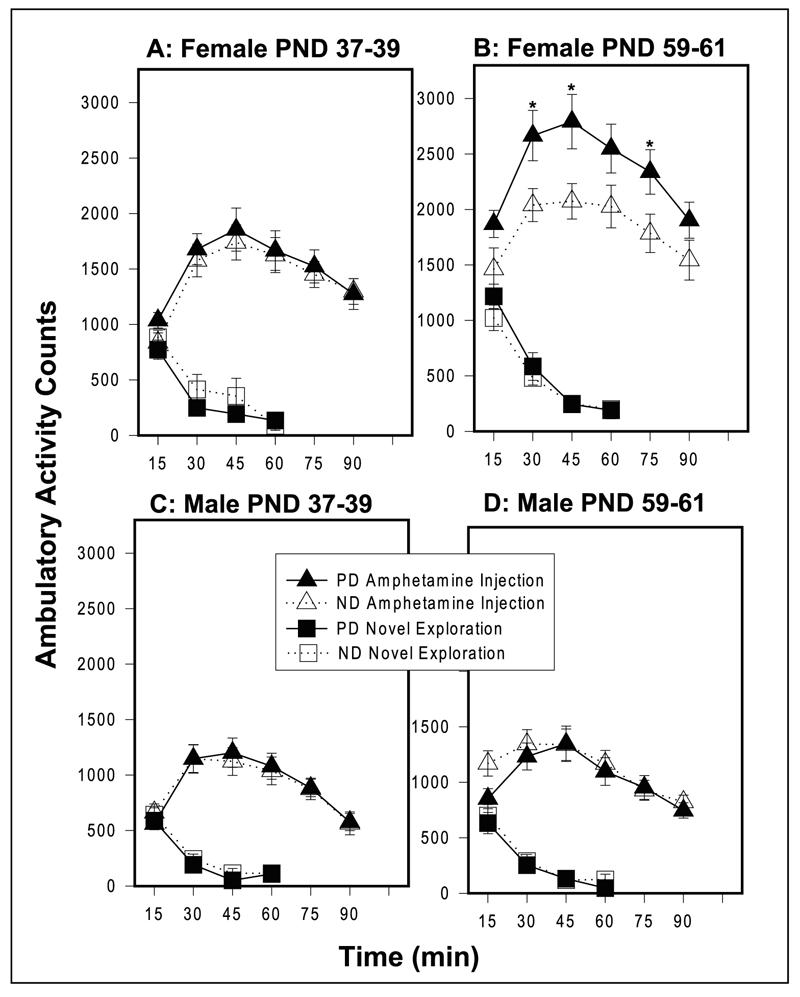
Novel and Amphetamine-induced (1.5 mg/kg) ambulatory activity measured in photocell cages in females at (A) PND 37-39 and at (B) PND 59-61 and in males at(C) PND 37-39 and at (D) PND 59-61. Values are mean ± sem. *p<0.05 (post hoc t-tests). PD females (n=16); ND females (n=22); PD males (n=18); ND males (n=22).
Body weight of PD female rats was not significantly correlated to total novel or amphetamine induced locomotion at either age. However, body weight of ND female rats was significantly related to total amphetamine locomotion at PND 59-61 (r=-0.46, n=21, p=0.04) but was not related to amphetamine locomotion at PND 37-39 or to novel locomotion at either age. Decrease in amphetamine locomotion thus does not appear to be related to decreased weight since there was no correlation between weight and amphetamine locomotion in the PD deprived group.
Body weight of PD male rats was significantly correlated with novel (r=-0.51, n=18, p=0.03) and amphetamine locomotion (r=-0.48, n=18, p=0.04) at PND 59-61 but not at PND 37-39. Body weight of ND male rats was significantly correlated with amphetamine locomotion (r=0.45, n=23, p=0.03) at PND 37-39 but no other significant correlations were seen with this group and novel or amphetamine locomotion at either age.
Haloperidol-Induced Catalepsy
PD females and males did not differ from their ND counterparts on time spent in a cataleptic position following injection of haloperidol at either age (Figure 3A-D).
Figure 3.
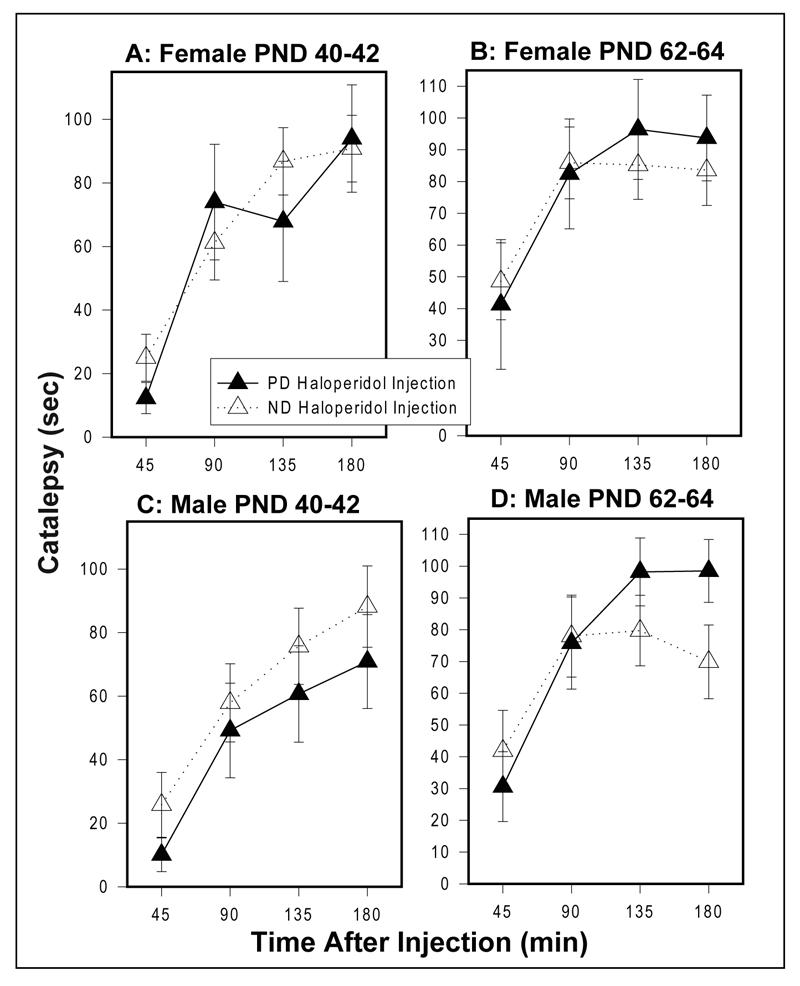
Haloperidol-induced (1 mg/kg) catalepsy in females at (A) PND 40-42 and at (B) PND 62-64 and in males at (C) PND 40-42 and at (D) PND 62 -64. Values are mean ± sem. PD females (n=7); ND females (n=17); PD males (n=11); ND males (n=15).
MK-801-Induced Locomotion
PD females and males did not differ from their ND counterparts on ambulatory activity following exposure to a novel environment or MK-801 injection at either age (Figures 4A-D).
Figure 4.
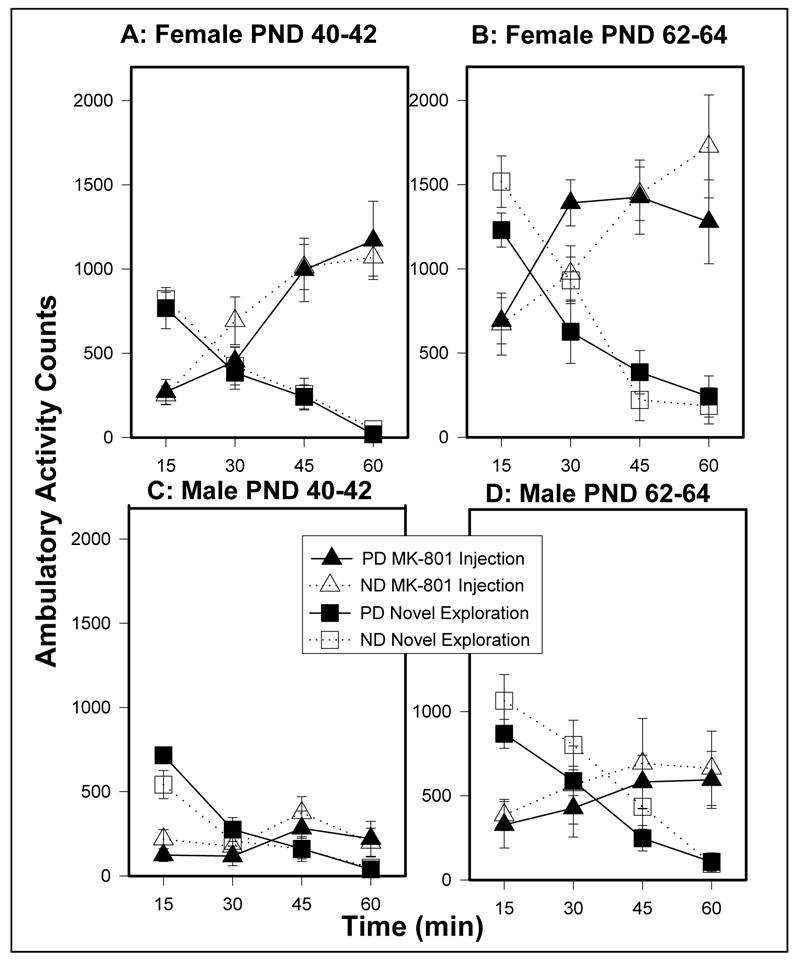
Novel and MK-801-induced (0.1 mg/kg) ambulatory activity measured in photocell cages in females at (A) PND 40-42 and at (B) PND 62-64 and males at (C) PND 40-42 and (D) PND 62-64. Values are mean ± sem. PD females (n=10); ND females (n=9); PD males (n=8); ND males (n=9).
Receptor Binding
Table 4 shows the results of receptor binding studies. PD females showed significant increases in 3H-MK-801 binding in striatum (35%;t(10)=5, p=.001), and hippocampus (67%; t(11)=12.04, p<0.001), but not cortex compared to ND rats. In addition, PD females showed a significant increase in striatal 3H-haloperidol binding (38%;t(11)=2.30, p=0.04) and a significant decrease in striatal 3H-GBR-12935 binding (33%;t(11)=5.88, p<0.001) compared to ND females. 3H-GBR-12935 binds to dopamine transporter sites. In contrast, PD males showed a significant increase in 3H-MK-801 binding in cortex (22%; t(11)=4.59, p=0.001) compared to ND rats, but showed no other changes in receptor binding.
Table 4.
Receptor and Transporter Binding Following Prenatal Protein Deprivation
| Females | Males | |||
|---|---|---|---|---|
| ND (n) | PD(n) | ND (n) | PD (n) | |
| 3H-MK-801 | ||||
| Striatum | 132.3 ± 7.1 (7) | 178.9 ± 4.6 (5)** | 169.5 ± 5.8 (7) | 157.0 ± 6.1 (5) |
| Hippocampus | 250.9 ± 7.9 (8) | 419.4 ± 12.5 (5)** | 49.9 ± 1.2 (8) | 49.1 ± 1.9 (5) |
| Cortex | 399.0 ± 10.7 (8) | 380.9 ± 8.2 (5) | 134.5 ± 4.0 (8) | 164.2 ± 5.1 (5)** |
| 3H-Haloperidol | ||||
| Striatum | 759.0 ± 72.6 (8) | 1048.7 ± 109.8 (5)* | 522.9 ± 25.8 (7) | 552.9 ± 17.1 (5) |
| 3H-GBR12935 | ||||
| Striatum | 2675.9 ± 110.5 (8) | 1793.6 ± 62.8 (5)** | 460.9 ± 111.4 (6) | 761.3 ± 53.2 (5) |
Results are expressed as pmol of 3H-radioligand bound/mg protein.
p<0.05
p<0.001
Discussion
The major findings of this study were that prenatally PD female rats showed an increased stereotypic response to apomorphine and an increased locomotor response to amphetamine which reached significance only in adulthood, and not during puberty. In addition, increased 3H-MK-801 binding was found in the striatum and hippocampus, but not cortex, of PD female rats and increased 3H-haloperidol binding and decreased dopamine transporter (i.e., 3H-GBR-12935) binding were found in striatum of PD female rats. PD females did not differ from ND females in MK-801-induced locomotion or haloperidol-induced catalepsy. No statistically significant behavioral or receptor binding changes were found in males with the exception of increased 3H-MK-801 binding in cortex.
These results extend those from our earlier study which showed that PD female rats developed deficits in prepulse inhibition at PND 56 that were not observed at PND 35. The present results add apomorphine induced stereotypy and amphetamine induced locomotor stimulation to the list of adult-onset changes observed following prenatal protein deprivation. Previous studies have examined the effects of early protein deprivation on dopamine mediated behaviors and have examined these behaviors only in adulthood (Brioni et al 1986; Leahy et al 1978; Shultz et al 1999). Brioni et al (1986) found a progressive increase in the locomotor stimulant response to repeated injections of amphetamine in PD male rats tested following pre and postnatal PD with a period of nutritional rehabilitation prior to testing. Similarly, Shultz et al. (1999) found increased sensitization to the stereotypic effects of repeated injections of cocaine in PD male and female rats tested following prenatal PD with nutritional rehabilitation until testing. In contrast, Leahy et al (1978) found no differences between PD and ND rats on amphetamine-mediated locomotion but a decrease in apomorphine-mediated stereotypy in PD male rats following a single injection of drug when protein deprivation began prenatally and was continued until the time of testing. As stated above, our effects were found only in females and we used only a single injection of each substance at each age. Based on the studies of Brioni et al (1986) and Shultz et al (1999), it may be that in males, behavioral sensitivity to dopaminergic agents requires repeated injections and/or a period of nutritional rehabilitation.
Unlike amphetamine and apomorphine, haloperidol did not alter behavior in PD rats. In their developmental model of schizophrenia, Lipska and Weinberger (1993) found decreased haloperidol catalepsy in adult rats receiving neonatal ventral hippocampal lesions, perhaps due to increased dopaminergic tone. Similarly, we hypothesized that we would observe a decrease in haloperidol catalepsy in prenatally PD rats in adulthood. The lack of a decrease in haloperidol catalepsy in our study may be related to our finding of increased 3H-haloperidol binding in striatum of PD female rats. This difference might counteract the expected effect by increasing haloperidol-induced catalepsy.
The increase in 3H-haloperidol binding in the striatum of PD females (Table 4) may be functionally related to the observed increases in dopamine mediated behaviors in adult PD females; however in our previous study of prenatal protein deprivation, we observed significant differences in prepulse inhibition in PD females at PND 56 (but not at PND 35), but no differences in haloperidol binding in the striatum in either male or females rats (Palmer et al 2004), suggesting that certain PD-induced behavioral differences can be dissociated from differential dopaminergic binding in the striatum. That study used Wistar Kyoto rats, where as the present study uses Sprague Dawley rats, so the discordant binding results could be due to genetic differences in the response to protein deprivation. Haloperidol is predominantly a D2 antagonist, but also binds to D3 and D4 receptor subtypes as well as to serotonin and noradrenergic receptors. Brioni et al. (1986) reported increased dopamine receptors in PD male rats following pre and postnatal PD with a period of nutritional rehabilitation prior to sacrifice in adulthood who had received repeated amphetamine injection in adulthood. While brain dopamine and metabolite levels have not been reported to be altered in striatum of PD rats (Chen et al 1997), our finding of increased 3H-haloperidol binding in striatum of PD female rats may be related to decreased tyrosine in this brain region following prenatal protein deprivation (Chen et al 1997).
Our findings of increased dopamine-mediated behaviors as well as our finding of increased striatal 3H-haloperidol binding in PD female rats are relevant to long-standing theories of dopamine dysfunction in schizophrenia (Stone et al 2007; Carlsson and Lindqvist 1963; Laruelle and Abi-Dargham 1999; Abi-Dargham et al 2004; Seeman and Lee 1975). Increases in striatal D2 receptor binding have been reported in post-mortem brains from schizophrenia patients (Joyce et al 1988; Seeman et al 1987) and imaging studies have provided further evidence that differences in dopamine dynamics play an important role in the pathophysiology of schizophrenia; nevertheless multiple neurotransmitter system are clearly important for schizophrenia (Abi-Dargham et al 2004; Toda and Abi-Dargham 2007; Stone et al 2007).
The increase in binding of the NMDA antagonist 3H-MK-801 in striatum and hippocampus of PD female rats in our study suggests that there may be decreased glutamatergic activity, leading to a compensatory increase in binding. In our previous study of PD rats we observed similar differences in 3H-MK-801 in the striatum but not in the hippocampus (Palmer et al 2004). This observation may be related to theories of NMDA receptor hypofunction in schizophrenia (Stone et al 2007; Javitt and Zukin 1991; Olney and Farber 1995; Coyle and Tsai 2004).
In light of our finding of increased 3H-MK-801 binding in PD female rats, it was surprising that we did not find increased MK-801-induced locomotion in these animals. Tonkiss and colleagues (Tonkiss et al 1998), using the same diet and protein deprivation regimen, found increased sensitivity to MK-801 in a differential reinforcement of low rates task in prenatally PD female, but not male, rats in adulthood. Very low doses of MK-801 were needed to disrupt learning in that study (e.g., 032 mg/kg) whereas higher doses are needed to increase locomotion (e.g., 0.1 mg/kg used in our study; (Fleischmann et al 1991; Honack and Loscher 1993; Lipska et al 1998). A ceiling effect may have occurred in our study because at the dose necessary to produce locomotion, ataxia is also seen. Ataxia could have prevented a further increase in locomotion in the PD rats.
Lipska and colleagues (Lipska et al 1993; Lipska et al 1995; Lipska and Weinberger 1993; Sams-Dodd et al 1997; Tseng et al 2007), and others (Chambers et al 1996; Schroeder et al 1999) have found increased dopamine- and glutamate-mediated locomotion and stereotypy, decreased dopamine-mediated catalepsy, disrupted prepulse inhibition of startle, cognitive and social deficits, as well as dopamine and glutamate binding and cell excitability changes in adult rats following neonatal ventral hippocampal lesions. Others have found similar results following amygdala lesions (Hanlon and Sutherland 2000), social isolation (Paulus et al 1998), and neonatal viral infection (Rothschild et al 1999). Our model extends this work, and, like viral infection (Rothschild et al 1999), utilizes a natural exposure that occurs in human populations and has been suggested to be a risk factor in schizophrenia (Susser et al 1996; Susser and Lin 1992; St. Clair et al 2005). Our finding of increased dopamine-mediated locomotion and stereotypy in adult PD female rats is consistent with the idea that prenatal nutritional insults can underlie much later behavioral changes. These behavioral changes were associated with biochemical changes, all of which involve neurotransmitter systems thought to be important in schizophrenia.
The decreased weight in male and female PD are broadly similar to that seen in other studies (Almeida et al 1996b; Almeida et al 1996c; Almeida et al 1996d; Shultz et al 1999; Tonkiss and Galler 1990; Tonkiss et al 1994), although there are some inconsistencies about whether these differences persist into adulthood, with some showing decreases (Almeida et al 1996b; Shultz et al 1999; Tonkiss and Galler 1990; Tonkiss et al 1991) and others not (Almeida et al 1996c; Tonkiss et al 1998; Tonkiss and Galler 1990; Tonkiss et al 1990; Tonkiss et al 1994). The differences in performance between PD and ND females on amphetamine-induced locomotion and apomorphine-induced stereotypy are probably not primarily due to weight differences. The correlations between body weight and behavior were relatively weak and inconsistent and would not have been significant had we applied a correction for the number of comparisons performed in this study. While the effect of PD on body weight was strongest in female rats (Table 3), we observed correlations between body weight and behavior in both male and female rats from both the PD and ND groups but never in female PD rats. Although PD females weighted significantly less than ND females, the magnitude of the difference was small.
Behavioral, receptor binding and body weight changes were most apparent in PD female rats replicating many of our earlier observations (Palmer et al 2004). One possibility is that these PD females have alterations in the estrus cycle or other aspects of reproductive function. Phase of the estrous cycle was not measured in the current study. The accompanying failure to account for an additional source of variability might obscure, rather than enhance the statistically significant results reported in this study; however, we cannot be sure that adult PD females had normal estrus cycles, and so it is possible that differences between female PD and ND mice are secondary to an abnormal or absent estrus cycle in PD females. This does not seem to be the case among humans exposed to famine; studies of the children born during and shortly after the Dutch Hunger Winter have not revealed any differences in age of first menstrual cycle, nor in a variety of other measures of reproductive function, with the exception of a small increase in perinatal deaths (Lumey and Stein 1997). Estradiol, a key hormone in the estrus cycle, is known to alter NMDA receptor binding and hippocampal CA1 dendritic spine density (Woolley 1998), DA transporter number (Attali et al 1997; Disshon et al 1998), D2 receptors (Bazzett and Becker 1994), as well as to affect amphetamine-induced behaviors (Becker 1990; Becker and Beer 1986). Schizophrenia occurs in both men and women with women showing a somewhat later onset. In our study, only female rats seemed to show an effect of PD. In contrast, an increased prevalence of schizophrenia was found in both male and female offspring of mothers exposed to famine during early pregnancy (Susser et al 1996). This difference is a limitation of our animal model.
There were several other limitations of this study. First, all animals in a group received all treatments (Table 1). It is possible that exposure to a drug prior to puberty could have altered the outcome after puberty. Similarly, the receptor binding studies were also conducted in animals that had previously received various drugs. Therefore, it is impossible to rule out the possibility that drug administration interacted with treatment to produce the observed binding results. Finally, in both this and our previous study (Palmer et al 2004), we have treated individuals as the unit of analysis whereas regarding each litter as the experimental unit might have been a more conservative approach. In the present study we took steps to address this final concern by sampling fewer individuals from a larger number of families. Future studies may further address some of these methodological issues.
In conclusion, this study shows that prenatal PD in females produces increased behavioral responsivity to the dopamine agonists amphetamine and apomorphine in adulthood, but not before puberty, and increased 3H-MK-801 and 3H-haloperidol binding post-puberty in PD versus ND female rats. This animal model of prenatal protein deprivation is generally consistent with epidemiological findings showing that prenatal nutritional deficiency is a risk factor for schizophrenia (St. Clair et al 2005; Susser et al 1996; Susser and Lin 1992) and may be a valuable tool to understand aberrant developmental processes that lead to brain pathology in adulthood such as occurs in schizophrenia. Enhanced amphetamine-induced locomotion and other anomalies related to dopaminergic and glutamatergic dysfunction have also been demonstrated in rodent models of maternal infection and immune activation (Shi et al, 2003; Zuckerman et al, 2003; Meyer et al, 2007). Our results are largely consistent with our previous study that examined behavioral and neuroanatomical correlates of prenatal protein deprivation (Palmer et al 2004). The current results may also be relevant in studying processes that lead to increased responsivity to stimulant drugs such as amphetamine.
Experimental Procedures
Nutritional Treatment, Mating, and Cross-Fostering
Sprague-Dawley rats were obtained viral and antibody free from Charles River Laboratories (Kingston, NY). Two diets were used, which had exactly the same composition as those utilized by Galler and colleagues (Tonkiss and Galler (1990); Teklad Laboratories, Madison, WI.). The pelleted diets were either of adequate protein content (25% casein) or of low protein content (6% casein) made isocaloric by addition of sucrose and cornstarch to the 6% diet.
Using the procedure of Galler and colleagues (Tonkiss and Galler 1990), one group of female rats (150-175 g) were randomly assigned to receive the adequate protein diet for five weeks prior to mating and throughout pregnancy. Another group of female rats (150-175 g) were randomly assigned to receive the low protein diet for five weeks prior to mating and during pregnancy. Male rats (300-325 g) received the adequate protein or the low protein, according to the females they were assigned to mate with, for one week prior to mating to acclimatize them to the diet. Each male was then placed with 2 to 3 females of the same diet group for a one week breeding period. Vaginal smears were taken each morning and stained to determine the presence of sperm. The day sperm was present, females were removed from breeding cages and housed singly in birthing cages. Birthing cages were inspected daily between 0800 and 1600 hrs, and the day pups were born was designated post-natal day 0 (PND 0).
All litters were cross-fostered whole within 36 hours of birth to mothers who had received the 25% diet. The experimental group was designated protein deprived (PD) and the control group non-deprived (ND). On the day they were fostered, litters were culled to 8 pups, making sex distribution as even as possible, and toe-clipped for identification. Rats were weaned at PND 25 and housed 2-3 to a cage with other rats of the same nutritional history and sex. There were two groups of rats used in this study, which will be referred to as Group 1 and Group 2. All animals in Group 1 were continued on the 25% diet during and after weaning whereas all animals in Group 2 were continued on standard rat chow during and after weaning, as standard rat chow has not been found to produce differential effects on weight from the 25% diet but is much less expensive (Dr. John Tonkiss, personal communication). Food was available ad libitum and animals were maintained on a 12-h light/12-h dark cycle. Offspring were weighed approximately every other day for the first two weeks and then again at the time of each behavioral test.
Stereotypy
Rats were removed from their home cages and placed in steel wire bottom, hanging cages for observation of stereotypy at 0900 hr on PND 34 and PND 56. After 2 hr of acclimatization, rats were injected with freshly prepared apomorphine (Sigma Chemical Co., 0.75 mg/kg, SC). Beginning five min following the injection, rats were observed for 15 seconds every 5 min during a 55 minute rating session. Stereotypy ratings were based on the procedures of Lipska and Weinberger (1993). Rats were scored from 0 to 5. Zero = no stereotypy, 1 = fixed sniffing, 2 = short occurrence, 3 = occasional bursts, 4 = intense intermittent, and 5 = continuous intense performance of oral stereotypies of licking, biting, or gnawing.
d-Amphetamine-Induced Locomotion
Ambulatory locomotor activity was examined in two conditions: after exposure to a novel environment and after d-amphetamine injection utilizing the procedure of Lipska et al (1993) on PND 37-39 and PND 59-61. Rats were placed in a clear plexiglass cage (43 × 43 × 33 cm) in a novel testing room and ambulatory photocell activity was recorded (Omnitech Digiscan Micro Animal Activity Monitor) every 15 min for 60 min. Then, after a d-amphetamine injection (Sigma Chemical Co., 1.5 mg/kg, IP) ambulatory activity was recorded every 15 min for 90 min. Because there were not enough photocell cages to test all rats, locomotor activity scores were not available for all animals. However, these animals received the appropriate injection.
Catalepsy
Rats in Group 1 were tested for haloperidol-induced catalepsy according to the procedure of Lipska and Weinberger (1993) on PND 40-42 and PND 62-64. A haloperidol solution of 20 mg/ml in 1% lactic acid was made and diluted with distilled H20 to yield a final concentration of 1.0 mg/ml (pH 5.2). After 3 hours of acclimatization to the test environment, rats received haloperidol injections (1 mg/kg, IP) at 1200 hr. Beginning 45 min after injection, both front paws were placed on a horizontal plastic bar that was elevated to 9.5 cm for PND 40-42 and 11 cm for PND 62-64. The time spent in a cataleptic position (i.e. both front paws on the bar) for up to 2 min was recorded. This was done four times at 45 min intervals.
MK-801-Induced Locomotion
Rats in Group 2 were tested for ambulatory activity in two consecutive conditions: after exposure to a novel environment and after an MK-801 injection on PND 40-42 and PND 62-64. Rats were placed in a clear plexiglass cage (43 × 43 × 33 cm) and ambulatory photocell activity was recorded (Omnitech Digiscan Micro Animal Activity Monitor) every 15 min for 60 min. Then, after an MK-801 injection (Sigma Chemical Co., 0.15 mg/kg, IP), ambulatory activity was recorded every 15 min for another 60 min.
Haloperidol, MK-801, and Dopamine Transporter Binding to Regional Rat Brain Membranes
At PND 85, after behavioral testing was completed, animals were sacrificed by decapitation, brains were rapidly removed and cerebral cortex, hippocampus, and striatum were carefully dissected. These brain regions were placed on dry ice and stored at -70°C until use. PD males and females were drawn from 3 litters. ND males and females were drawn from 5 litters. Binding was done in Group 1 animals. Membrane fractions were prepared as described below and assayed for radioligand binding to cortical, hippocampal, and striatal receptor binding and transporter sites. Three different radiolabeled ligands were used to quantify the binding of dopamine receptors (3H-haloperidol, NEN specific activity: 15.0 Ci/mmol), NMDA-sensitive glutamate receptors (3H-MK-801, NEN, specific activity: 23.9 Ci/mmol), and dopamine transporter sites (3H-GBR-12935, NEN, specific activity: 22.0 Ci/mmol).
On the day of assay, samples were homogenized in a 60× v/w Tris-EDTA buffer containing (mM): Tris HCl (38.5); Tris-Base (11.5), ethylenediaminetetraammonium (EDTA; 1.0) at pH 7.4. using a Brinkman polytron. The tissue was centrifuged at 30,000g for 20 minutes at +4°C and the resultant pellet was resuspended in 60× v/w radioligand-specific buffer. Tissue for 3H-GBR-12935 binding was resuspended in a Tris-NaCl buffer containing (mM): Tris HCl (38.5); Tris-Base (11.5); NaCl (120); and KCl (5.6) at pH 7.4, while tissue for 3H-MK-801 and 3H-haloperidol binding were resuspended in the above Tris-EDTA buffer. For 3H-GBR-12935, 3haloperidol, and 3MK-801 binding to rat brain membrane homogenates, 300 μl of membrane homogenate was incubated in the presence of either 600 μl Tris buffer (Tris-NaCl for3H-GBR-12935 and Tris-EDTA for 3H-haloperidol and 3H-MK-801; total binding) or a final concentration of 10 μM unlabeled nomifensine (RBI, Natick, MA), haloperidol (Sigma, St. Louis, MO), or MK-801 (RBI, Natick, MA) for competition to the dopamine uptake transporter, dopamine receptors, and NMDA receptors, respectively (non-specific binding) for five minutes at room temperature. 100 μl of 3H-MK-801, 3H-haloperidol, or 3H-GBR-12935 (final concentration for each ligand 3 nM) was added and allowed to equilibrate in the dark for 120 minutes at 22°C.
The tissue was harvested onto Titertek filtermats, coated with 0.1% polyethylimine (PEI) to reduce non-specific binding, using a Titertek cell harvester and the filters were placed in scintillation vials containing 8.0 ml Liquiscint (National Diagnostics). Samples were counted for 10 minutes in a Beckman liquid scintillation counter (efficiency: 50%). CPM data were converted to pmol of 3H-radioligand bound/mg protein.
The 3H-MK-801 binding assay was done without the addition of glycine. While some studies have utilized glycine in 3H-MK-801 binding assays to enhance binding or to saturate the glycine site to control for varying amounts of endogenous glycine which can affect 3H-MK-801 binding (Sakurai et al 1993), previous studies have successfully been carried out without this agent (Woolley et al 1997) and have found similar results with enhanced or unenhanced binding (McCoy and Richfield 1996).
Statistical Analysis
The data for apomorphine-induced stereotypy, baseline, saline, and amphetamine-induced locomotion, haloperidol-induced catalepsy, and baseline and MK-801-induced locomotion were analyzed by separate ANOVAs for each sex at each age with repeated measures for test time after injection. We analyzed the data in this manner, rather than starting with more complex multi-way ANOVAs due to our a priori interest in these comparisons based on our previous study of protein deprivation in rats (Palmer et al 2004). Significant main effects for diet or a significant diet × time interactions were followed up using t-tests to compare PD and ND groups. In some instances, behavioral data were missing for one particular test; in these cases all behavioral data for that animal were excluded from all analyses.
To examine the relationship between body weight and behavior, Pearson’s Product Moment Correlation Coefficient was used. Total locomotor or stereotypy scores for each animal were used.
Acknowledgements
We thank Drs. Ezra Susser, Myron Hofer and Jack Gorman for their invaluable input in the development of this project. We also thank Dr. H. Ken Kramer for advice on binding assays and Dr. Leonid Yatzkar, Ms. Debra Ciplet and Ms. Jennifer Schultz for technical help. This work was supported by NIMH SDAC K08MH01206, RSA MH00416 and DCRC MK50727.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7:S1–5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Rodenhiser J, Printz D, et al. From the Cover: Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney W, Jr., Jones EG. Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry. 1996;53:425–436. doi: 10.1001/archpsyc.1996.01830050061010. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Vinuela A, Kim JJ, Potkin SG, Bunney WE, Jr., Jones EG. Distorted distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase neurons in temporal lobe of schizophrenics implies anomalous cortical development. Archives of General Psychiatry. 1993;50:178–187. doi: 10.1001/archpsyc.1993.01820150016002. [DOI] [PubMed] [Google Scholar]
- Al-Amin HA, Weinberger DR, Lipska BK. Exaggerated MK-801-induced motor hyperactivity in rats with the neonatal lesion of the ventral hippocampus. Behavioral Pharmacology. 2000;11:269–278. doi: 10.1097/00008877-200006000-00010. [DOI] [PubMed] [Google Scholar]
- Almeida SS, Tonkiss J, Galler JR. Malnutrition and reactivity to drugs acting in the central nervous system. Neurosci Biobehav Rev. 1996a;20:389–402. doi: 10.1016/0149-7634(95)00054-2. [DOI] [PubMed] [Google Scholar]
- Almeida SS, Tonkiss J, Galler JR. Prenatal protein malnutrition affects avoidance but not escape behavior in the elevated T-maze test. Physiol Behav. 1996b;60:191–5. doi: 10.1016/0031-9384(95)02209-0. [DOI] [PubMed] [Google Scholar]
- Almeida SS, Tonkiss J, Galler JR. Prenatal protein malnutrition affects exploratory behavior of female rats in the elevated plus-maze test. Physiol Behav. 1996c;60:675–80. doi: 10.1016/s0031-9384(96)80047-3. [DOI] [PubMed] [Google Scholar]
- Almeida SS, Tonkiss J, Galler JR. Prenatal protein malnutrition affects the social interactions of juvenile rats. Physiol Behav. 1996d;60:197–201. doi: 10.1016/0031-9384(95)02236-8. [DOI] [PubMed] [Google Scholar]
- Arnold SE. Neurodevelopmental abnormalities in schizophrenia: insights from neuropathology. Dev Psychopathol. 1999;11:439–56. doi: 10.1017/s095457949900214x. [DOI] [PubMed] [Google Scholar]
- Attali G, Weizman A, Gil-Ad I, Rehavi M. Opposite modulatory effects of ovarian hormones on rat brain dopamine and serotonin transporters. Brain Res. 1997;756:153–9. doi: 10.1016/s0006-8993(97)00136-4. [DOI] [PubMed] [Google Scholar]
- Barch DM, Smith E. The Cognitive Neuroscience of Working Memory: Relevance to CNTRICS and Schizophrenia. Biol Psychiatry. 2008;64:11–17. doi: 10.1016/j.biopsych.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–72. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990;118:169–71. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity: behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res. 1986;19:27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Keller EA, Levin LE, Cordoba N, Orsingher OA. Reactivity to amphetamine in perinatally undernourished rats: behavioral and neurochemical correlates. Pharmacol Biochem Behav. 1986;24:449–54. doi: 10.1016/0091-3057(86)90540-x. [DOI] [PubMed] [Google Scholar]
- Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophrenia Bulletin. 2006;32:200–2. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Bottigleri T, Schaefer CA, Quesenberry CP, Liu L, Bresnahan M, Susser ES. Elevated prenatal homocysteine levels as a risk factor for schizophrenia. Arch Gen Psychiatry. 2007;64:31–9. doi: 10.1001/archpsyc.64.1.31. [DOI] [PubMed] [Google Scholar]
- Butler PD, Printz D, Klugewicz D, Brown AS, Susser ES. Plausibility of early nutritional deficiency as a risk factor for schizophrenia. In: Susser ES, Brown AS, Gorman JM, editors. Prenatal Exposures in Schizophrenia. American Psychiatric Press; Washington, D.C.: 1999. pp. 163–193. [Google Scholar]
- Carlsson A, Lindqvist M. Effect of chlorpromazine or haloperidol on formation of 3-methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol. 1963;20:140–4. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15:587–94. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- Chen JC, Turiak G, Galler J, Volicer L. Effect of prenatal malnutrition on release of monoamines from hippocampal slices. Life Sci. 1995;57:1467–75. doi: 10.1016/0024-3205(95)02119-4. [DOI] [PubMed] [Google Scholar]
- Chen JC, Turiak G, Galler J, Volicer L. Postnatal changes of brain monoamine levels in prenatally malnourished and control rats. Int J Dev Neurosci. 1997;15:257–63. doi: 10.1016/s0736-5748(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Cintra L, Aguilar A, Granados L, et al. Effects of prenatal protein malnutrition on hippocampal CA1 pyramidal cells in rats of four age groups. Hippocampus. 1997;7:192–203. doi: 10.1002/(SICI)1098-1063(1997)7:2<192::AID-HIPO6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Tsai G. NMDA receptor function, neuroplasticity, and the pathophysiology of schizophrenia. Int Rev Neurobiol. 2004;59:491–515. doi: 10.1016/S0074-7742(04)59019-0. [DOI] [PubMed] [Google Scholar]
- Diaz-Cintra S, Cintra L, Galvan A, Aguilar A, Kemper T, Morgane PJ. Effects of prenatal protein deprivation on postnatal development of granule cells in the fascia dentata. J Comp Neurol. 1991;310:356–64. doi: 10.1002/cne.903100306. [DOI] [PubMed] [Google Scholar]
- Diaz-Cintra S, Garcia-Ruiz M, Corkidi G, Cintra L. Effects of prenatal malnutrition and postnatal nutritional rehabilitation on CA3 hippocampal pyramidal cells in rats of four ages. Brain Res. 1994;662:117–26. doi: 10.1016/0006-8993(94)90803-6. [DOI] [PubMed] [Google Scholar]
- Disshon KA, Boja JW, Dluzen DE. Inhibition of striatal dopamine transporter activity by 17beta-estradiol. Eur J Pharmacol. 1998;345:207–11. doi: 10.1016/s0014-2999(98)00008-9. [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Vincent PA, Etgen AM. Effects of non-competitive NMDA receptor antagonists on reproductive and motor behaviors in female rats. Brain Res. 1991;568:138–46. doi: 10.1016/0006-8993(91)91389-i. [DOI] [PubMed] [Google Scholar]
- Hanlon FM, Sutherland RJ. Changes in adult brain and behavior caused by neonatal limbic damage: implications for the etiology of schizophrenia. Behav Brain Res. 2000;107:71–83. doi: 10.1016/s0166-4328(99)00114-x. [DOI] [PubMed] [Google Scholar]
- Hoek HW, Susser E, Buck KA, Lumey LH, Lin SP, Gorman JM. Schizoid personality disorder after prenatal exposure to famine. Am J Psychiatry. 1996;153:1637–1639. doi: 10.1176/ajp.153.12.1637. [DOI] [PubMed] [Google Scholar]
- Hoek HW, Brown AS, Susser E. The Dutch Famine and schizophrenia spectrum disorders. Soc Psychiatry Psychiatr Epidemiol. 1998;33:373–379. doi: 10.1007/s001270050068. [DOI] [PubMed] [Google Scholar]
- Honack D, Loscher W. Sex differences in NMDA receptor mediated responses in rats. Brain Res. 1993;620:167–70. doi: 10.1016/0006-8993(93)90287-w. [DOI] [PubMed] [Google Scholar]
- Hultman CM, Sparen P, Takei N, Murray RM, Cnattingius S. Prenatal and perinatal risk factors for schizophrenia, affective psychosis, and reactive psychosis of early onset: case-control study. BMJ. 1999;318:421–6. doi: 10.1136/bmj.318.7181.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia [see comments] Am J Psychiatry. 1991;148:1301–8. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discovery. 2008;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JN, Lexow N, Bird E, Winokur A. Organization of dopamine D1 and D2 receptors in human striatum: receptor autoradiographic studies in Huntington’s disease and schizophrenia. Synapse. 1988;2:546–57. doi: 10.1002/syn.890020511. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–71. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Grottick AJ, Higgins GA, Martin JR, Jenck F, Moreau J-L. Spatial and associative learning deficits induced by neonatal excitotoxic hippocampal damage in rats: further evaluation of an animal model of schizophrenia. Behav Pharmacol. 2000;11:257–268. doi: 10.1097/00008877-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Leahy JP, Stern WC, Resnick O, Morgane PJ. A neuropharmacological analysis of central nervous system catecholamine systems in development protein malnutrition. Dev Psychobiol. 1978;11:361–70. doi: 10.1002/dev.420110409. [DOI] [PubMed] [Google Scholar]
- Lipska BK, al-Amin HA, Weinberger DR. Excitotoxic lesions of the rat medial prefrontal cortex. Effects on abnormal behaviors associated with neonatal hippocampal damage. Neuropsychopharmacology. 1998;19:451–64. doi: 10.1016/S0893-133X(98)00045-1. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: A potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR. Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology. 1995;122:35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. Delayed effects of neonatal hippocampal damage on haloperidol-induced catalepsy and apomorphine-induced stereotypic behaviors in the rat. Dev Brain Res. 1993;75:213–222. doi: 10.1016/0165-3806(93)90026-7. [DOI] [PubMed] [Google Scholar]
- Lumey, Stein In Utero Exposure to Famine and Subsequent Fertility: The Dutch Famine Birth Cohort Study. Am J Public Health. 1997;87:1962–6. doi: 10.2105/ajph.87.12.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy L, Richfield EK. Chronic antipsychotic treatment alters glycine-stimulated NMDA receptor binding in rat brain. Neurosci Lett. 1996;213:137–41. doi: 10.1016/0304-3940(96)12834-2. [DOI] [PubMed] [Google Scholar]
- McGrath J, Castle D. Does influenza cause schizophrenia? A five year review. Aust N Z J Psychiatry. 1995;29:23–31. doi: 10.3109/00048679509075888. [DOI] [PubMed] [Google Scholar]
- McNeil TF, Cantor-Graae E, Ismail B. Obstetric complications and congenital malformation in schizophrenia. Brain Res Brain Res Rev. 2000a;31:166–78. doi: 10.1016/s0165-0173(99)00034-x. [DOI] [PubMed] [Google Scholar]
- McNeil TF, Cantor-Graae E, Weinberger DR. Relationship of obstetric complications and differences in size of brain structures in monozygotic twin pairs discordant for schizophrenia. Am J Psychiatry. 2000b;157:203–12. doi: 10.1176/appi.ajp.157.2.203. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Healy DJ. Glutamate receptor expression in schizophrenic brain. Brain Res Brain Res Rev. 2000;31:288–94. doi: 10.1016/s0165-0173(99)00044-2. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology. 2007;33:441–6. doi: 10.1038/sj.npp.1301413. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia [see comments] Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–33. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Printz DJ, Butler PD, Dulawa SC, Printz MP. Prenatal protein deprivation in rats induces changes in prepulse inhibition and NMDA receptor binding. Brain Res. 2004;996:193–201. doi: 10.1016/j.brainres.2003.09.077. [DOI] [PubMed] [Google Scholar]
- Pasamanick B, Rogers ME, Lilienfeld AM. Pregnancy experience and the development of behavior disorder in children. Am J Psychiatry. 1956;112:613–618. doi: 10.1176/ajp.112.8.613. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Bakshi VP, Geyer MA. Isolation rearing affects sequential organization of motor behavior in post-pubertal but not pre-pubertal Lister and Sprague-Dawley rats. Behav Brain Res. 1998;94:271–80. doi: 10.1016/s0166-4328(97)00158-7. [DOI] [PubMed] [Google Scholar]
- Pilowsky LS, Kerwin RW, Murry RM. Schizophrenia: a neurodevelopmental perspective. Neuropsychopharmacology. 1993;9:83–91. doi: 10.1038/npp.1993.46. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Minzenberg MJ, Ragland JD. The Cognitive Neuroscience of Memory Function and Dysfunction in Schizophrenia. Biol Psychiatry. 2008;64:18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoklija G, Toomayan G, Ellis SP, et al. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–56. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- Rothschild DM, O’Grady M, Wecker L. Neonatal cytomegalovirus exposure decreases prepulse inhibition in adult rats: implications for schizophrenia. J Neurosci Res. 1999;57:429–34. [PubMed] [Google Scholar]
- Romero C, Guaza B, Castellano, Borrell J.Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia Mol Psych 2008. doi:10.1038/mp.2008.44 [DOI] [PubMed] [Google Scholar]
- Sakurai SY, Penney JB, Young AB. Regionally distinct N-methyl-D-aspartate receptors distinguished by quantitative autoradiography of [3H]MK-801 binding in rat brain. J Neurochem. 1993;60:1344–53. doi: 10.1111/j.1471-4159.1993.tb03295.x. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F, Lipska BK, Weinberger DR. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology (Berl) 1997;132:303–10. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- Schroeder H, Grecksch G, Becker A, Bogerts B, Hoellt V. Alterations of the dopaminergic and glutamatergic neurotransmission in adult rats with postnatal ibotenic acid hippocampal lesion. Psychopharmacology (Berl) 1999;145:61–6. doi: 10.1007/s002130051032. [DOI] [PubMed] [Google Scholar]
- Seeman P, Bzowej NH, Guan HC, et al. Human brain D1 and D2 dopamine receptors in schizophrenia, Alzheimer’s, Parkinson’s, and Huntington’s diseases. Neuropsychopharmacology. 1987;1:5–15. doi: 10.1016/0893-133x(87)90004-2. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–9. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz PL, Galler JR, Tonkiss J. Prenatal protein restriction increases sensitization to cocaine-induced stereotypy [In Process Citation] Behav Pharmacol. 1999;10:379–87. doi: 10.1097/00008877-199907000-00005. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser E, Neugebauer R, Hoek HW, et al. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- Susser ES, Lin SP. Schizophrenia after prenatal exposure to the Dutch hunger winter of 1944- 45. Arch Gen Psychiatry. 1992;49:983–988. doi: 10.1001/archpsyc.1992.01820120071010. [DOI] [PubMed] [Google Scholar]
- Susser E, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine: The story of the Dutch Famine Study. Am J Epidemiol. 1998;147:213–216. doi: 10.1093/oxfordjournals.aje.a009439. [DOI] [PubMed] [Google Scholar]
- Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, Zheng X, Gu N, Feng G, Sham P, He L. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA. 2005;294:557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia--a synthesis and selective review. J Psychopharmacol. 2007;21:440–52. doi: 10.1177/0269881106073126. [DOI] [PubMed] [Google Scholar]
- Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep. 2007;(4):329–36. doi: 10.1007/s11920-007-0041-7. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Almeida SS, Galler JR. Prenatally malnourished female but not male rats show increased sensitivity to MK-801 in a differential reinforcement of low rates task. Behav Pharmacol. 1998;9:49–60. [PubMed] [Google Scholar]
- Tonkiss J, Galler JR. Prenatal protein malnutrition and working memory performance in adult rats. Behav Brain Res. 1990;40:95–107. doi: 10.1016/0166-4328(90)90002-v. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Galler JR, Formica RN, Shukitt-Hale B, Timm RR. Fetal protein malnutrition impairs acquistion of a DRL task in adult rats. Physiol Behav. 1990;48:73–77. doi: 10.1016/0031-9384(90)90263-4. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Galler JR, Shukitt-Hale B, Rocco FJ. Prenatal protein malnutrition impairs visual discrimination learning in adult rats. Psychobiology. 1991;19:247–250. [Google Scholar]
- Tonkiss J, Shultz P, Galler JR. An analysis of spatial navigation in prenatally protein malnourished rats. Physiol Behav. 1994;55:217–24. doi: 10.1016/0031-9384(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Lipska BK, O’Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biol Psychiatry. 2007;62(7):730–8. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursula G, Kyle, Pichard Claude. The Dutch Famine of 1944–1945: a pathophysiological model of long-term consequences of wasting disease. Curr Opin Clin Nutr Metab Care. 2006;9:388–394. doi: 10.1097/01.mco.0000232898.74415.42. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Wilkins A, Cunningham C, Perry VH, Seet MJ, Osmond C, Eckert JJ, Torrens C, Cagampang FR, Cleal J, Gray WP, Hanson MA, Fleming TP. Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. J Physiol. 2008;586:2231–44. doi: 10.1113/jphysiol.2007.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. On the plausibility of “the neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacology. 1996;14:1S–11S. doi: 10.1016/0893-133X(95)00199-N. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34:140–8. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–59. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner Immune activation during pregnancy in rats leads to a post-pubertal emergence of latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: A novel neurodevelopmental model of schizophrenia. Neuropsychpharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]


