Alcoholism–the chronic and excessive consumption of alcohol–is a syndrome characterized by untoward somatic and nervous system sequelae. Often unrecognized is the extent of the neurobehavioral deficits of alcoholism and their impact on the well-being of the individual. Further, the mechanism of alcoholism-induced neuropathology is not fully understood. Among possible causative factors are neurotoxicity of the ethanol molecule itself or its metabolic products (e.g., acetaldehyde), nutritional deficiencies (e.g., B vitamins), head trauma, and repeated withdrawals. The article by He and Crews poses yet another mechanism – alcohol-induced neuroinflammation.
This review of chronic alcohol consumption as a substrate of neurodegeneration provides a context for our commentary on the clinical and basic neuroscience significance of the postmortem study of human alcoholic brains by He and Crews (this issue). Toward this end, we review brain structure, function, and chemistry studies that have led to the common belief of the neurodegenerative effects of alcoholism. We attempt to distinguish between evidence for clinically detected abnormalities as attributable to permanent or transient neural damage and whether such damage is pervasive or restricted. Finally, we proffer ideas for further neuropathological studies to explicate findings from in vivo longitudinal studies conducted in human alcoholism and animal models.
A perspective on the clinical significance of alcohol-induced neuroinflammation as a potential mechanism of alcohol-induced neuropathology requires an appreciation of the terms used (or misused). According to Adams and Victor (Adams and Victor, 1993), “Atrophy specifies a gradual decay and loss of neurons, leaving in their wake no degradative products and only a sparsely cellular, fibrous gliosis while degeneration refers to a more rapid process of neuronal, myelin or tissue breakdown with resulting degradative products that evoke a more vigorous reaction of phagocytosis and cellular gliosis” (page 921). Evidence for permanent loss of neurons in alcoholism is sparse, and alcohol-induced damage to brain tissue can usually be attributable to neurodegenerative rather than atrophic processes. When neuronal death does occur, it is usually considered to be necrosis, which “occurs when neurons are damaged by a trauma or metabolic injury and typically involves the concurrent death of groups of adjacent cells. Cells undergoing necrosis initially swell and their internal components, or orgenelles, break down. The cells eventually rupture and spill debris that leads to local inflammation” (page 177) (Goodlett and Horn, 2001). Figure 1 present examples of presumed acute edematous and chronic necrotic mammillary bodies of two men diagnosed with Wernicke's encephalopathy (Sullivan and Pfefferbaum, 2008) and a rat model of pyrithiamine-induced WE (Pfefferbaum, et al., 2007).
Figure 1.
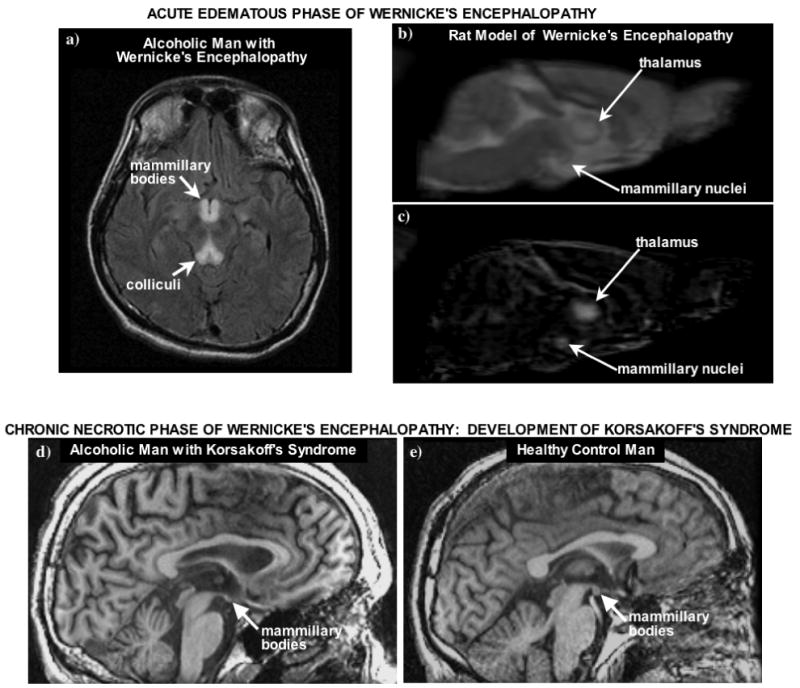
a) Axial MR fluid attenuated inversion recovery (FLAIR) image of an acute a 35 year-old man with schizophrenia and acute nutritional deficiency-induced Wernicke's Encephalopathy (WE). Prominent are the hyperintense signals in the mammillary bodies and colliculi, indicative of inflammation in the acute phase of WE (taken from Sullivan and Pfefferbaum, 2008). b-c) Sagittal slice of b) an early-echo structural image and c) of a grand average post-pre difference image showing hyperintense areas in the thalamus and mammillary nuclei in pyrithiamine-treated rats (taken from Pfefferbaum, et al., 2007). d-e) T1-weighted SPoiled GRadient echo (SPGR) MR images of 53 year old man with a history of Wernicke's Encephalopathy, which developed into Korsakoff's Syndrome (KS). Note the shrunken mammillary bodies (arrows), indicative of necrosis, in the man with KS compared with the 59 year old healthy, nonalcoholic control man (taken from Sullivan and Pfefferbaum, 2008).
A potential mechanism of the neurotoxic effects of chronic excessive alcohol consumption
The study by He and Crews (2008) is an hypothesis-driven work with the objective of isolating a potential mechanism of neural damage caused by extensive, chronic alcohol consumption. To this end, the authors obtained human brain tissue of alcoholics and moderate drinking controls from the New South Wales Tissue Resource Center, which provided a clinical characterization through systematic postmortem “interview” (Harper, et al., 2003). Markers of inflammation sought were proinflammatory cytokines, specifically, monocyte chemoattractant protein 1 (MCP-1, also known as CCL2) and microglia. MCP-1 is a particularly promising candidate because it has been shown to regulate alcohol consumption in mice (Blednov, et al., 2005). Perhaps relevant to the human condition, the regulation of drinking was limited to female rats; although controversial (c.f., Hommer, et al., 2001; Pfefferbaum, et al., 2001), some studies have found that women have radiological evidence for great brain damage from chronic alcoholism than men (e.g., Hommer, 2003; Mann, et al., 2005; for review of sex differences in alcohol use disorders Witt, 2007). MCP-1 is also involved in demyelination in a variety of experimental animal models (Kim and Perlman, 2005). MCP-1 protein levels were measured in brain homogenate in the ventral tegmental area (VTA), substantia nigra (SN), hippocampus, and amygdala and found to be elevated in alcoholics relative to controls. Thus, in all regions examined, albeit not the entire brain, MCP-1 levels were abnormally high in alcoholics. In their immunohistochemistry analysis seeking microglial markers of inflammation in cingulate cortex, amygdala, VTA, and brainstem, the authors found differential regional patterns depending on marker type: ionized calcium binding adaptor protein-1 (Iba-1) and Glucose transporter-5 (GluT5). Both markers have a microglial-restricted expression confirmed with RNA analysis of rat brain-derived primary-cultured cells (Imai, et al., 1996) and immunocytochemistry of adult rat brain sections for Iba-1 (Ito, et al., 1998) and immunocytochemistry of human tissue for GluT5 (Horikoshi, et al., 2003; Payne, et al., 1997; Sasaki, et al., 2003). Enhanced expression of Iba-1 in neuroinflammatory processes has been demonstrated in models of ischemia (Ito, et al., 2001), hippocampal denervation (Babcock, et al., 2003), Parkinson's disease (Wada, et al., 2006), stress (Frank, et al., 2007), and aging (Choi, et al., 2007; Hwang, et al., 2008). In the He and Crews study, although neither marker detected greater microglial density in the alcoholics than the controls in the amygdala, both markers revealed signs of greater microglial presence in the alcoholic cingulate cortex, and GluT5 detected such presence in VTA and midbrain structures. These positive signs of neuroinflammation provide cross-sectional support for their mechanistic role in alcoholism-related neurodegeneration. That the neurodegenerative effects had a profile of action suggests that selective brain structures carry a special vulnerability to the throes of alcoholism.
As most worthy research endeavors do, this study raises as many questions as it answers. To wit: What other brain regions might be vulnerable or resilient? Why should alcohol excesses have differential regional effects? Can identification of steps in the neurodegenerative cascade to cell death (which was not examined by He and Crews) lead to mechanisms of arrest or reversal of damage prior to neural death? Can this cascade of events, associated with very high doses of alcohol, whether consumed in massive binges or chronically over a lifetime, contribute to remodeling brain systems to be dependent on alcohol? What roles do genetics, family history, or historical events (e.g., age of alcoholism onset, bouts of nutritional deficiency, number of withdrawals) play in the ultimate outcome observed postmortem? Given that neuroinflammatory processes are capable of insulting both gray and white matter, are clinically significant sequelae of alcoholism necessarily a result of neuronal death, or alternatively, can they arise from “incomplete” lesions of white matter or cell processes?
Alcohol-induced neurodegeneration: Evidence from neuropathological, neuroelectrophysiological, neuropsychological, and neuroradiological human studies
Neuropathological evidence
Neuropathological studies of chronic alcoholism indicate that loss of neurons is selective to frontal cortex (Courville, 1955; Harper and Kril, 1990) with little evidence for widespread cell loss (Harper, 1998; Harper, et al., 1987; Kril and Harper, 1989). Although more recent work using TUNEL staining, an indicator of damaged DNA and potentially indicative of apoptosis, reveals TUNEL-positive cells in the superior frontal cortex of human alcoholics, rarely observed in control brains, morphological and immunohistochemical evidence suggests that the majority of TUNEL-stained cells were glial, not neuronal (Ikegami, et al., 2003).
Subcortical gray matter has also been examined. Although neuronal dysmorphology can be present in hippocampus (Harding, et al., 1997; Kril, et al., 1997), thalamus (Harding, et al., 2000), and cerebellum (Pentney, 1993; Phillips, et al., 1987; Torvik and Torp, 1986), neuronal loss is rarely reported in postmortem human tissue. “Atrophy” of the anterior vermis and adjoining portions of the anterior lobes of the cerebellum was described in alcoholic human patients as early as the 1950's (Victor, et al., 1959), and reduced Purkinje cell density was reported with histopathological examination of the anterior superior segment of the cerebellar vermis of human alcoholics (Phillips, et al., 1987; Torvik and Torp, 1986). More recent work, however, suggests that Purkinje cell loss in the vermis occurs only in chronic alcoholics with cerebellar dysfunction (Baker, et al., 1999).
With the exception of the frontal lobes, evidence for neuronal loss in response to chronic alcoholism is scarce. By contrast, postmortem studies of brains of human alcoholics often report that white matter is especially affected (Badsberg-Jensen and Pakkenberg, 1993; De la Monte, 1988; Harper and Kril, 1991; Harper and Kril, 1994) regardless of sex (Harper, et al., 1990). Abnormalities identified in white matter on postmortem examination include volume shrinkage, demyelination, loss of myelinated fibers, and axonal deletion possibly arising from regional neuronal loss (Alling and Bostrom, 1980; Harper, et al., 1988; Harper and Kril, 1989; Kril, et al., 1997). The corpus callosum is also affected (Harper and Kril, 1988; Tarnowska-Dziduszko, et al., 1995), especially in alcoholics who have experienced nutritional deficiencies. In their most extreme forms, these white matter conditions can be life-threatening and are associated with Marchiafava-Bignami disease and central pontine myelinolysis (Charness, 1993; Victor, et al., 1989).
Neuroelectrophysiological evidence
The P300 component of the event-related potential (ERP) elicited in behavioral paradigms requiring response inhibition to prepotent stimuli (e.g., NOGO stimuli in the GO/NOGO paradigm) is commonly diminished in recovering alcoholics (Ceballos, et al., 2003; Cohen, et al., 1997; Ford, et al., 1991; Hada, et al., 2000; Rodriguez Holguin, et al., 1999) and also nonalcoholic children of alcoholic fathers (Cohen, et al., 1997; Rodriguez Holguin, et al., 1999). This electrophysiological measure of response inhibition has a frontal scalp distribution and suggests a focal eminence of the alcoholism-related impairment (Bokura, et al., 2001; Filipovic, et al., 1999; Pfefferbaum and Ford, 1988; Pfefferbaum, et al., 1985; Shibata, et al., 1997; Shibata, et al., 1998). Given that response inhibition is controlled by frontal systems, appropriate response inhibition to a NOGO stimulus requires functionally intact frontally-based corticocortical pathways, which are affected in alcoholics and may even sustain neuronal loss (Harper, et al., 2003).
Neuropsychological evidence
The acute behavioral effects of alcohol intoxication are well-known and recognized as ataxia of gait, slurred speech, prolonged reaction time, poor memory consolidation, impaired emotional modulation, and compromised judgment. Chronic alcoholics can continue to exhibit a subset of this constellation of cognitive and motor impairment, often in a milder form, even after a month's abstinence from alcohol and without alcohol on board. Functions tend to be impaired but not completely lost in sober “uncomplicated” alcoholics (Victor, et al., 1989). Typically, the processes affected are visuospatial abilities, executive functions, and gait and balance (for reviews, Fein, et al., 1990; Moselhy, et al., 2001; Oscar-Berman, 2000; Oscar-Berman, et al., 2004; Sullivan, 2000), evidenced in both alcoholic men (Sullivan, et al., 2000) and women (Sullivan, et al., 2002). Executive functions affected include working memory, problem solving, temporal ordering, response inhibition, and psychomotor speed (Johnson-Greene, et al., 1997; Nixon, et al., 2002; Oscar-Berman and Hutner, 1993; Sullivan, 2000). These symptoms characterize impairment arising from lesions of cerebellum, frontostriatal circuitry (Sullivan, et al., 2003), and frontolimbic circuitry and occasionally have been formally correlated with measurements of local brain structure (Rosenbloom, et al., 2007), metabolite concentrations (Bendszus, et al., 2001; Meyerhoff, et al., 2004), or glucose metabolism (Adams, et al., 1993; Gilman, et al., 1996; Wang, et al., 1993).
Neuroradiological evidence
CT and MRI
Early observations using computerized tomography (CT) identified “shrinkage” in the brains of chronic alcoholics (Cala, et al., 1981) that were later verified with higher resolution imaging based on magnetic resonance (MR) techniques. Cross-sectional MR imaging (MRI) studies of chronically dependent alcoholic human adults, without obvious complications from nutritional deficiencies or hepatic disorders, demonstrated a comprised cortical mantle with thinner gyri and wider sulci than controls (for reviews Oscar-Berman and Marinkovic, 2007; Sullivan and Pfefferbaum, 2005). Specific brain regions affected by chronic alcohol exposure and described by structural MRI include cortical gray and white matter (Jernigan, et al., 1991; Pfefferbaum, et al., 1992), particularly prefrontal areas in older alcoholic individuals (Cardenas, et al., 2007; Pfefferbaum, et al., 1997), mammillary bodies (Davila, et al., 1994; Shear, et al., 1996; Sullivan, et al., 2000), anterior hippocampus (Agartz, et al., 1999; Sullivan and Marsh, 2003; Sullivan, et al., 1995), thalamus (De Bellis, et al., 2005; Sullivan, et al., 2003), pons (Pfefferbaum, et al., 2002; Sullivan, 2003), and cerebellum (De Bellis, et al., 2005; Sullivan, et al., 2000). A subset of these and other studies have tested the relationship between regional brain volume and performance on specific neuropsychological tests and have been successful in demonstrating associations or even double dissociations, for example, between olfactory discrimination and thalamic volume (Shear, et al., 1992), postural stability and cerebellar vermis (Sullivan, et al., 2000; Sullivan, et al., 2006), and perseverative errors in problem solving and prefrontal cortical volume (Chanraud, et al., 2007).
In search of clues to factors that either cause or exacerbate the untoward effect of chronic alcohol consumption, we noted a graded effect in ostensibly “uncomplicated” alcoholics, that is, those without clinically obvious symptoms of any of the serious medical conditions commonly associated with alcoholism (e.g., Wernicke's Encephalopathy (WE), Korsakoff's Syndrome (KS), alcoholic dementia, hepatic encephalopathy, cirrhosis). When compared with healthy controls, uncomplicated alcoholics had mild to moderate volume deficits and alcoholics with KS and historical evidence for WE had moderate to severe volume deficits in the mammillary bodies, hippocampus, thalamus, cerebellum, and pons (Sullivan and Pfefferbaum, 2008). That the uncomplicated alcoholics showed the same pattern of focal brain volume deficits as did the KS alcoholics but in a milder form implicates a nutritional deficiency component, likely thiamine, as contributing to the constellation of brain regions affected (Butters, 1981; Thomson, 2000). Whether bouts of subclinical vitamin deficiencies fully account for or simply contribute to regional brain volume shrinkage in alcoholism remains controversial (Impeduglia, et al., 1987; Martin, et al., 2003; Pfefferbaum, et al., 2007; Thomson, 2000).
MR Spectroscopy (MRS)
MRS enables in vivo investigation of the biochemical composition of the brain and how the brain's principal proton metabolites are altered by chronic alcohol exposure. The proton spectrum provides quantitative measures of vital brain metabolites: N-acetyl compounds primarily N-acetyl-aspartate (NAA), a marker of living, mature neurons; creatine+phosphocreatine (Cr), a reflection of high-energy phosphate metabolism; choline (Cho), an index of membrane turnover; myo-inositol (mI), an index of gliosis; and the neurotransmitter glutamate. An assumption, then, is that decline or deficits in gray matter NAA should reflect neuronal damage or loss, as it apparently does in conditions like Alzheimer's disease (Adalsteinsson, et al., 1998), which is marked by known neuronal death (Braak and Braak, 1994). Other metabolites, including Cho and mI, may reflect neuroinflammatory processes.
Exemplary studies report abnormally low levels of NAA in frontal (Bendszus, et al., 2001; Fein, et al., 1994; Jagannathan, et al., 1996) and cerebellar (Bendszus, et al., 2001; Jagannathan, et al., 1996; Mann, et al., 1998) regions in alcoholics than controls, suggesting neuronal loss. A comparison between currently drinking and recently abstinent (3 week) alcoholics found only a volume deficit in frontal white matter in the former, but no difference in levels of NAA, Cr, or Cho (O'Neill, et al., 2001). Another group reported that NAA was nearly 15% lower in a frontal white matter region and mI was higher in frontal and parietal regions in recently abstinent (4 weeks) alcoholics than controls (Schweinsburg, et al., 2000). An NAA deficit in frontal white matter and elevated parietal gray matter Cr and mI was observed to be especially strong in older alcoholics (Meyerhoff, et al., 2004).
MR Diffusion Tensor Imaging (DTI)
Rigorous study of white matter integrity is enhanced by examination with DTI, which permits characterization of myelin integrity and axon density (Basser and Jones, 2002; Le Bihan, 2003; Moseley, et al., 1990). In alcoholism, microstructural white matter abnormalities involve callosal and pericallosal degradation that occur in men (Pfefferbaum and Sullivan, 2005; Pfefferbaum, et al., 2000) and women (Pfefferbaum and Sullivan, 2002). These abnormalities correlate with working memory, visuospatial ability, and gait and balance (Pfefferbaum, et al., 2006) and demonstrated an age-alcohol interaction (Pfefferbaum, et al., 2006). More recently, we showed that FA deficits are widespread throughout left and right hemispheres in men and women (Pfefferbaum, et al., 2006). This widespread distribution of white matter microstructural compromise contrasts with the regional-specific deficits seen in nutritional deficiency syndromes that can accompany alcoholism. Results from rodent models of alcoholism and thiamine deficiency suggest that some white matter pathology in alcoholism is due to the neurotoxicity of alcohol per se (He, et al., 2007; Langlais and Savage, 1995; Pfefferbaum, et al., 2006; Zimatkin and Zimatkina, 1996).
Evidence for recovery with abstinence from alcohol
From the earliest CT studies to current MRI studies aimed at tracking evidence for brain structural recovery, positive support for at least partial reversal of brain tissue shrinkage with abstinence from alcohol (CT: Cala, et al., 1983; Carlen, et al., 1986; Carlen, et al., 1984) (MRI: Cardenas, et al., 2007; Pfefferbaum, et al., 1995; Pfefferbaum, et al., 1998). We assume that the shrinkage with drinking does not necessarily reflect “loss,” nor does the tissue expansion with abstinence reflect neurogenesis. Rather, the controlled longitudinal structural imaging studies likely reflect non-neuronal loss and neuronal cell body and process shrinkage.
To account for changes in brain tissue volume over the course of alcoholism, through bouts of drinking and abstinence, Carlen posed a two factor process, whereby one process reflected shrinkage without cell death thereby permitting volume change (up or down), and the other reflected true, irreversible neuronal cell death (Carlen, et al., 1984). That brain volume can increase and that this increase predicts improvement on neuropsychological test performance (Rosenbloom, et al., 2007; Sullivan, et al., 2000) again supports the contention that little neuronal death occurs with alcoholism. Recovery of NAA, the spectroscopic marker of neuronal integrity, can also occur and has been documented in frontal lobes over short-term supervised abstinence (Bendszus, et al., 2001) and in cerebellum over longer-term abstinence (Parks, et al., 2002) with complementary improvement in cognitive and motor performance. Higher mI, the spectroscopic marker of glia, observed in the anterior cingulate gyrus of recently detoxified alcoholics is not observed in longer-term sober alcoholics or controls (Schweinsburg, et al., 2001). Probably, low NAA levels reflect neurodegeneration without cell death, and increases with abstinence may reflect healing without cell generation. Further, alcohol-related increases in mI together with perturbation of NAA levels prominent in white matter support the possibility that white matter even more than gray matter is affected by alcoholism, a conclusion embraced in human neuropathological studies (De la Monte, 1988; Harper, et al., 2003).
Axonal (Wallerian) degeneration can lead to a permanent reduction in white matter volume. Consistent with these results are molecular studies of human brains that report expression of genes encoding myelin proteins (Lewohl, et al., 2000; Mayfield, et al., 2002) and actual levels of myelin-associated proteins as being low in alcoholic relative to control cases (Hasin, et al., 2006; Lewohl, et al., 2005). Frequent withdrawals from alcohol may also contribute to disruption of white matter integrity (Phillips, et al., 1987; Sullivan, et al., 1996). Specific white matter structures of the brain, such as the corpus callosum, are particularly affected by alcoholism (Harper and Kril, 1988; Hommer, et al., 1996; Pfefferbaum, et al., 1996). The precise structural changes underlying the white matter volume shrinkage, restoration with alcohol abstinence, and disruption of microstructural integrity remain unclear, but in vivo DTI studies (Pfefferbaum, et al., 2006; Pfefferbaum, et al., 2006; Pfefferbaum and Sullivan, 2002; Pfefferbaum and Sullivan, 2005; Pfefferbaum, et al., 2000) implicate compromise of both myelin and axonal integrity and may explain why tissue volume recovery appears incomplete with abstinence. Definitive pathology accounting for reversible brain shrinkage remains unidentified, indicating the need for further investigation of the potential interaction of alcohol exposure pattern, withdrawal experience, and nutritional deficiency in white matter pathology.
How He and Crews fill a lacuna of evidence for alcoholism neurotoxicity
The hypothesis tested by He and Crews was that alcohol induces inflammatory processes in the brain leading to neurodegeneration. To the extent that He and Crews sought to demonstrate an overly active immune response in the brains of alcoholics compared with controls, they were successful. Greater expression of MCP-1, Iba-1, or GluT5 in several brain regions of alcoholics relative to controls supports the concept that neuroinflammation occurs in response to chronic and excessive alcohol consumption.
That neuroinflammation may lead to neurodegeneration is supported by several converging lines of evidence. Neuroinflammation is a feed-forward/feed-back process. For example, an immune reaction primes microglia to activate proinflammatory cytokines (e.g., IL1β and TNFα), which in turn stimulate microglia to produce MCP-1, which in turn leads to excessive production of proinflammatory cytokines, and potentially neurodegeneration (Qin, et al., 2007). Systemic cytokines, particularly TNFα, may enter the brain to initiate the inflammatory process (Qin, et al., 2007) and lead to neuronal loss by increasing brain glutamate levels (Zou and Crews, 2005). Alcohol may also modify the glutamatergic system (Krystal, et al., 2003; Melendez, et al., 2005), but direct evidence for a hyperglutamatergic state in chronic alcoholism is lacking. Significant clues to the conditions sufficient to produce a hyperglutamatergic state derives from animal studies, indicating a dramatic increase in extracellular glutamate in the striatum during withdrawal from alcohol in chronically treated rats but not during alcohol treatment itself (Rossetti and Carboni, 1995). Whether withdrawal with related neurological signs is a necessary condition for the change in glutamatergic state remains to be established. Future in vivo MRS studies focusing on additional excitotoxic and inflammatory markers, notably glutamate, may be revealing of locus and mechanism of alcohol neurotoxicity and even neuronal death.
Another untoward outcome of excessive alcohol consumption on the brain is compromise of neurovascular health (Hillbom and Kaste, 1990; Regan, 1990). Epidemiological studies in Europe, China, and the U.S. indicate an undeniable increased risk of ischemic or hemorrhagic stroke in men and women who declare drinking, on average, more than 21 drinks per week or more than 3 drinks per day. Although some reports suggest a protective effect of low drinking (no more that 1 drink per day for women and no more than 2 drinks per day for men) from ischemic stroke (Elkind, et al., 2006; Reynolds, et al., 2003), a 10-year prospective study of 64,338 Chinese men found little evidence for such protection but did note a linear relation between greater number of drinks consumed and higher relative risk for stroke and mortality from stroke (Bazzano, et al., 2007). It has been speculated that alcohol-induced stroke and inflammation may be stimulated or exacerbated by alcohol-induced injury to the blood brain barrier (BBB), which includes brain microvascular endothelial cells (BMVEC) and astrocytes (Haorah, et al., 2007). To examine this possibility, BMVEC, harvested from human epileptics during temporal lobe tissue resection, were exposed to alcohol for 2-48 hours (Haorah, et al., 2008). Through astrocytic secretion (Haorah, et al., 2008) or oxidative stress (Haorah, et al., 2007), alcohol treatment increased metalloproteinase activity; also observed was a parallel decrease in the collagen content of the BMVEC's basement membrane, the essential component of the BBB. This experiment indicates either oxidative stress or glial activation as contributing to BBB injury, which in turn could be a potential cause of stroke in alcoholism.
The in vivo, postmortem, and animal studies of chronic alcohol consumption indicate the importance of pursuing evidence for alcohol's neurotoxic effect on white matter, which can also sustain damage through neuroinflammatory processes. Although not yet established in alcoholism, there is evidence that neuroinflammation can selectively harm white matter. The “Father of Microglia,” Pio del Rio-Hortega, characterized microglial response to brain lesions describing “fountains of microglia” in the corpus callosum and other perinatal white matter (del Rio-Hortega and Penfield, 1892). Additionally, the extent of axonal damage in the primary demyelinating lesion of multiple sclerosis patients is associated with the number of activated microglia (Neumann, 2003). Potential microglial-mediated vehicles of damage to white matter include MCP-1, shown to be involved in demyelination in a variety of experimental animal models (Kim and Perlman, 2005); cytokines, such as IL-1, which result in reversible demyelination of axons (Ferrari, et al., 2004; Hartung, et al., 1992); and potentiation of glutamate release through impairment of glutamate uptake and reduction in the expression of glutamate transporters (Matute, et al., 2007; Matute, et al., 2006). These leads should serve to generate testable hypotheses about alcohol's role in mediating neuroinflammation-induced white matter degeneration.
Significance of basic research on alcoholism to medicine and society
According to the 2001-2 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) survey, more than 20% of men, age 18 to 29, met criteria for a diagnosable alcohol use disorder (AUD): 9.3% Alcohol Abuse and 13% Alcohol Dependence. The lower prevalence in women and a declining prevalence with older age reduce the population-wide mean prevalence of an AUD in the past year to less than 10% (Grant, et al., 2004). The socioeconomic, health, and mortality costs of alcohol use in the US, whether diagnosed as an AUD or not, exceed $184 billion and have increased by 25% over estimates made for 1992 (Harwood, 2000). Alcoholism is pervasive in medical settings, estimated as upwards of 30% in university hospitals and 50% in Veterans hospitals (Beresford, et al., 1990), and often undetected, ignored, and under-treated despite its being a treatable condition. Surely, isolating specific mechanisms of alcoholism neurotoxicity is essential and carries the hope of stemming its adverse affects on brain structure and function and identifying targets for pharmacological therapy, whether for arrest, amelioration, or reversal of damage or for reduction of craving or of dependency.
Acknowledgments
This work was supported by NIAAA (AA010723, AA005965, AA012388, AA017347). We would like to thank Adolf Pfefferbaum for his support and discussion in preparing this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adalsteinsson E, Spielman D, Lim KO, Sullivan EV, Pfefferbaum A. Alzheimer's disease vs. normal aging: Brain metabolite concentrations as measured by volumetric 1H spectroscopic imaging (abs) Radiological Society of North America 1998 [Google Scholar]
- Adams KM, Gilman S, Koeppe RA, Kluin KJ, Brunberg JA, Dede D, Berent S, Kroll PD. Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcoholism: Clinical and Experimental Research. 1993;17:205–210. doi: 10.1111/j.1530-0277.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Adams RD, Victor M. Principles of Neurology. McGraw-Hill Inc.; New York: 1993. [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Archives of General Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Alling C, Bostrom K. Demyelination of the mamillary bodies in alcoholism. A combined morphological and biochemical study. Acta Neuropathologica (Berl) 1980;50:77–80. doi: 10.1007/BF00688539. [DOI] [PubMed] [Google Scholar]
- Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badsberg-Jensen G, Pakkenberg B. Do alcoholics drink their neurons away? Lancet. 1993;342:1201–1204. doi: 10.1016/0140-6736(93)92185-v. [DOI] [PubMed] [Google Scholar]
- Baker K, Harding A, Halliday G, Kril J, Harper C. Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke's encephalopathy. Neuroscience. 1999;91:429–438. doi: 10.1016/s0306-4522(98)90664-9. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Bazzano LA, Gu D, Reynolds K, Wu X, Chen CS, Duan X, Chen J, Wildman RP, Klag MJ, He J. Alcohol consumption and risk for stroke among Chinese men. Ann Neurol. 2007;62:569–578. doi: 10.1002/ana.21194. [DOI] [PubMed] [Google Scholar]
- Bendszus M, Weijers HG, Wiesbeck G, Warmuth-Metz M, Bartsch AJ, Engels S, Boning J, Solymosi L. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. American Journal of Neuroradiology. 2001;22:1926–1932. [PMC free article] [PubMed] [Google Scholar]
- Beresford TP, Blow FC, Hill E, Singer K, Lucey MR. Comparison of CAGE questionnaire and computer-assisted laboratory profiles in screening for covert alcoholism. Lancet. 1990;336:482–485. doi: 10.1016/0140-6736(90)92022-a. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav Brain Res. 2005;165:110–125. doi: 10.1016/j.bbr.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, Kobayashi S. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clinical Neurophysiology. 2001;112:2224–2232. doi: 10.1016/s1388-2457(01)00691-5. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Morphological criteria for the recognition of Alzheimer's disease and the distribution pattern of cortical changes related to this disorder. Neurobiology of Aging. 1994;15:355–356. doi: 10.1016/0197-4580(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Butters N. The Wernicke-Korsakoff Syndrome: A Review of Psychological, Neuropathological and Etiological Factors. Currents in Alcoholism. 1981;8:205–232. [PubMed] [Google Scholar]
- Cala LA, Jones B, Burns P, Davis RE, Stenhouse N, Mastaglia FL. Results of computerized tomography, psychometric testing and dietary studies in social drinkers, with emphasis on reversibility after abstinence. Med J Aust. 1983;2:264–269. doi: 10.5694/j.1326-5377.1983.tb122460.x. [DOI] [PubMed] [Google Scholar]
- Cala LA, Thickbroom GW, Black JL, Collins DWK, Mastaglia FL. Brain density and cerebrospinal fluid space size: CT of normal volunteers. American Journal of Neuroradiology. 1981;2:41–47. [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen PL, Penn RD, Fornazzari L, Bennett J, Wilkinson DA, Wortzman G. Computerized tomographic scan assessment of alcoholic brain damage and its potential reversibility. Alcoholism: Clinical and Experimental Research. 1986;10:226–232. doi: 10.1111/j.1530-0277.1986.tb05080.x. [DOI] [PubMed] [Google Scholar]
- Carlen PL, Wilkinson DA, Wortzman G, Holgate R. Partially reversible cerebral atrophy and functional improvement in recently abstinent alcoholics. Can J Neurol Sci. 1984;11:441–446. doi: 10.1017/s0317167100045972. [DOI] [PubMed] [Google Scholar]
- Carlen PL, Wilkinson DA, Wortzman G, Holgate R. Partially reversible cerebral atrophy and functional improvement in recently abstinent alcoholics. Canadian Journal of Neurological Sciences. 1984;11:441–446. doi: 10.1017/s0317167100045972. [DOI] [PubMed] [Google Scholar]
- Ceballos NA, Nixon SJ, Tivis R. Substance abuse-related P300 differences in response to an implicit memory task. Prog Neuropsychopharmacol Biol Psychiatry. 2003;21:157–164. doi: 10.1016/S0278-5846(02)00347-0. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Charness ME. Brain lesions in alcoholics. Alcoholism: Clinical and Experimental Research. 1993;17:2–11. doi: 10.1111/j.1530-0277.1993.tb00718.x. [DOI] [PubMed] [Google Scholar]
- Choi JH, Lee CH, Hwang IK, Won MH, Seong JK, Yoon YS, Lee HS, Lee IS. Age-related changes in ionized calcium-binding adapter molecule 1 immunoreactivity and protein level in the gerbil hippocampal CA1 region. J Vet Med Sci. 2007;69:1131–1136. doi: 10.1292/jvms.69.1131. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neurophysiological correlates of response production and inhibition in alcoholics. Alcohol Clin Exp Res. 1997;21:1398–1406. [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang WY. Neuroelectric correlates of response production and inhibition in individuals at risk to develop alcoholism. Biological Psychiatry. 1997;42:57–67. doi: 10.1016/S0006-3223(96)00221-1. [DOI] [PubMed] [Google Scholar]
- Courville CB. Effects of Alcohol on the Nervous System of Man. San Lucas Press; Los Angeles: 1955. [Google Scholar]
- Davila MD, Shear PK, Lane B, Sullivan EV, Pfefferbaum A. Mammillary body and cerebellar shrinkage in chronic alcoholics: An MRI and neuropsychological study. Neuropsychology. 1994;8:433–444. [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- De la Monte SM. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Archives of Neurology. 1988;45:990–992. doi: 10.1001/archneur.1988.00520330076013. [DOI] [PubMed] [Google Scholar]
- del Rio-Hortega P, Penfield W. Cerebral Cicatrix: the reaction of neuroglia and microglia to brain wounds. Bulletin of the Johns Hopkins Hospital. 1892;41:278–303. [Google Scholar]
- Elkind MS, Sciacca R, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Moderate alcohol consumption reduces risk of ischemic stroke: the Northern Manhattan Study. Stroke. 2006;37:13–19. doi: 10.1161/01.STR.0000195048.86810.5b. [DOI] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. Western Journal of Medicine. 1990;152:531–537. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Meyerhoff D, Di Sclafani V, Ezekiel F, Poole N, MacKay S, Dillon WP, Constans JM, Weiner MW. 1H magnetic resonance spectroscopic imaging separates neuronal from glial changes in alcohol-related brain atrophy. In: Lancaster F, editor. Alcohol and Glial Cells, NIAAA Research Monograph # 27. US Government Printing Office; Bethesda, MD: 1994. pp. 227–241. [Google Scholar]
- Ferrari CC, Depino AM, Prada F, Muraro N, Campbell S, Podhajcer O, Perry VH, Anthony DC, Pitossi FJ. Reversible demyelination, blood-brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. Am J Pathol. 2004;165:1827–1837. doi: 10.1016/S0002-9440(10)63438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovic SR, Jahanshahi M, Rothwell JC. Cortical potentials related to decision-making: comparison of two types of go/no-go decision. Neuroreport. 1999;10:3583–3587. doi: 10.1097/00001756-199911260-00022. [DOI] [PubMed] [Google Scholar]
- Ford JM, Rosenbloom MJ, Sullivan EV, Pfefferbaum A. ERPs and brain structure: Relationships across the adult age span, in alcoholics, and in a patient with herpes simplex encephalitis. In: Brunia CHM, Mulder G, Verbaten MN, editors. Electroencephalography & Clinical Neurophysiology Supplement 42. Event Related Brain Research; Elsevier, Amsterdam: 1991. pp. 342–354. [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Gilman S, Adams KM, Johnson-Greene D, Koeppe RA, Junck L, Kluin KJ, Martorello S, Heumann N, Hill E. Effects of disulfiram on positron emission tomography and neuropsychological studies in severe chronic alcoholism. Alcoholism: Clinical and Experimental Research. 1996;20:1456–1461. doi: 10.1111/j.1530-0277.1996.tb01149.x. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Res Health. 2001;25:175–184. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug and Alcohol Dependence. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Hada M, Porjesz B, Begleiter H, Polich J. Auditory P3a assessment of male alcoholics. Biological Psychiatry. 2000;48:276–286. doi: 10.1016/s0006-3223(00)00236-5. [DOI] [PubMed] [Google Scholar]
- Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem. 2007;101:566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- Haorah J, Schall K, Ramirez SH, Persidsky Y. Activation of protein tyrosine kinases and matrix metalloproteinases causes blood-brain barrier injury: Novel mechanism for neurodegeneration associated with alcohol abuse. Glia. 2008;56:78–88. doi: 10.1002/glia.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123:141–154. doi: 10.1093/brain/123.1.141. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Wong A, Svoboda M, Kril JJ, Halliday GM. Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus. 1997;7:78–87. doi: 10.1002/(SICI)1098-1063(1997)7:1<78::AID-HIPO8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? Neuropathology and Experimental Neurology. 1998;57:101–110. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Harper C, Dixon G, Sheedy D, Garrick T. Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:951–961. doi: 10.1016/S0278-5846(03)00155-6. [DOI] [PubMed] [Google Scholar]
- Harper C, Garrick T, Matsumoto I, Pfefferbaum A, Sullivan EV, Adalsteinsson E, Dodd P, Lewohl J, Butterworth R. How important are brain banks for alcohol research? Alcoholism: Clinical and Experimental Research. 2003;27:310–323. doi: 10.1097/01.ALC.0000052585.81056.CA. [DOI] [PubMed] [Google Scholar]
- Harper C, Kril J. If you drink your brain will shrink: Neuropathological considerations. Alcohol and Alcoholism Supplement. 1991;1:375–380. [PubMed] [Google Scholar]
- Harper C, Kril J. An introduction to alcohol-induced brain damage and its causes. Alcohol Alcohol Suppl. 1994;2:237–243. [PubMed] [Google Scholar]
- Harper C, Kril J, Daly J. Does a “moderate” alcohol intake damage the brain? Journal of Neurology, Neurosurgery, and Psychiatry. 1988;51:909–913. doi: 10.1136/jnnp.51.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Kril JJ. Patterns of neuronal loss in the cerebral cortex in chronic alcoholic patients. Journal of Neurological Science. 1989;92:81–89. doi: 10.1016/0022-510x(89)90177-9. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ. Corpus callosal thickness in alcoholics. British Journal of Addiction. 1988;83:577–580. doi: 10.1111/j.1360-0443.1988.tb02577.x. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ. Neuropathology of alcoholism. Alcohol and Alcoholism. 1990;25:207–216. doi: 10.1093/oxfordjournals.alcalc.a044994. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Daly JM. Are we drinking our neurones away? British Medical Journal. 1987;294:534–536. doi: 10.1136/bmj.294.6571.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Smith NA, Kril JJ. The effects of alcohol on the female brain - A neuropathological study. Alcohol and Alcoholism. 1990;25:445–448. [PubMed] [Google Scholar]
- Hartung HP, Jung S, Stoll G, Zielasek J, Schmidt B, Archelos JJ, Toyka KV. Inflammatory mediators in demyelinating disorders of the CNS and PNS. J Neuroimmunol. 1992;40:197–210. doi: 10.1016/0165-5728(92)90134-7. [DOI] [PubMed] [Google Scholar]
- Harwood H. Updating Estimates of the Economic Costs of Alcohol Abuse in the United States - Report prepared for NIAAA. In: Public Health Service, editor. Services, U.D.o H.a H. 2000. [Google Scholar]
- Hasin DS, Liu X, Alderson D, Grant BF. DSM-IV alcohol dependence: a categorical or dimensional phenotype? Psychological Medicine. 2006;36:1695–16705. doi: 10.1017/S0033291706009068. [DOI] [PubMed] [Google Scholar]
- He X, Sullivan EV, Stankovic RK, Harper CG, Pfefferbaum A. Interaction of thiamine deficiency and voluntary alcohol consumption disrupts rat corpus callosum ultrastructure. Neuropsychopharmacology. 2007;32:2207–2216. doi: 10.1038/sj.npp.1301332. [DOI] [PubMed] [Google Scholar]
- Hillbom M, Kaste M. Alcohol abuse and brain infarction. Ann Med. 1990;22:347–352. doi: 10.3109/07853899009147918. [DOI] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Rawlings R, Ragan P, Williams W, Rio D, Eckardt M. Decreased corpus callosum size among alcoholic women. Archives of Neurology. 1996;53:359–363. doi: 10.1001/archneur.1996.00550040099019. [DOI] [PubMed] [Google Scholar]
- Hommer DW. Male and female sensitive to alcohol-induced brain damage. Alcohol Research and Health. 2003;27:181–185. [PMC free article] [PubMed] [Google Scholar]
- Hommer DW, Momenan R, Kaiser E, Rawlings RR. Evidence for a gender-related effect of alcoholism on brain volumes. American Journal of Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Horikoshi Y, Sasaki A, Taguchi N, Maeda M, Tsukagoshi H, Sato K, Yamaguchi H. Human GLUT5 immunolabeling is useful for evaluating microglial status in neuropathological study using paraffin sections. Acta Neuropathol. 2003;105:157–162. doi: 10.1007/s00401-002-0627-4. [DOI] [PubMed] [Google Scholar]
- Hwang IK, Lee CH, Li H, Yoo KY, Choi JH, Kim DW, Kim DW, Suh HW, Won MH. Comparison of Ionized Calcium-binding Adapter Molecule 1 Immunoreactivity of the Hippocampal Dentate Gyrus and CA1 Region in Adult and Aged Dogs. Neurochem Res. 2008 doi: 10.1007/s11064-007-9584-6. [DOI] [PubMed] [Google Scholar]
- Ikegami Y, Goodenough S, Inoue Y, Dodd PR, Wilce PA, Matsumoto I. Increased TUNEL positive cells in human alcoholic brains. Neuroscience Letters. 2003;349:201–205. doi: 10.1016/s0304-3940(03)00826-7. [DOI] [PubMed] [Google Scholar]
- Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996;224:855–862. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- Impeduglia G, Martin PR, Kwast M, Hohlstein LA, Roehrich L, Majchrowicz E. Influence of thiamine deficiency on the response to ethanol in two inbred rat strains. Journal of Pharmacology and Experimental Therapeutics. 1987;240:754–763. [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke. 2001;32:1208–1215. doi: 10.1161/01.str.32.5.1208. [DOI] [PubMed] [Google Scholar]
- Jagannathan NR, Desai NG, Raghunathan P. Brain metabolite changes in alcoholism: An in vivo proton magnetic resonance spectroscopy (MRS) study. Magnetic Resonance Imaging. 1996;14:553–557. doi: 10.1016/0730-725x(96)00048-3. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Grant I, Schuckit M, Cermak L. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcoholism: Clinical and Experimental Research. 1991;15:418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Johnson-Greene D, Adams KM, Gilman S, Kluin KJ, Junck L, Martorello S, Heumann M. Impaired upper limb coordination in alcoholic cerebellar degeneration. Archives of Neurology. 1997;54:436–439. doi: 10.1001/archneur.1997.00550160070018. [DOI] [PubMed] [Google Scholar]
- Kim TS, Perlman S. Viral expression of CCL2 is sufficient to induce demyelination in RAG1-/- mice infected with a neurotropic coronavirus. J Virol. 2005;79:7113–7120. doi: 10.1128/JVI.79.11.7113-7120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Harper CG. Neuronal counts from four cortical regions of the alcoholic brain. Acta Neuropathologica. 1989;79:200–204. doi: 10.1007/BF00294379. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Krupitsky E, Schutz C, Trevisan L, D'Souza DC. NMDA receptor antagonism and the ethanol intoxication signal: from alcoholism risk to pharmacotherapy. Ann N Y Acad Sci. 2003;1003:176–184. doi: 10.1196/annals.1300.010. [DOI] [PubMed] [Google Scholar]
- Langlais PJ, Savage LM. Thiamine deficiency in rats produces cognitive and memory deficits on spatial tasks that correlate with tissue loss in diencephalon, cortex and white matter. Behav Brain Res. 1995;68:75–89. doi: 10.1016/0166-4328(94)00162-9. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Lewohl J, Wang L, Miles M, Zhang L, Dodd P, Harris R. Gene expression in human alcoholism: Microarray analysis of frontal cortex. Alcoholism: Clinical and Experimental Research. 2000;24:1873–1882. [PubMed] [Google Scholar]
- Lewohl JM, Wixey J, Harper CG, Dodd PR. Expression of MBP, PLP, MAG, CNP, and GFAP in the Human Alcoholic Brain. Alcohol Clin Exp Res. 2005;29:1698–1705. doi: 10.1097/01.alc.0000179406.98868.59. [DOI] [PubMed] [Google Scholar]
- Mann K, Seitz D, Widman U, Grodd W. Proton MR spectroscopy of the cerebellum in detoxified alcoholics and healthy controls (abs) American College of Neuropsychopharmacology 1998 [Google Scholar]
- Mann KF, Ackermann K, Croissan B, Mundle G, Diehl A. Neuroimaging of gender differences in alcoholism: Are women more vulnerable? Alcoholism: Clinical and Experimental Research. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- Martin PR, Singleton CK, Hiller-Sturmhofel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27:134–142. [PMC free article] [PubMed] [Google Scholar]
- Matute C, Alberdi E, Domercq M, Sanchez-Gomez MV, Perez-Samartin A, Rodriguez-Antiguedad A, Perez-Cerda F. Excitotoxic damage to white matter. J Anat. 2007;210:693–702. doi: 10.1111/j.1469-7580.2007.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Domercq M, Sanchez-Gomez MV. Glutamate-mediated glial injury: mechanisms and clinical importance. Glia. 2006;53:212–224. doi: 10.1002/glia.20275. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Lewohl JM, Dodd PR, Herlihy A, Liu J, Harris RA. Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. Journal of Neurochemistry. 2002;81:802–813. doi: 10.1046/j.1471-4159.2002.00860.x. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcoholism Clinical and Experimental Research. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner MW. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcoholism: Clinical and Experimental Research. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley ME, Cohen Y, Kucharczyk J, Mintorovitch J, Asgari HS, Wendland MF, Tsuruda J, Norman D. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176:439–445. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- Neumann H. Molecular mechanisms of axonal damage in inflammatory central nervous system diseases. Curr Opin Neurol. 2003;16:267–273. doi: 10.1097/01.wco.0000073926.19076.29. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Tivis R, Ceballos N, Varner JL, Rohrbaugh J. Neurophysiological efficiency in male and female alcoholics. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2002;26:919–927. doi: 10.1016/s0278-5846(02)00206-3. [DOI] [PubMed] [Google Scholar]
- O'Neill J, Cardenas VA, Meyerhoff DJ. Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcoholism: Clinical and Experimental Research. 2001;25:1673–1682. [PubMed] [Google Scholar]
- Oscar-Berman M. Neuropsychological vulnerabilities in chronic alcoholism. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's Neuroscience and Behavioral Research Portfolio, NIAAA Research Monograph No 34. National Institutes of Health; Bethesda, MD: 2000. pp. 437–472. [Google Scholar]
- Oscar-Berman M, Hutner N. Frontal lobe changes after chronic alcohol ingestion. In: Hunt WA, Nixon SJ, editors. Alcohol-Induced Brain Damage, NIAAA Research Monographs #22. National Institutes of Health; Rockville, MD: 1993. pp. 121–156. [Google Scholar]
- Oscar-Berman M, Kirkley SM, Gansler DA, Couture A. Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcoholism: Clinical and Experimental Research. 2004;28:667–675. doi: 10.1097/01.alc.0000122761.09179.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks MH, Dawant BM, Riddle WR, Hartmann SL, Dietrich MS, Nickel MK, Price RR, Martin PR. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcoholism: Clinical and Experimental Research. 2002;26:1368–1380. doi: 10.1097/01.ALC.0000029598.07833.2D. [DOI] [PubMed] [Google Scholar]
- Payne J, Maher F, Simpson I, Mattice L, Davies P. Glucose transporter Glut 5 expression in microglial cells. Glia. 1997;21:327–331. doi: 10.1002/(sici)1098-1136(199711)21:3<327::aid-glia7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pentney RJ. Alterations in the structure of the cerebellum after long-term ethanol consumption. In: Hunt WA, Nixon SJ, editors. Alcohol-Induced Brain Damage: NIAAA Research Monograph No 22. National Institute of Health; Rockville, MD: 1993. pp. 249–276. [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Bell RL, Sullivan EV. Development and resolution of brain lesions caused by pyrithiamine- and dietary-Induced thiamine deficiency and alcohol exposure in the alcohol-preferring rat: A longitudinal magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2007;32:1149–1177. doi: 10.1038/sj.npp.1301107. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sood R, Mayer D, Bell R, McBride WJ, Li TK, Sullivan EV. Longitudinal brain MRI study of the alcohol-preferring rat: Part II: Effects of voluntary chronic alcohol consumption. Alcoholism: Clinical and Experimental Research. 2006;30:1248–1261. doi: 10.1111/j.1530-0277.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiol Aging. 2006;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiology of Aging. 2006;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biological Psychiatry. 2006;59:364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM. ERPs to stimuli requiring response production and inhibition: The effects of age, probability and visual noise. Electroencephalography and clinical Neurophysiology. 1988;71:55–63. doi: 10.1016/0168-5597(88)90019-6. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Kopell BS. ERPs to response production and inhibition. Electroencephalography and clinical Neurophysiology. 1985;60:423–434. doi: 10.1016/0013-4694(85)91017-x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: A magnetic resonance imaging study. Alcoholism: Clinical and Experimental Research. 1996;20:752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcoholism: Clinical and Experimental Research. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Deshmukh A, Sullivan EV. Sex differences in the effects of alcohol on brain structure. American Journal of Psychiatry. 2001;158:188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Serventi K, Sullivan EV. Corpus callosum, pons and cortical white matter in alcoholic women. Alcoholism: Clinical and Experimental Research. 2002;26:400–405. [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage. 2002;15:708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: Evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcoholism: Clinical and Experimental Research. 2000;24:1214–1221. [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a five year interval. Archives of General Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Phillips SC, Harper CG, Kril J. A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain. 1987;110:301–314. doi: 10.1093/brain/110.2.301. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan TJ. Alcohol and the cardiovascular system. Jama. 1990;264:377–381. [PubMed] [Google Scholar]
- Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J. Alcohol consumption and risk of stroke: a meta-analysis. Jama. 2003;289:579–588. doi: 10.1001/jama.289.5.579. [DOI] [PubMed] [Google Scholar]
- Rodriguez Holguin S, Porjesz B, Chorlian DB, Polich J, Begleiter H. Visual P3a in male alcoholics and controls. Alcoholism: Clinical and Experimental Research. 1999;23:582–591. [PubMed] [Google Scholar]
- Rodriguez Holguin S, Porjesz B, Chorlian DB, Polich J, Begleiter H. Visual P3a in male subjects at high risk for alcoholism. Biological Psychiatry. 1999;46:281–291. doi: 10.1016/s0006-3223(98)00247-9. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Rohlfing T, O'Reilly A, Sassoon SA, Pfefferbaum A, Sullivan EV. Improvement in memory and static balance with abstinence in alcoholic men and women: Selective relations with changes in regional ventricular volumes. Psychiatry Research: Neuroimaging. 2007;155:91–102. doi: 10.1016/j.pscychresns.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. European Journal of Pharmacology. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Horikoshi Y, Yokoo H, Nakazato Y, Yamaguchi H. Antiserum against human glucose transporter 5 is highly specific for microglia among cells of the mononuclear phagocyte system. Neurosci Lett. 2003;338:17–20. doi: 10.1016/s0304-3940(02)01332-0. [DOI] [PubMed] [Google Scholar]
- Schweinsburg B, Taylor M, Videen J, Alhassoon O, Patterson T, Grant I. Elevated myo-inositol in gray matter of recently detoxified but not long-term abstinent alcoholics: A preliminary MR spectroscopy study. Alcoholism: Clinical and Experimental Research. 2000;24:699–705. [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Videen JS, Brown GG, Patterson TL, Berger F, Grant I. Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcoholism: Clinical and Experimental Research. 2001;25:924–934. [PubMed] [Google Scholar]
- Shear PK, Butters N, Jernigan TL, DiTraglia GM, Irwin M, Schuckit MA, Cermak LS. Olfactory loss in alcoholics: correlations with cortical and subcortical MRI indices. Alcohol. 1992;9:247–255. doi: 10.1016/0741-8329(92)90061-e. [DOI] [PubMed] [Google Scholar]
- Shear PK, Sullivan EV, Lane B, Pfefferbaum A. Mammillary body and cerebellar shrinkage in chronic alcoholics with and without amnesia. Alcoholism: Clinical and Experimental Research. 1996;20:1489–1495. doi: 10.1111/j.1530-0277.1996.tb01153.x. [DOI] [PubMed] [Google Scholar]
- Shibata T, Shimoyama I, Ito T, Abla D, Iwasa H, Koseki K, Yamanouchi N, Sato T, Nakajima Y. The time course of interhemispheric EEG coherence during a GO/NO-GO task in humans. Neurosci Lett. 1997;233:117–120. doi: 10.1016/s0304-3940(97)00652-6. [DOI] [PubMed] [Google Scholar]
- Shibata T, Shimoyama I, Ito T, Abla D, Iwasa H, Koseki K, Yamanouchi N, Sato T, Nakajima Y. The synchronization between brain areas under motor inhibition process in humans estimated by event-related EEG coherence. Neurosci Res. 1998;31:265–271. doi: 10.1016/s0168-0102(98)00046-7. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. Human brain vulnerability to alcoholism: Evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's Neuroscience and Behavioral Research Portfolio, NIAAA Research Monograph No 34. National Institutes of Health; Bethesda, MD: 2000. pp. 473–508. [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcoholism: Clinical and Experimental Research. 2003;27:1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: Relation to ataxia. Neuropsychology. 2000;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: relation to ataxia. Neuropsychology. 2000;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Mathalon DH, Rosenbloom MJ, Lim KO, Pfefferbaum A. Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Archives of General Psychiatry. 2000;57:894–902. doi: 10.1001/archpsyc.57.9.894. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16:74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, Desmond JE, Chen SHA, Pryor MR, De Rosa E, Pfefferbaum A. Disruption of frontocerebellar circuitry and function in alcoholism. Alcoholism: Clinical and Experimental Research. 2003;27:301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L. Hippocampal volume deficits in alcoholic Korsakoff's syndrome. Neurology. 2003;61:1716–1719. doi: 10.1212/01.wnl.0000098940.31882.bb. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Relationship between alcohol withdrawal seizures and temporal lobe white matter volume deficits. Alcoholism: Clinical and Experimental Research. 1996;20:348–354. doi: 10.1111/j.1530-0277.1996.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neuroimaging of the Wernicke Korsakoff Syndrome. Alcohol and Alcoholism: accepted. 2008 doi: 10.1093/alcalc/agn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A. Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: a quantitative physiological and MRI study. Cereb Cortex. 2006;16:1077–1086. doi: 10.1093/cercor/bhj048. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: Relationships to changes in brain structure. Neuropsychology. 2000;14:178–188. [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism: Clinical and Experimental Research. 2000;24:611–621. [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Serventi KL, Deshmukh A, Pfefferbaum A. The effects of alcohol dependence comorbidity and anti-psychotic medication on volumes of the thalamus and pons in schizophrenia. American Journal of Psychiatry. 2003;160:1110–1116. doi: 10.1176/appi.ajp.160.6.1110. [DOI] [PubMed] [Google Scholar]
- Tarnowska-Dziduszko E, Bertrand E, Szpak G. Morphological changes in the corpus callosum in chronic alcoholism. Folia Neuropathologica. 1995;33:25–29. [PubMed] [Google Scholar]
- Thomson AD. Mechanisms of vitamin deficiency in chronic alcohol misusers and the development of the Wernicke-Korsakoff syndrome. Alcohol and Alcoholism Suppl. 2000;35:2–7. doi: 10.1093/alcalc/35.supplement_1.2. [DOI] [PubMed] [Google Scholar]
- Torvik A, Torp S. The prevalence of alcoholic cerebellar atrophy: A morphometric and histological study of an autopsy material. Journal of Neurological Science. 1986;75:43–51. doi: 10.1016/0022-510x(86)90049-3. [DOI] [PubMed] [Google Scholar]
- Victor M, Adam RD, Mancall EL. A restricted form of cerebellar degeneration occurring in alcoholic patients. Archives of Neurology. 1959;1:577–688. [Google Scholar]
- Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. 2nd. F.A. Davis Co; Philadelphia: 1989. [Google Scholar]
- Wada M, Yoshimi K, Higo N, Ren YR, Mochizuki H, Mizuno Y, Kitazawa S. Statistical parametric mapping of immunopositive cell density. Neurosci Res. 2006;56:96–102. doi: 10.1016/j.neures.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Roque CT, Cestaro VL, Hitzemann RJ, Cantos EL, Levy AV, Dhawan AP. Functional importance of ventricular enlargement and cortical atrophy in healthy subjects and alcoholics as assessed with PET, MR imaging, and neuropsychologic testing. Radiology. 1993;186:59–65. doi: 10.1148/radiology.186.1.8416587. [DOI] [PubMed] [Google Scholar]
- Witt ED. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol Teratol. 2007;29:81–95. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Zimatkina TI. Thiamine deficiency as predisposition to, and consequence of, increased alcohol consumption. Alcohol Alcohol. 1996;31:421–427. doi: 10.1093/oxfordjournals.alcalc.a008172. [DOI] [PubMed] [Google Scholar]
- Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]


