Abstract
The body’s defense against schistosome infection can take many forms. For example, upon developing acute schistosomiasis, patients often have fever coinciding with larval maturation, migration and early oviposition. As the infection becomes established, the parasite comes under oxidative stress generated by the host immune system. The most common treatment for schistosomiasis is the anti-helminthic drug praziquantel. Its effectiveness, however, is limited due to its inability to kill schistosomes 2 – 4 weeks post-infection. Clearly there is a need for new antischistosomal drugs. We hypothesize that gene products expressed as part of a protective response against heat and/or oxidative stress are potential therapeutic targets for future drug development. Using a 12,166 element oligonucleotide microarray to characterize Schistosoma mansoni genes induced by heat and oxidative stress we found that 1,878 S. mansoni elements were significantly induced by heat stress. These included previously reported heat-shock genes expressing homologs of HSP40, HSP70 and HSP86. One thousand and one elements were induced by oxidative stress including those expressing homologs of superoxide dismutase, glutathione peroxidase and aldehyde dehydrogenase. Seventy-two elements were common to both stressors and could potentially be exploited in the development of novel anti-schistosomal therapeutics.
Keywords: Schistosoma mansoni, temperature stress, oxidative stress, transcriptome
1. Introduction
Schistosomiasis is an intravascular infection that affects approximately 200 million people, 85% of whom live on the African continent [1]. The three species of schistosome most closely associated with the disease in humans are Schistosoma mansoni, S. haematobium and S. japonicum. Children are particularly prone to infection, often suffering stunted physical growth and mental development [2–4].
Upon infecting their definitive host, S. mansoni schistosomula migrate to the hepatic portal and mesenteric veins. Male and female worms pair about 28 to 35 days after infection, resulting in the release of an estimated 300 eggs per day [5]. Presently, the drug of choice for all forms of schistosomiasis is praziquantel. Its effectiveness has been shown to be biphasic, however, being unable to kill worms during the third and fourth weeks of a mouse infection [6–8]. In addition, a number of clinical and laboratory studies have suggested the emergence of praziquantel resistant parasites (reviewed by [9,10]). Although the interpretation of such studies remains controversial [11], the continued reliance on a single drug is, nonetheless, perturbing.
Once established, schistosome infections can be extremely long-lived. For example, Cross and colleagues have reported an active S. mansoni infection in a former Portuguese soldier 34 years after contracting the disease in Angola [12]. Similarly, Harris and colleagues reported 16 Polish patients with schistosomiasis in Western Australia, a non-endemic area, who had previously lived in refugee camps in East Africa in the early 1950’s [13]. Three of these patients had been living in Western Australia for more than 31 years while 10 had been there for more than 20 years. Clearly, schistosomes have evolved very successful defense strategies to counter the host immune system.
With acute human schistosomiasis, fever may develop as soon as 2 weeks after initial infection, but this appears to have no discernable effect on worm survival [14]. In an analysis of 163,000 S. mansoni ESTs, Verjovski-Almeida and colleagues, were able to identify 23 assembled ESTs encoding heat-shock proteins [15]. This suggests that molecular mechanisms are present to deal with the rapid temperature transitions schistosomes must undergo throughout all stages of their lifecycle. Acute schistosomiasis is most easily identifiable in tourists and travelers and often resolves spontaneously. Chronic exposure of those living in endemic areas to schistosomes, however, generally leads to a much more severe pathology primarily associated with the parasite’s eggs. An important component of the chronic anti-schistosomal response is due to reactive oxygen species (ROS), such as the superoxide radical anion. This is highly toxic to cells and is converted to H2O2 by superoxide dismutase [16]. H2O2 is also dangerous to cells as it can be converted into highly destructive hydroxyl radicals. In many species, H2O2 is removed from cells by catalase but this enzyme appears to be missing in S. mansoni. In addition to catalase, H2O2 can be removed from cells by glutathione and thioredoxin based systems. These pathways rely on glutathione reductase and thioredoxin reductase respectively to maintain glutathione and thioredoxin in their reduced state [17,18]. Alger and colleagues have provided strong evidence, however, that glutathione reductase and thioredoxin reductase have been replaced in S. mansoni by a single enzyme, thioredoxin glutathione reductase [19]. Interestingly, inhibition of this enzyme is able to kill schistosomes in culture and partially cure infected mice [20,21].
While the inhibition of thioredoxin glutathione reductase is a welcome step forward in the development of new anti-schistosomal therapeutics it is our belief that a more complete understanding of the molecular response of schistosomes to stressors such as heat and ROS will present new strategies for attacking them. In this paper we report the transcriptional response of S. mansoni to temperature and oxidative stress, and describe the induced transcripts that are unique to, and shared by, each stressor.
2. Materials and methods
2.1. Schistosoma mansoni
Schistosome infected mice and snails were supplied by Dr. Fred A. Lewis, NIAID Schistosomiasis Resource Center at the Biomedical Research Institute (Rockville, MD), through NIAID contract NO1-A1-30026. The abdomens of female SW mice were exposed to 125 S. mansoni PR-1 cercaria. Six weeks post exposure, mice were anesthetized and worms harvested by cardiac perfusion with enriched RPMI media (RPMI 1640 containing 20% fetal calf serum, 100 IU penicillin, 10 μg/mL streptomycin). Mice were subsequently euthanized by cervical dislocation. All animal experimentation complied with the policies, regulations and guidelines mandated by the Institutional Animal Care and Use Committee, University of New Mexico. Prior to all experiments, adult worms were allowed to recover overnight in enriched RPMI media at 37°C. This and all subsequent procedures that required worms to be maintained at 37 or 42°C (see below) were performed using a water jacketed incubator with 5% CO2.
2.2. Stress experiments
Individual groups of 12 male and 12 female worms were placed into six Petri dishes containing 5 mL enriched RPMI media pre-warmed to 37°C. One dish was immediately harvested (0 h sample) by replacing the media with 600 μL of RLT buffer (Qiagen) containing 1 % β-ME (RLT/β-ME buffer) and homogenizing the worms (Kontes Glass Company). The homogenate was then frozen at −80°C until RNA was extracted as described below. The media in the remaining 5 dishes was replaced by 5 mL enriched RPMI media pre-warmed to 42°C and the dishes incubated at 42°C. Worms were then harvested as described above after 5, 15, 30, 60, and 240 min. A control group of 12 male and 12 female worms in 5 mL enriched RPMI media was maintained at 37°C and harvested after 4.0 h.
Oxidative stress was induced in schistosomes using H2O2 [22]. Individual groups of 12 male and 12 female worms were each placed into six Petri dishes containing 5 mL enriched RPMI media and H2O2 added to a final concentration of 100 μM. Worms were then incubated at 37°C and harvested after 0 (prior to the addition of H2O2), 5, 15, 30, 60, and 240 min as described above.
Each of these experiments was performed in duplicate.
2.3. Reference RNA
Six to seven weeks after infection of female SW mice, schistosomes were collected by cardiac perfusion to provide a universal reference RNA for the normalization of gene expression data in both the heat-shock and oxidative stress experiments. A universal reference RNA should provide a positive hybridization signal at as many elements on a microarray as possible [23]. To achieve this we mixed total RNA from untreated worms incubated at 37°C for 4.0 h (approximately 99% of the final amount) with that of worms incubated at 42°C for 4.0 h, along with worms treated with pathogen associated molecular patterns (PAMPs; 50 μg/mL lipopolysaccharide derived from Escherichia coli, 50 μg/mL peptidoglycan from Staphylococcus aureus and 50 μg/mL laminarin from Laminaria digitata) for 6.0 h at 37°C and a group treated with a sub-lethal dose of PZQ (50 μg/mL) for 0.5 h at 37°C.
2.4. RNA extraction
RNA was isolated from thawed worms using an RNeasy® Mini kit (Qiagen) according to the manufacturer’s instructions, eluted with 30 μL of RNase free distilled water (dH2O) and quantified using a NanoDrop® ND-1000 spectrophotometer using ND-1000 3.3 software (NanoDrop Technologies). RNA integrity was evaluated using an Agilent 2100 Bioanalyzer with a RNA 6000 Nano LabChip® Kit (Agilent Technologies).
2.5. cDNA synthesis, amplification, labeling and hybridization to microarrays
Complementary DNA was synthesized, amplified, labeled and hybridized using the modified SMART (Clontech) cDNA labeling protocol described by Petalidis and colleagues [24]. Briefly, 300 ng total RNA were mixed with 3 μL of 10 μM 3′ SMART CDS primer IIA (5′-AAGCAGTGGTATCAACGCAGAGTACT30VN-3′) and 3 μL 10 μM template switching primer (5′-d(AAGCAGTGGTATCAACGCAGAGTACGC)r(GGG)-3′) and brought to a final volume of 15 μL with RNase free dH2O. The template switching primer is a DNA:RNA hybrid where the last 3 bases are RNA. The reaction was then incubated at 72°C for 2 min and then placed on ice. Six microliters of 5× first-strand buffer (Clontech), 3 μL DTT (20 mM), 3 μL dNTPs (10 mM), and 3 μL and PowerScript™ reverse transcriptase (Clontech) were then added to the reaction and the mixture incubated at 42°C for 1.0 h to generate first strand cDNA. Second-strand cDNA was then amplified by mixing 15 μL of the first-strand cDNA reaction with 57 μL dH2O, 10 μL 10× PCR buffer II (Applied Biosystems), 10 μL 25 mM MgCl2, 2 μL 10 mM dNTPs, 4 μL 10 μM 5′ PCR primer (5′-AAGCAGTGGTATCAACGCAGAGT-3′) and 2 μl AmpliTaq® (40 U/μL). Amplification conditions were 95°C for 1 min for one cycle followed by 95°C for 5 s, 65°C for 5 s, and 68°C for 6 min for 15 cycles. Second strand cDNA was then purified using a QIAquick® PCR Purification Kit (Qiagen), quantified using a NanoDrop® ND-1000 spectrophotometer and labeled with Cy3/Cy5 d-CTPs (GE Healthcare-Amersham) using BioPrime® DNA Labeling System (Invitrogen). For labeling, 200 ng of second-strand cDNA suspended in 21 μL dH2O was mixed with 20 μL of 2.5× random primer reaction buffer (Invitrogen) and incubated at 95°C for 5 min, then placed on ice. While on ice, 2 μL dH2O, 5 μL low-C dNTP mix (5 mM dATP, 5 mM dGTP, 5 mM dTTP, 2 mM dCTP), 1 μL Cy3 or Cy5 dCTP and 1 μL Klenow enzyme (40 U/μL; Invitrogen) were mixed and incubated at 37°C for 2.0 h. The labeling reaction was stopped by adding 5 μL stop buffer (Invitrogen). For all experiments, reference samples were labeled with Cy3 and experimental samples with Cy5. Cy3 and Cy5 labeled probes were purified separately using an AutoSeq™G-50 Dye Terminator Removal Kit (GE Healthcare) and labeling efficiency quantified using a NanoDrop® ND-1000 spectrophotometer. Purified Cy3 and Cy5 labeled products were then pooled, ethanol precipitated, resuspended in 100 μL hybridization buffer (40 % formamide, 5× Denhardt’s, 5× SSC, 1 mM sodium pyrophosphate, 50 mM Tris (pH 7.4) and 0.1 % SDS) and incubated at 95°C for 5 min followed by 50°C for 5 min. Arrays were pre-hybridized for 3.0 h at 42°C in hybridization buffer prior to hybridization with labeled probes. Labeled cDNA was hybridized overnight at 42°C, to 7,066 S. japonicum and 12,166 S. mansoni element oligonucleotide arrays purchased from Agilent Technologies and described by Gobert and colleagues [25]. Only the S. mansoni elements were considered in the analyses.
2.6. Microarray scanning and analyses
Microarray slides were scanned with a GenePix® 4000B scanner (Axon Instruments) with GenePix® Pro 6.0 (Axon Instruments) software using a modified protocol as described by Aragon and colleagues [26]. A preloaded S. mansoni grid was used to align and identify array spots. Alignment diameter minimum and maximums were set at 50% and 200%, respectively. Nearest negative control spots were selected for background subtraction. Acuity version 4.0 (Axon Instruments) software was used in array analyses and arrays were normalized using a ratio of medians.
Microarray analysis was performed using samples derived from the 0 (untreated), 30, 60 and 240 min time points from both stress experiments. Raw expression data for each element was expressed as a ratio of the treatment (Cy5) to the common reference sample (Cy3). Expression ratios obtained for genes with the untreated samples were then subtracted from all sample ratios. Transcripts expressed ±1.0 log2, in both biological replicates, in either the 30 and 60 min, 60 and 240 min or 30, 60 and 240 min samples after this subtraction step were considered to be differentially expressed [27]. Unsupervised hierarchical clustering analyses were performed on all microarrays. All information is MIAME compliant (http://www.mged.org/Workgroups/MIAME/miame.html) and all data were submitted to Gene Express Omnibus (http://www.ncbi.nlm.nih.gov/geo/) with accession number GSE11706.
The accession (TC) numbers associated with elements binding differentially expressed transcripts were used to obtain their associated sequences from the S. mansoni Genome Index (http://compbio.dfci.harvard.edu/tgi/tgipage.html). These sequences were then analyzed using the gene ontology program Blast2GO (http://www.blast2go.de/) [28] with a cut off of ≤ 1e-5. Sequences with ascribed functional annotations were further analyzed by blastx (www.ncbi.nlm.nih.gov/blast/Blast.cgi). Elements with an E value greater than 1e-5 were considered as having no known database homolog.
2.7. Quantitative real-time PCR analysis
For quantitative real-time PCR (qRT-PCR) reactions, 250 ng of total RNA was reverse transcribed using TaqMan® Reverse Transcription Reagents (Applied Biosystems) with random hexamers as primers. Exogenous Arabidopsis thaliana CAB mRNA (Stratagene) was spiked into each RT reaction as a control. qRT-PCR reactions (95°C for 10 min followed by 40 cycles of 95°C for 15 s and 58°C for 1 min) were performed, in triplicate (technical replicates) for each sample from both biological replicates, using Power SYBR® Green PCR Master Mix (Applied Biosystems), forward and reverse primers (0.6 μM each), and a cDNA template (1–2 ng). Primer pairs were designed using Primer Express 2.0 (Applied Biosystems). Cycle threshold values for each reaction were determined using the ABI Prism 7000 SDS 1.1 software package (Applied Biosystems). The mean fold change was calculated using the 2−ΔΔCT method [29].
3. Results and discussion
3.1. Transcript induction after heat stress
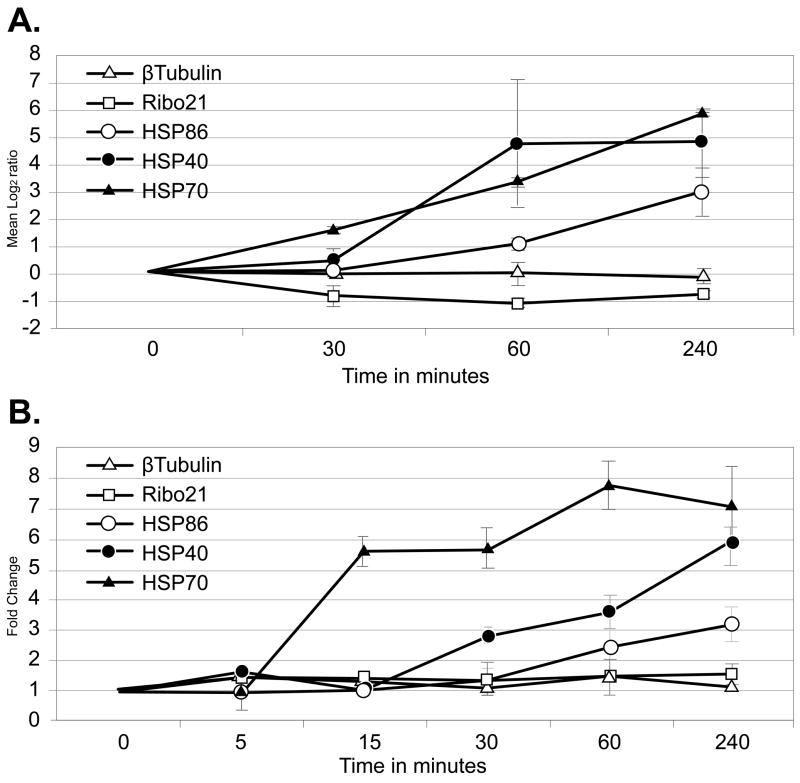
S. mansoni worms, obtained 6 weeks after infection of a mouse host, were maintained at 42°C for 0.5, 1.0, and 4.0 h in vitro and changes in their transcriptome analyzed using an oligonucleotide microarray containing 12,166 unique S. mansoni elements. The significant induction of transcripts encoding 3 homologs of HSP40 (major egg antigen, TC16575), HSP70 (TC16542), and HSP86 (TC16552), known from previously published work to be induced in response to heat-shock was confirmed in this analysis [30–32]. This suggests that the treatment was successful in inducing the heat-shock stress response (Fig. 1A). HSP70 transcripts were induced significantly 30 min after the ambient temperature was raised to 42°C, while HSP40 and HSP86 transcripts were induced after 60 and 240 min respectively. At their peak, HSP40 and HSP70 transcript levels were both induced at least 40-fold and HSP86 at least 6-fold. Transcripts encoding the constitutively expressed β-tubulin (TC8581) and Ribo21 (TC17090) homologs remained stable during the period of heat-shock (Fig. 1A). In the case of HSP70, this is a significantly higher level of induction than that described by Neumann and colleagues who, using Northern blot hybridization analysis, reported a 5.1 and 7.1-fold increases in adult worm HSP70 mRNA after 3 and 6 h at 42°C respectively [32]. This paper also reported a 6.3-fold increase in HSP70 levels 6 h after the production of schistosomula by mechanical transformation in the absence of heat shock. Similarly, Chai and colleagues [33] have demonstrated using microarrays that a S. japonicum HSP70 homolog is induced 3.5-fold in lung schistosomula compared to adult parasites suggesting that the up-regulation of this gene may be associated with the induction of heat tolerance as the parasites had only been in the host 3 days and may still have been adjusting to the thermal shift or were reacting to host immune attack. Changes in the transcriptome of samples from untreated (0 min) worms and worms maintained at 37°C for 240 min were also analyzed. One percent of array elements were differentially regulated, well within the expected error range (data not shown). These elements were removed from our analyses.
Figure 1.
Comparison of microarray data and qRT-PCR measurements for representative mRNAs from schistosomes exposed to heat-shock. A. Microarray expression data are plotted as mean log2 Cy5/Cy3 as a function of time after stress. Log2 transformed data collected from array elements at 0, 0.5, 1.0, and 4.0 h were subtracted from log2 transformed data collected from 0 h arrays. Error bars represent the standard deviation of three measurements from biological replicates. Log2 > 1 is considered a significant increase in transcript abundance. B. qRT-PCR data were plotted as fold change calculated using the 2−ΔΔCT method [29] over time after heat stress. Error bars represent the standard deviation of three measurements. Fold change > 1 indicates an increase in transcript abundance.
Microarray expression data were validated for the transcripts described above using qRT-PCR. Transcript abundance was compared between control (37°C) and heat-shocked (42°C) worms (Fig. 1B). For these analyses, two additional time points (5 and 15 min post heat-shock) were included to judge how quickly the schistosomes were able to respond to the stressor. HSP70 transcripts had greater than two-fold induction after 15 min, HSP40 after 30 min and HSP86 after 60 min, while β-tubulin and Ribo21 transcripts showed no change. The overall changes in the levels of expression of these 5 transcripts, as measured by microarray and qRT-PCR analyses, were closely matched.
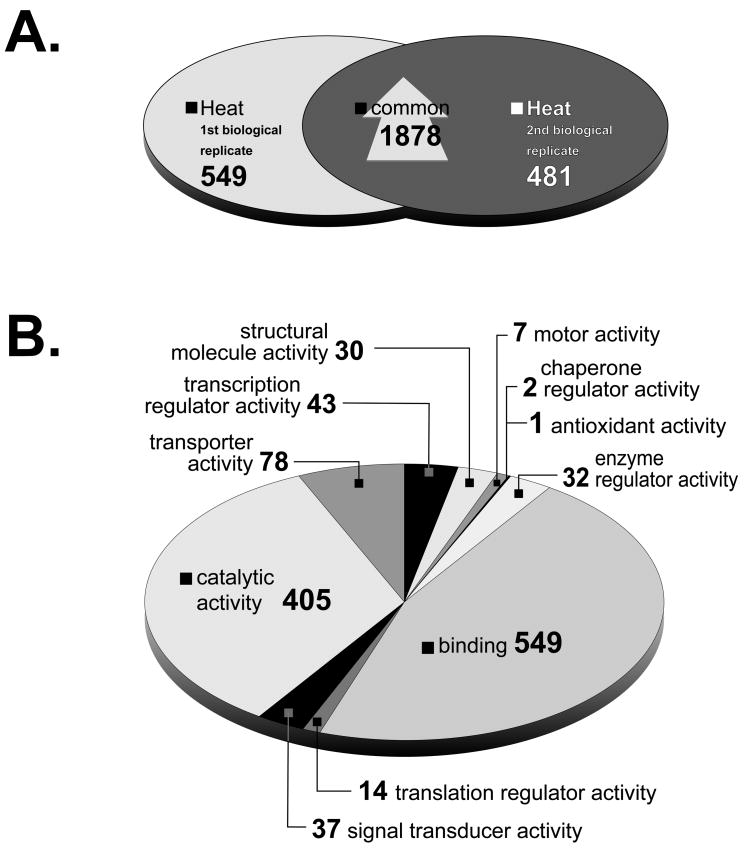
The heat-shock experiment was performed on two separate occasions. There was a significant overlap of 1,878 elements when comparing the microarray elements reporting a significant induction in their bound transcript between these two biological replicates. (Fig. 2A). Gene ontology analysis of molecular function was determined for 825 of these elements (Fig. 2B). Genes with E values less than, or equal to 1e-5 were considered as having a known database homolog (See supplemental material. All supplemental material including a complete list of induced (and down-regulated) genes can be found at http://biology.unm.edu/Cunningham/Papers/SP.htm). The majority of genes were determined to be involved in ‘binding’ (46%), ‘catalytic’ (34%) and ‘transporter’ (7%) activities.
Figure 2.
A. Venn diagram of elements whose bound transcript increased after schistosomes were exposed to heat-shock. A total of 2,427 and 2,359 elements were up-regulated two-fold at two time points in the 1st and 2nd biological replicates respectively, 1,878 of which were shared by both experiments. S. japonicum probes were excluded from the analysis. B. Gene ontology annotation following heat-shock. Predicted molecular functions of 825 elements induced in response to heat-shock. S. japonicum elements were excluded from gene ontology analysis. Elements with multiple annotated functions were included and could be in more than one category.
The ‘binding’ activity category, which encompasses 64% of all annotated S. mansoni genes, included those encoding HSP40 (TC16575), HSP70 (TC16542), HSP86 (TC16547) and an HSP70 associated DnaJ homolog (TC16732) [34,35]. Several genes encoding homologs of DNA break repair proteins were also included in this category. These proteins included MRE11A (TC11269), required for dsDNA break initiation and repair functions in yeast [36], Rad1 (TC13061) responsible for cell cycle checkpoint control in response to DNA damage and incomplete DNA replication [37] and endonuclease III-like protein 1 (TC19669) that functions in the repair of damaged DNA as a result of oxidative stress [38]. The ‘catalytic’ and ‘transporter’ categories included homologs of synovial apoptosis inhibitor (TC17764), whose expression renders cells resistant to cell death by apoptosis induced by endoplasmic reticulum (ER) related stress [39]; lactate dehydrogenase-B chain protein (TC16736) that is involved in anaerobic glycolysis and is induced in gastric mucosa of humans infected with Helicobacter pylori [40]; natural killer cell-enhancing factor B, which protects cells from oxidative stress [41]; double-strand DNA break repair protein Rad21 (TC8470), required for dsDNA break repair in yeast [42]; AMPactivated protein kinase (TC11310), induced in response to hypoxia, ischemia, and osmotic stress in cardiac and skeletal muscle tissue [43]; Derlin-2 (TC14077), induced in response to ER stress and ER associated degradation which is, itself, enhanced by heat-shock [44]; endoplasmic reticulum oxidoreductin-1 (TC18435), induced in response to heat, ethanol, and oxidative stressors in yeast [45]; DNA repair protein XRCC1 (TC8486), which functions in base excision repair and single-strand DNA break repair [46]; histone chaperone Asf1 (TC11916), which has a role in protecting against replicational stress [47] and P2X4 purinoceptor (TC9754) that has been shown to activate calcium ion influxes in sheer stressed endothelial cells [48].
3.2. Transcript induction after oxidative stress
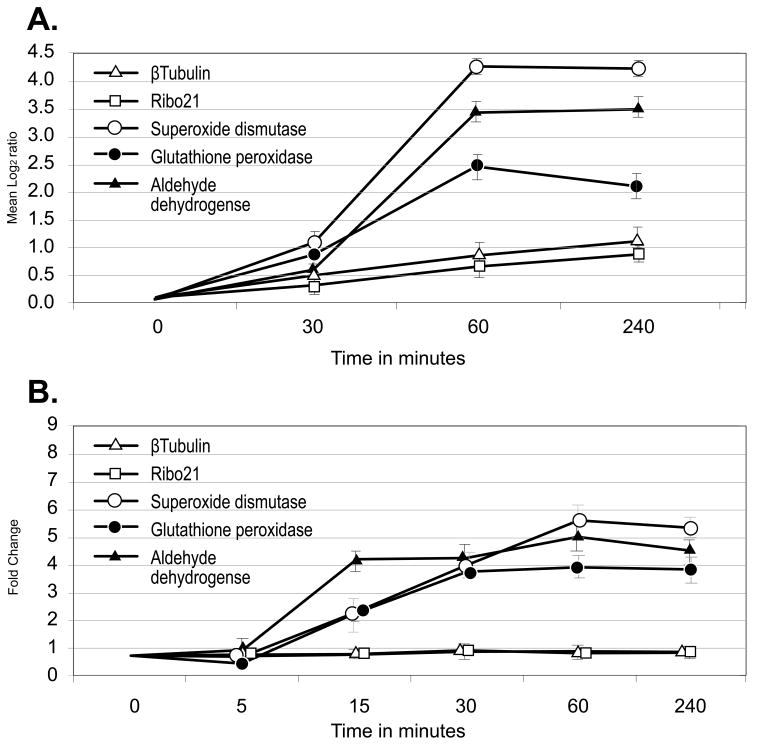
Reactive oxygen species (ROS) pose a constant threat to all organisms living in an aerobic environment due to their ability to damage nucleic acids, proteins, and lipids. Such ROS are produced as a natural by-product of cellular respiration; however, schistosomes also encounter host generated oxygen radicals aimed at killing the parasite. To determine how the transcriptome of schistosomes changes in response to oxidative stress, worms were examined 30, 60 and 240 min after exposure to 100 μM H2O2 in vitro. The significant induction of 3 homologs known from previously published work to be induced by the presence of ROS was confirmed in this analysis suggesting that the treatment was successful in inducing the anti-oxidative stress response (Fig. 3A). These were extracellular superoxide dismutase (TC16777) which protects tissues against oxygen toxicity in a variety of organisms [49], glutathione peroxidase (TC10653) which possesses hydrogen-peroxide oxidoreductase activity [50] and aldehyde dehydrogenase (TC13692) which is required for detoxification of oxygen radicals [51]. In contrast, the expression of transcripts encoding β-tubulin (TC8581) and Ribo21 (TC17090) were unaffected. These results were confirmed by qRT-PCR (Fig. 3B) where all three anti-ROS transcripts were significantly induced after 15 min and were reproducible in both biological replicates for each of the elements examined. Interestingly, in an analysis of gender associated gene expression using a different microarray from that employed in this paper, Fitzpatrick and colleagues have demonstrated that 5 oligonucleotides representing exon regions of extracellular superoxide dismutase were highly enriched in adult female worms while 4 oligonucleotides encoding cytosolic superoxide dismutase were equally expressed between both sexes [52]. Glutathione peroxidase was also significantly enriched in female worms suggesting perhaps that this enzyme and cytosolic superoxide dismutase may play a role in detoxification of the by-products of hemoglobin digestion as females greatly increase red blood cell consumption as egg production begins. In an examination of strain- and gender-associated differences in gene expression, Moertel and colleagues [53] also found extracellular superoxide dismutase to be significantly up-regulated in Chinese strain S. japonicum adult female compared to adult male worms, however, this induction was absent when both sexes of Philippine strain S. japonicum were compared.
Figure 3.
Comparison of microarray data and quantitative RT-PCR measurements for representative mRNAs from schistosomes exposed to oxidative stress. A. Microarray expression data are plotted as mean log2 Cy5/Cy3 as a function of time after stress. Log2 transformed data collected from array elements at 0, 0.5, 1.0, and 4.0 h were subtracted from log2 transformed data collected from 0 h arrays. Error bars represent the standard deviation of three measurements from biological replicates. Log2 > 1 is considered a significant increase in transcript abundance. B. qRT-PCR results plotted as fold change calculated using the 2− ΔΔCT method [29] as a function of time after stress. Error bars represent the standard deviation of three measurements. Fold change > 1 indicates an increase in transcript abundance.
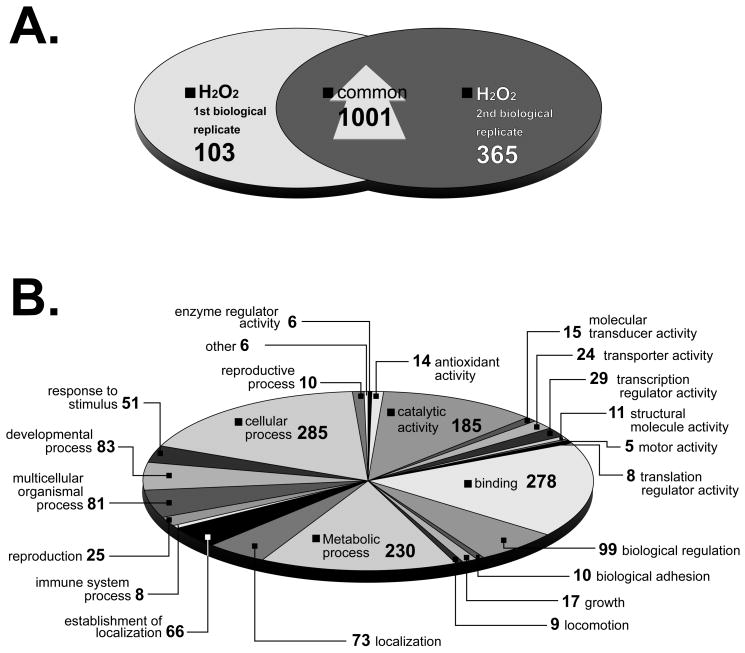
The oxidative stress experiment was performed on two separate occasions. A total of 1,001 microarray elements reported a significant increase in their bound transcript when both biological replicates were compared (Fig. 4A). Gene ontology was determined for 459 of these elements which fell into 25 categories (Fig. 4B). These included ‘antioxidant’, ‘transcription regulator’, and ‘response to stimulus’ activities. Genes with E values less than or equal to 1e–5 were considered as having a known database homolog (supplemental material) and some of these are discussed below. Fourteen transcripts were partitioned into the ‘antioxidant’ category. These included the superoxide dismutase, glutathione peroxidase and aldehyde dehydrogenase homologs described above, as well as L-2-hydroxyglutarate dehydrogenase (TC16490), an FAD-dependent enzyme catalyzing the oxidation of L-2-hydroxyglutarate to α-ketoglutarate [54]; ferredoxin NADP+ reductase (TC8516), which is involved in the oxidative stress response in E. coli and Salmonella enterica [55] and thioredoxin glutathione reductase (TC7521), a flavoenzyme expressed by schistosomes that bridges two detoxification pathways crucial for the parasite survival in the host, and which has been suggested as a potential therapeutic target [20,21].
Figure 4.
A. Venn diagram of elements that increased after schistosomes were exposed to oxidative stress (100 μM H2O2). A total of 1,104 and 1,366 elements were induced two-fold in two time points in 1st and 2nd biological replicates respectively, 1,001 of which were found in common. S. japonicum probes were excluded from the analysis. B. Gene ontology following oxidative stress. Predicted molecular functions of 459 elements induced in response to oxidative stress. S. japonicum elements were excluded from gene ontology analysis. Elements with multiple annotated functions were included and could be in more than one category.
There were 29 transcripts categorized as active in the ‘regulation of transcription’ that increased in abundance during oxidative stress. These include transcripts encoding G protein-coupled receptor kinase type 2 (TC17610), which has been shown to be active during oxidative stress in rats [56]; nuclear receptor superfamily member TR4/TR2 [57]; retinoic acid receptor RXR (TC17352) that is a regulator of genes important for homeostasis and development [58]; an insulin receptor tyrosine kinase (TC14300) that has been shown to function during oxidative stress [59]; epidermal growth factor receptor (TC14983) that functions in cell differentiation and development [60]; a mediator of RNA polymerase II transcription (TC15461), which associates with core polymerase subunits to form the RNA polymerase II holoenzyme that is essential for transcriptional regulation [61]; the YY1 transcription factor (TC18482) that directs histone deacetylases and histone acetyltransferases for promoter regulation [62]; the SNF2 family N-terminal domain containing protein (TC9884) that is the catalytic subunit of the SWI/SNF chromatin remodeling complex involved in transcriptional regulation [63]; a TAF13 RNA polymerase II, TATA box binding protein (TBP) (TC14686), involved in RNA polymerase II transcription initiation [64] and a transcriptional cofactor CA150 (TC7375) that regulates RNA Polymerase II elongation [65].
In the ‘response to stimulus’ category there were 51 transcripts that increased in abundance. These transcripts include those encoding DNA mismatch repair protein MSH2 (TC11333) that binds to DNA mismatches to initiate the mismatch repair process [66]; G/T mismatch-specific thymine DNA glycosylase (TC19118) that functions in excising thymine and uracil from G:T and G:U mismatches [67]; DNA-repair protein complementing Xeroderma pigmentosum group A protein (XPA) (TC11443) that functions during nucleotide excision repair [68]; a CREB-binding protein (TC13907) that modifies chromatin to an active relaxed state increasing transcription [69] and Rac GTPase, (TC7355) which has been shown to function in response to oxidative stress in fibroblast cells [70].
3.3. Transcripts induced in response to both heat and oxidative stress
Seventy-two transcripts were commonly induced in worms in response to both heat and oxidative stress. Forty-five of these had no known significant homologs. Of the remaining 27, there were several for which homologs have been previously reported to be active in stress responses (Table 1). A telomerase reverse transcriptase homolog (TC10026) is induced and translocated from the nucleus to the cytoplasm in human cells after oxidative stress [71]. It has been suggested that retinol dehydrogenase 13 (TC11759) protects mitochondria from oxidative stress in humans [72]. Yeast phosphoglycerate kinase (PGK; TC16651) has been shown to be induced following heat-shock, and to contain a heat-shock promoter element [73,74]. In addition, the PGK promoter has been shown to be induced following sub-lethal oxidative stress exposure [75]. Finally, alpha-glucosidase (TC16965) catalyzes the final step of glucose release during carbohydrate digestion in mammalian intestinal cells and serves as a target for blood glucose management in diabetic individuals [76].
Table 1.
Commonly induced genes in response to heat and oxidative stress
| Transcript* | Description |
|---|---|
| TC10026, TC11632, TC14826 | Homolog to endonuclease-reverse transcriptase |
| TC10560 | S. mansoni cathepsin B2 (smB2) protease |
| TC10640 | Homolog to serine/threonine phosphatase 1 regulatory subunit 10 |
| TC10763 | Homolog to ankyrin 3 (ANK-3) |
| TC11295 | Similar to yeast retrotransposable element |
| TC11425 | Homolog to sulfite oxidase |
| TC11759 | Homolog to retinol dehydrogenase |
| TC12705 | Homolog to aspartate carbamoyltransferase, CAD protein |
| TC13469 | Homolog to RNA-binding protein 40, snRNP 65 |
| TC14047 | Homolog to alpha-actinin, F-actin |
| TC14057 | Homolog to serine/threonine-protein kinase PRP4 |
| TC14202 | Homolog to zinc finger protein |
| TC14204 | Homolog to ATP-dependent RNA helicase DDX3Y |
| TC14312 | Homolog to DNA replication licensing factor MCM2 |
| TC16651 | Homolog to phosphoglycerate kinase |
| TC16843 | Homolog to plasminogen precursor |
| TC16965 | Homolog to alpha-glucosidase |
| TC17032 | Homolog to 1-AGP acyltransferase 2, (1-AGPAT 2) |
| TC17139 | Homolog to PRPP synthetase-associated protein 2 |
| TC18034 | Homolog to mitochondrial dicarboxylate carrier |
| TC7461 | Homolog to spliceosome RNA helicase BAT1, UAP56 |
| TC7721 | Homolog to oligosaccharyl transferase subunit STT3A |
| TC7745 | Homolog to spliceosome-associated protein 155 (SAP 155) |
| TC8694 | Homolog to high-affinity cationic amino acid transporter 1 (CAT-1) |
| TC9925 | Homolog to aminopeptidase P3 (APP3) |
| 45 transcripts | Unknown |
Transcript sequences were used to search Swissprot protein data base using NCBL blastx tool. Transcripts with blastx E values > 10 −5 were not included.
It has been previously observed that some organisms use similar molecular mechanism to respond to heat-shock and oxidative stress. For example, both stressors induce the expression of a variety of heat-shock proteins such as DnaK (HSP70 homolog) and DnaJ (HSP40 homolog) in E. coli [77], HSP40 in human cell line SH-SY5Y [78], as well as HSP70 and HSP28 in human breast carcinoma MCF-7/ADR cells [79]. It is conceivable that ROS-damaged proteins might induce the heat-shock response in order to repair, or degrade any mis-folded, and/or non-functional molecules. Alternatively, oxidative stress has also been shown to play a major role in heat-shock induced cell death, most likely due to the thermal instability of antioxidant proteins [80]. Finally, the deletion of antioxidant proteins, such as superoxide dismutase, or catalase, makes cells more sensitive to the lethal effects of heat, while the over-expression of these enzymes significantly increases the ability of cells to survive heat-shock [81].
It has been suggested that the identification of schistosome proteins that may help counter the host immune response, such as heat-shock proteins and antioxidants, could be potential chemotherapeutic targets. For example, HSP40 (major egg antigen) has been proposed as an ‘anti-pathology’ schistosomal vaccine candidate due to its ability to stimulate IL-5 and IL-10 but not IL-4 and IL-13. Such a cytokine profile is associated with reduced collagen deposition, decreased fibrosis and granuloma inhibition formation which are hallmarks of the ultimately destructive host immune response against schistosome eggs [82]. Shalaby and colleagues [83] were able to achieve significant reductions in worm burden by DNA vaccination against enzymes such as superoxide dismutase and glutathione peroxidase. More recently, thioredoxin glutathione reductase, which has been shown to be essential for schistosome survival during oxidative stress, has been exploited as a target for the development of a new generation of anti-schistosomal compounds [20,21].
The work presented in this manuscript focused on the changes in gene expression that occur in both sexes in response to temperature and oxidative stress. We believe that the transcripts and proteins that are induced by these stressors should make excellent candidates as targets for future novel anti-schistosomal therapeutics. Interestingly, there is also some indication from the work of Fitzpatrick and colleagues [52] and Moertel and colleagues [53] that there may be differences in the capacity of adult male and female worms to respond to stress. This would serve not only to highlight the dioecious nature of the adult parasite but also suggest another avenue of investigation to identify sex-specific therapeutics.
Acknowledgments
We thank Professor Sam Loker and Dr. Coen Adema of the Dept. of Biology, University of New Mexico, for valuable discussions about this work. We would also like to thank Geoffrey N. Gobert of the Queensland Institute of Medical Research and Australian Centre for International Health and Nutrition, Brisbane, Qld, Australia for his permission to use the microarrays employed in this study and Dr. Karl F. Hoffmann, University of Cambridge, UK for generously providing the microarray protocols used in this paper. Technical support was supplied by the Molecular Biology Core Facility of the Dept. of Biology, University of New Mexico. This work was funded by NIH grant number 1P20RR18754 from the Institute Development Award (IDeA) Program of the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77:41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000;121:S23–S38. doi: 10.1017/s0031182000006491. [DOI] [PubMed] [Google Scholar]
- 3.Guyatt HL, Brooker S, Kihamia CM, Hall A, Bundy DAP. Evaluation of efficacy of school-based antihelmintic treatments against anaemia in children in the United Republic of Tanzania. Bull World Health Organ. 2001;79:695–703. [PMC free article] [PubMed] [Google Scholar]
- 4.Ezeamama AE, Friedman JF, Acosta LP, Bellinger DC, Langdon GC, Manalo DL, Olveda RM, Kurtis JD, McGarvey ST. Helminth infection and cognitive impairment among Filipino children. Am J Trop Med Hyg. 2005;72:540–48. [PMC free article] [PubMed] [Google Scholar]
- 5.Moore DV, Sandground JH. The relative egg producing capacity of Schistosoma mansoni and Schistosoma japonicum. Am J Trop Med Hyg. 1956;5:831–40. doi: 10.4269/ajtmh.1956.5.831. [DOI] [PubMed] [Google Scholar]
- 6.Gonnert R, Andrews P. Praziquantel, a new broad-spectrum antischistosomal agent. Z Parasitenkd. 1977;52:129–50. doi: 10.1007/BF00389899. [DOI] [PubMed] [Google Scholar]
- 7.Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. Schistosoma mansoni- Chemotherapy of infections of different ages. Exp Parasitol. 1986;61:294–303. doi: 10.1016/0014-4894(86)90184-0. [DOI] [PubMed] [Google Scholar]
- 8.Pica-Mattoccia L, Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int J Parasitol. 2004;34:527–33. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Doenhoff MJ, Kusel JR, Coles GC, Cioli D. Resistance of Schistosoma mansoni to praziquantel: is there a problem? Trans R Soc Trop Med Hyg. 2002;96:465–69. doi: 10.1016/s0035-9203(02)90405-0. [DOI] [PubMed] [Google Scholar]
- 10.Cioli D, Botros SS, Wheatcroft-Francklow K, Mbaye A, Southgate V, Tchuente LAT, Pica-Mattoccia L, Troiani AR, El-Din SHS, Sabra ANA, Albin J, Engels D, Doenhoff MJ. Determination of ED50 values for praziquantel in praziquantelresistant and -susceptible Schistosoma mansoni isolates. Int J Parasitol. 2004;34:979–87. doi: 10.1016/j.ijpara.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Gryseels B, Mbaye A, De Vlas SJ, Stelma FF, Guisse F, Van Lieshout L, Faye D, Diop M, Ly A, Tchuem-Tchuente LA, Engels D, Polman K. Are poor responses to praziquantel for the treatment of Schistosoma mansoni infections in Senegal due to resistance? An overview of the evidence. Trop Med Int Health. 2001;6:864–73. doi: 10.1046/j.1365-3156.2001.00811.x. [DOI] [PubMed] [Google Scholar]
- 12.Cross JH, Vieira P, Miranda HP, Cerqueira M, Delgado MD, Coelho H, Antunes D, de Costa JMC. Latent schistosomiasis in Portuguese solders. Mil Med. 2007;172:144–46. doi: 10.7205/milmed.172.2.144. [DOI] [PubMed] [Google Scholar]
- 13.Harris ARC, Russell RJ, Charters AD. A review of schistosomiasis in immigrants in Western Australia, demonstrating the unusual longevity of Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1984;78:385–88. doi: 10.1016/0035-9203(84)90129-9. [DOI] [PubMed] [Google Scholar]
- 14.Bottieau E, Clerinx J, de Vega MR, Van den Enden E, Colebunders R, Van Esbroeck M, Vervoort T, Van Gompel A, Van den Ende J. Imported Katayama fever: Clinical and biological features at presentation and during treatment. J Infect. 2006;52:339–45. doi: 10.1016/j.jinf.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Verjovski-Almeida S, DeMarco R, Martins EAL, Guimaraes PEM, Ojopi EPB, Paquola ACM, Piazza JP, Nishiyama MY, Kitajima JP, Adamson RE, Ashton PD, Bonaldo MF, Coulson PS, Dillon GP, Farias LP, Gregorio SP, Ho PL, Leite RA, Malaquias LCC, Marques RCP, Miyasato PA, Nascimento ALTO, Ohlweiler FP, Reis EM, Ribeiro MA, Sa RG, Stukart GC, Soares MB, Gargioni C, Kawano T, Rodrigues V, Madeira AMBN, Wilson RA, Menck CFM, Setubal JC, Leite LCC, Dias-Neto E. Transcriptome analysis of the acoelomate human parasite Schistosoma mansoni. Nat Genet. 2003;35:148–57. doi: 10.1038/ng1237. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira HD, Schumacher RI, Meneghini R. Lower intracellular hydrogen peroxide levels in cells overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci USA. 1998;95:7872–75. doi: 10.1073/pnas.95.14.7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–55. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lillig CH, Holmgren A. Thioredoxin and related molecules - From biology to health and disease. Antioxid Redox Signal. 2007;9:25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 19.Alger HM, Sayed AA, Stadecker MJ, Williams DL. Molecular and enzymatic characterisation of Schistosoma mansoni thioredoxin. Int J Parasitol. 2002;32:1285–92. doi: 10.1016/s0020-7519(02)00108-x. [DOI] [PubMed] [Google Scholar]
- 20.Kuntz AN, Davioud-Charvet E, Sayed AA, Califf LL, Dessolin J, Arner ESJ, Williams DL. Thioredoxin glutathione reductase from Schistosoma mansoni: An essential parasite enzyme and a key drug target. PloS Med. 2007;4:1071–86. doi: 10.1371/journal.pmed.0040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayed AA, Simeonov A, Thomas CJ, Inglese J, Austin CP, Williams DL. Identification of oxadiazoles as new drug leads for the control of schistosomiasis. Nat Med. 2008;14:407–12. doi: 10.1038/nm1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sayed AA, Cook SK, Williams DL. Redox balance mechanisms in Schistosoma mansoni rely on peroxiredoxins and albumin and implicate peroxiredoxins as novel drug targets. J Biol Chem. 2006;281:17001–10. doi: 10.1074/jbc.M512601200. [DOI] [PubMed] [Google Scholar]
- 23.Novoradovskaya N, Whitfield ML, Basehore LS, Novoradovsky A, Pesich R, Usary J, Karaca M, Wong WK, Aprelikova O, Fero M, Perou CM, Botstein D, Braman J. Universal Reference RNA as a standard for microarray experiments. BMC Genomics. 2004;5:20. doi: 10.1186/1471-2164-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petalidis L, Bhattacharyya S, Morris GA, Collins VP, Freeman TC, Lyons PA. Global amplification of mRNA by template-switching PCR: linearity and application to microarray analysis. Nucleic Acids Res. 2003;31:e142. doi: 10.1093/nar/gng142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gobert GN, McInnes R, Moertel L, Nelson C, Jones MK, Hu W, McManus DP. Transcriptomics tool for the human Schistosoma blood flukes using microarray gene expression profiling. Exp Parasitol. 2006;114:160–72. doi: 10.1016/j.exppara.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Aragon AD, Quinones GA, Thomas EV, Roy S, Werner-Washburne M. Release of extraction-resistant mRNA in stationary phase Saccharomyces cerevisiae produces a massive increase in transcript abundance in response to stress. Genome Biol. 2006;7:R9. doi: 10.1186/gb-2006-7-2-r9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vêncio RZN, Koide T. HTself: Self-self based statistical test for low replication microarray studies. DNA Res. 2005;12:211–14. doi: 10.1093/dnares/dsi007. [DOI] [PubMed] [Google Scholar]
- 28.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–76. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2(T)(−Delta Delta C) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Nene V, Dunne DW, Johnson KS, Taylor DW, Cordingley JS. Sequence and expression of a major egg antigen from Schistosoma mansoni - Homologies to heat-shock proteins and alpha-crystallins. Mol Biochem Parasitol. 1986;21:179–88. doi: 10.1016/0166-6851(86)90021-6. [DOI] [PubMed] [Google Scholar]
- 31.Johnson KS, Wells K, Bock JV, Nene V, Taylor DW, Cordingley JS. The 86- Kilodalton antigen from Schistosoma mansoni is a heat-shock protein homologous to yeast HSP90. Mol Biochem Parasitol. 1989;36:19–28. doi: 10.1016/0166-6851(89)90196-5. [DOI] [PubMed] [Google Scholar]
- 32.Neumann S, Ziv E, Lantner F, Schechter I. Regulation of HSP70 gene-expression during the life-cycle of the parasitic helminth Schistosoma mansoni. Eur J Bioch. 1993;212:589–96. doi: 10.1111/j.1432-1033.1993.tb17697.x. [DOI] [PubMed] [Google Scholar]
- 33.Chai M, McManus DP, McInnes R, Moertel L, Tran M, Loukas A, Jones MK, Gobert GN. Transcriptome profiling of lung schistosomula, in vitro cultured schistosomula and adult Schistosoma japonicum. Cell Mol Life Sci. 2006;63:919–29. doi: 10.1007/s00018-005-5578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suh WC, Burkholder WF, Lu CZ, Zhao X, Gottesman ME, Gross CA. Interaction of the HSP70 molecular chaperone, DnaK, with its cochaperone DnaJ. Proc Natl Acad Sci USA. 1998;95:15223–8. doi: 10.1073/pnas.95.26.15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–82. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 36.Nairz K, Klein F. mre11S - a yeast mutation that blocks double-strand-break processing and permits nonhomologous synapsis in meiosis. Genes Dev. 1997;11:2272–90. doi: 10.1101/gad.11.17.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bluyssen HA, van Os RI, Naus NC, Jaspers I, Hoeijmakers JHJ, de Klein A. A human and mouse homolog of the Schizosaccharomyces pombe rad1(+) cell cycle checkpoint control gene. Genomics. 1998;54:331–7. doi: 10.1006/geno.1998.5582. [DOI] [PubMed] [Google Scholar]
- 38.Akbari M, Otterlei M, Pena-Diaz J, Krokan HE. Different organization of base excision repair of uracil in DNA in nuclei and mitochondria and selective upregulation of mitochondrial uracil-DNA glycosylase after oxidative stress. Neuroscience. 2007;145:1201–12. doi: 10.1016/j.neuroscience.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Kaneko M, Ishiguro M, Niinuma Y, Uesugi M, Nomura Y. Human HRD1 protects against ER stress-induced apoptosis through ER-associated degradation. FEBS Lett. 2002;532:147–52. doi: 10.1016/s0014-5793(02)03660-8. [DOI] [PubMed] [Google Scholar]
- 40.Baek HY, Lim JW, Kim H, Kim JM, Kim JS, Jung HC, Kim KH. Oxidative-stressrelated proteome changes in Helicobacter pylori-infected human gastric mucosa. Biochem J. 2004;379:291–9. doi: 10.1042/BJ20031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shau HY, Kim AT, Hedrick CC, Lusis AJ, Tompkins C, Finney R, Leung DW, Paglia DE. Endogenous natural killer enhancing factor-B increases cellular resistance to oxidative stresses. Free Radic Biol Med. 1997;22:497–507. doi: 10.1016/s0891-5849(96)00372-3. [DOI] [PubMed] [Google Scholar]
- 42.Birkenbihl RP, Subramani S. Cloning and Characterization of Rad21 an Essential Gene of Schizosaccharomyces pombe involved in DNA Double-Strand- Break Repair. Nucleic Acids Res. 1992;20:6605–11. doi: 10.1093/nar/20.24.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujii N, Jessen N, Goodyear LJ. AMP-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab. 2006;291:E867– E877. doi: 10.1152/ajpendo.00207.2006. [DOI] [PubMed] [Google Scholar]
- 44.Han S, Liu Y, Chang A. Cytoplasmic hsp70 promotes ubiquitination for endoplasmic reticulum-associated degradation of a misfolded mutant of the yeast plasma membrane ATPase, PMA1. J Biol Chem. 2007;282:26140–9. doi: 10.1074/jbc.M701969200. [DOI] [PubMed] [Google Scholar]
- 45.Takemori Y, Sakaguchi A, Matsuda S, Mizukami Y, Sakurai H. Stress-induced transcription of the endoplasmic reticulum oxidoreductin gene ERO1 in the yeast Saccharomyces cerevisiae. Mol Genet Genomics. 2006;275:89–96. doi: 10.1007/s00438-005-0065-9. [DOI] [PubMed] [Google Scholar]
- 46.Mitra S, Izumi T, Boldogh I, Bhakat KK, Hill JW, Hazra TK. Choreography of oxidative damage repair in mammalian genomes. Free Radic Biol Med. 2002;33:15–28. doi: 10.1016/s0891-5849(02)00819-5. [DOI] [PubMed] [Google Scholar]
- 47.Groth A, Ray-Gallet D, Quivy JP, Lukas J, Bartek J, Almouzni G. Human Asf1 regulates the flow of S phase histories during replicational stress. Mol Cell. 2005;17:301–11. doi: 10.1016/j.molcel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto K, Korenaga R, Kamiya A, Ando J. Fluid shear stress activates Ca2+ influx into human endothelial cells via P2X4 purinoceptors. Circ Res. 2000;87:385–91. doi: 10.1161/01.res.87.5.385. [DOI] [PubMed] [Google Scholar]
- 49.Henkle-Duhrsen K, Kampkotter A. Antioxidant enzyme families in parasitic nematodes. Mol Bioch Parasitol. 2001;114:129–42. doi: 10.1016/s0166-6851(01)00252-3. [DOI] [PubMed] [Google Scholar]
- 50.Maiorino M, Roche C, Kiess M, Koenig K, Gawlik D, Matthes M, Naldini E, Pierce R, Flohe L. A selenium-containing phospholipid-hydroperoxide glutathione peroxidase in Schistosoma mansoni. Eur J Biochem. 1996;238:838–44. doi: 10.1111/j.1432-1033.1996.0838w.x. [DOI] [PubMed] [Google Scholar]
- 51.Vasiliou V, Pappa A, Estey T. Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug Metab Rev. 2004;36:279–99. doi: 10.1081/dmr-120034001. [DOI] [PubMed] [Google Scholar]
- 52.Fitzpatrick JM, Johnston DA, Williams GW, Williams DJ, Freeman TC, Dunne DW, Hoffmann KF. An oligonucleotide microarray for transcriptome analysis of Schistosoma mansoni and its application/use to investigate gender-associated gene expression. Mol Biochem Parasitol. 2005;141:1–13. doi: 10.1016/j.molbiopara.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Moertel L, McManus DP, Piva TJ, Young L, McInnes RL, Gobert GN. Oligonucleotide microarray analysis of strain- and gender-associated gene expression in the human blood fluke, Schistosoma japonicum. Mol Cell Probes. 2006;20:280–9. doi: 10.1016/j.mcp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Rzem R, Veiga-da-Cunha M, Noel G, Goffette S, Nassogne MC, Tabarki B, Scholler C, Marquardt T, Vikkula M, Van Schaftingen E. A gene encoding a putative FAD-dependent L-2-hydroxyglutarate dehydrogenase is mutated in L- 2-hydroxyglutaric aciduria. Proc Natl Acad Sci USA. 2004;101:16849–54. doi: 10.1073/pnas.0404840101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pomposiello PJ, Demple B. Identification of SoxS-regulated genes in Salmonella enterica serovar Typhimurium. J Bacteriol. 2000;182:23–9. doi: 10.1128/jb.182.1.23-29.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banday AA, Lokhandwala MF. Oxidative stress reduces renal dopamine D1 receptor-G(q/11)alpha G protein-phospholipase C signaling involving G protein-coupled receptor kinase 2. Am J Physiol Renal Physiol. 2007;293:F306–F315. doi: 10.1152/ajprenal.00108.2007. [DOI] [PubMed] [Google Scholar]
- 57.Hu R, Wu WJ, Niles EG, LoVerde PT. SmTR2/4, a Schistosoma mansoni homolog of TR2/TR4 orphan nuclear receptor. Int J Parasitol. 2006;36:1113–22. doi: 10.1016/j.ijpara.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Fantappie MR, Freebern WJ, Osman A, LaDuca J, Niles EG, LoVerde PT. Evaluation of Schistosoma mansoni retinoid X receptor (SmRXR1 and SmRXR2) activity and tissue distribution. Mol Biochem Parasitol. 2001;115:87–99. doi: 10.1016/s0166-6851(01)00274-2. [DOI] [PubMed] [Google Scholar]
- 59.Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stressinduced insulin resistance. Antioxid Redox Signal. 2005;7:1040–52. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 60.Vicogne J, Cailliau K, Tulasne D, Browaeys E, Yan YT, Fafeur V, Vilain JP, Legrand D, Trolet J, Dissous C. Conservation of epidermal growth factor receptor function in the human parasitic helminth Schistosoma mansoni. J Biol Chem. 2004;279:37407–14. doi: 10.1074/jbc.M313738200. [DOI] [PubMed] [Google Scholar]
- 61.Kornberg RD. Mediator and the mechanism of transcriptional activation. Trends Biochem Sci. 2005;30:235–9. doi: 10.1016/j.tibs.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 62.Thomas MJ, Seto E. Unlocking the mechanisms of transcription factor YY1: are chromatin modifying enzymes the key? Gene. 1999;236:197–208. doi: 10.1016/s0378-1119(99)00261-9. [DOI] [PubMed] [Google Scholar]
- 63.Flanagan JF, Peterson CL. A role for the yeast SWI/SNF complex in DNA replication. Nucleic Acids Res. 1999;27:2022–8. doi: 10.1093/nar/27.9.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tora L. A unified nomenclature for TATA box binding protein (TBP)-associated factors (TAFs) involved in RNA polymerase II transcription. Genes Dev. 2002;16:673–5. doi: 10.1101/gad.976402. [DOI] [PubMed] [Google Scholar]
- 65.Sune C, Hayashi T, Liu Y, Lane WS, Young RA, GarciaBlanco MA. CA150, a nuclear protein associated with the RNA polymerase II holoenzyme, is involved in Tat-activated human immunodeficiency virus type 1 transcription. Mol Cell Biol. 1997;17:6029–39. doi: 10.1128/mcb.17.10.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kijas AW, Studamire B, Alani E. msh2 separation of function mutations confer defects in the initiation steps of mismatch repair. J Mol Biol. 2003;331:123–38. doi: 10.1016/s0022-2836(03)00694-6. [DOI] [PubMed] [Google Scholar]
- 67.Neddermann P, Jiricny J. The purification of a mismatch-specific thymine-DNA glycosylase from HeLa-cells. J Biol Chem. 1993;268:21218–24. [PubMed] [Google Scholar]
- 68.Iakoucheva LM, Kimzey AL, Masselon CD, Smith RD, Dunker AK, Ackerman EJ. Aberrant mobility phenomena of the DNA repair protein XPA. Protein Sci. 2001;10:1353–62. doi: 10.1110/ps.ps.40101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bertin B, Oger F, Cornette J, Caby S, Noel C, Capron M, Fantappie MR, Rumjanek FD, Pierce RJ. Schistosoma mansoni CBP/p300 has a conserved domain structure and interacts functionally with the nuclear receptor SmFtz-F1. Mol Biochem Parasitol. 2006;146:180–91. doi: 10.1016/j.molbiopara.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 70.Embade N, Valeron PF, Aznar S, Lopez-Collazo E, Lacal JC. Apoptosis induced by Rac GTPase correlates with induction of FasL and ceramides production. Mol Biol Cell. 2000;11:4347–58. doi: 10.1091/mbc.11.12.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haendeler J, Hoffmann J, Brandes RP, Zeiher AM, Dimmeler S. Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphorylation of tyrosine 707. Mol Cell Biol. 2003;23:4598–610. doi: 10.1128/MCB.23.13.4598-4610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Belyaeva OV, Chetyrkin SV, Clark AL, Kostereva NV, SantaCruz KS, Chronwall BM, Kedishvili NY. Role of microsomal retinol/sterol dehydrogenase-like short-chain dehydrogenases/reductases in the oxidation and epimerization of 3 alpha-hydroxysteroids in human tissues. Endocrinology. 2007;148:2148–56. doi: 10.1210/en.2006-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Piper PW, Curran B, Davies MW, Lockheart A, Reid G. Transcription of the phosphoglycerate kinase gene of Saccharomyces cervisiae increase when fermentative cultures are stressed by heat-shock. Eur J Biochem. 1986;161:525–31. doi: 10.1111/j.1432-1033.1986.tb10474.x. [DOI] [PubMed] [Google Scholar]
- 74.Piper PW, Curran B, Davies MW, Hirst K, Lockheart A, Ogden JE, Stanway CA, Kingsman AJ, Kingsman SM. A heat-shock element in the phosphoglycerate kinase gene promoter of yeast. Nucleic Acids Res. 1988;16:1333–48. doi: 10.1093/nar/16.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vassallo N, Galea DR, Bannister WH, Balzan R. Stimulation of yeast 3- phosphoglycerate kinase gene promoter by paraquat. Biochem Biophys Res Commun. 2000;270:1036–40. doi: 10.1006/bbrc.2000.2566. [DOI] [PubMed] [Google Scholar]
- 76.Scheen AJ. Is there a role for alpha-glucosidase inhibitors in the prevention of type 2 diabetes mellitus? Drugs. 2003;63:933–51. doi: 10.2165/00003495-200363100-00002. [DOI] [PubMed] [Google Scholar]
- 77.Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5:781–91. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- 78.Ding QX, Keller JN. Proteasome inhibition in oxidative stress neurotoxicity: implications for heat-shock proteins. J Neurochem. 2001;77:1010–17. doi: 10.1046/j.1471-4159.2001.00302.x. [DOI] [PubMed] [Google Scholar]
- 79.Lee YJ, Corry PM. Metabolic oxidative stress-induced HSP70 gene expression is mediated through SAPK pathway - Role of Bcl-2 and c-Jun NH2-terminal kinase. J Biol Chem. 1998;273:29857–63. doi: 10.1074/jbc.273.45.29857. [DOI] [PubMed] [Google Scholar]
- 80.Davidson JF, Whyte B, Bissinger PH, Schiestl RH. Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:5116–21. doi: 10.1073/pnas.93.10.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Benov L, Fridovich I. Superoxide-dismutase protects against aerobic heat-shock in Escherichia coli. J Bacteriol. 1995;177:3344–6. doi: 10.1128/jb.177.11.3344-3346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abouel-Nour MF, Lotfy M, Attallah AM, Doughty BL. Schistosoma mansoni major egg antigen Smp40: molecular modeling and potential immunoreactivity for anti-pathology vaccine development. Mem Inst Oswaldo Cruz. 2006;101:365–72. doi: 10.1590/s0074-02762006000400004. [DOI] [PubMed] [Google Scholar]
- 83.Shalaby KA, Yin L, Thakur A, Christen L, Niles EG, LoVerde PT. Protection against Schistosoma mansoni utilizing DNA vaccination with genes encoding Cu/Zn cytosolic superoxide dismutase, signal peptide-containing superoxide dismutase and glutathione peroxidase enzymes. Vaccine. 2003;22:130–6. doi: 10.1016/s0264-410x(03)00535-8. [DOI] [PubMed] [Google Scholar]