Abstract
Since utilization of menaquinone in the electron transport system is a characteristic of Gram-positive organisms, the 1,4-dihydroxy-2-naphthoate prenyltransferase (MenA) inhibitors 1a and 2a act as selective antibacterial agents against organisms such as methicillin-resistant Stapylococcus aureus (MRSA), Staphylococcus epidermidis (MRSE), and Mycobacterium spp. Growth of drug-resistant Gram-positive organisms was sensitive to the MenA inhibitors, indicating that menaquinone synthesis is a valid new drug target in Gram-positive organisms.
Antimicrobial resistance of pathogens is a global problem. Each year worldwide, more than 11 million people die from the major infectious killers (i.e., MDRa tuberculosis, malaria, HIV, diarrhea diseases, and pneumonia).1 The increasing drug resistance among Gram-positive bacteria is a significant problem because they are responsible for one-third of nosocomial infections; drug resistance in Gram-positive organisms (i.e., staphylococci, pneumococci, vancomycin resistance in enterococci, and mycobacteria) have achieved prominence in the past 15 years. Methicillin-resistant S. aureus (MRSA) is one of most frequent nosocomial pathogens in developed countries.2 In addition, Mycobacterium tuberculosis (Mtb) is responsible for nearly 2 million deaths annually and one-third of the world population is infected with latent Mtb. In particular, people who are malnourished or have HIV-AIDS are susceptible to TB infection. Moreover, the emergence multidrug-resistant strains of Mtb (MDR-TB) seriously threatens TB control and prevention efforts.3 The results of over 10 years of screening of strains and molecular targets (existing and new) from traditional product sources (randomly generated library molecules, secondary metabolites, and drug libraries) have been disappointing.4 Therefore, identification of new molecular targets and mechanisms of action that involved identifying essential, ubiquitous bacterial genes in pathogens that are prokaryote and eukaryote selective to prevent side effects in the host has been studied.
The lipid-soluble electron carriers (lipoquinones) occupy a central and essential role in electron transport coupled ATP synthesis. The lipoquinones involved in the respiratory chains of bacteria consist of menaquinones and ubiquinones. From the taxonomic studies it is evident that a majority of Gram-positive bacteria including Mycobacterium spp. utilize only menaquinone in their electron transport systems,5 and menaquinone biosynthesis is essential for survival of nonfermenting Gram-positive bacteria.6 On the other hand, Gram-negative organisms such as E. coli utilize ubiquinone (CoQ) under aerobic conditions and utilize menaquinone under anaerobic conditions. Moreover, the electron transport chain in humans does not utilize menaquinone.7 Therefore, inhibitors of menaquinone biosynthesis have great potential for the development of novel and selective drugs against MDR Gram-positive pathogens.8 However, no study on the development of inhibitors for menaquinone biosynthetic enzymes has been reported. In this communication, we report that inhibition of 1,4-dihydroxy-2-naphthoate prenyltransferase (MenA), which catalyzes a formal decarboxylative prenylation of 1,4-dihydroxy-2-napthoate (DHNA) (Figure 1),9 showed significant growth inhibitory activities against drug-resistant Gram-positive bacteria.
Figure 1.
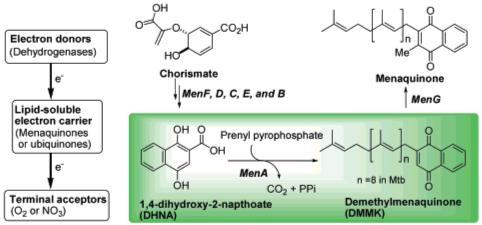
Schematic bacterial electron transport chain and menaquinone biosynthesis.
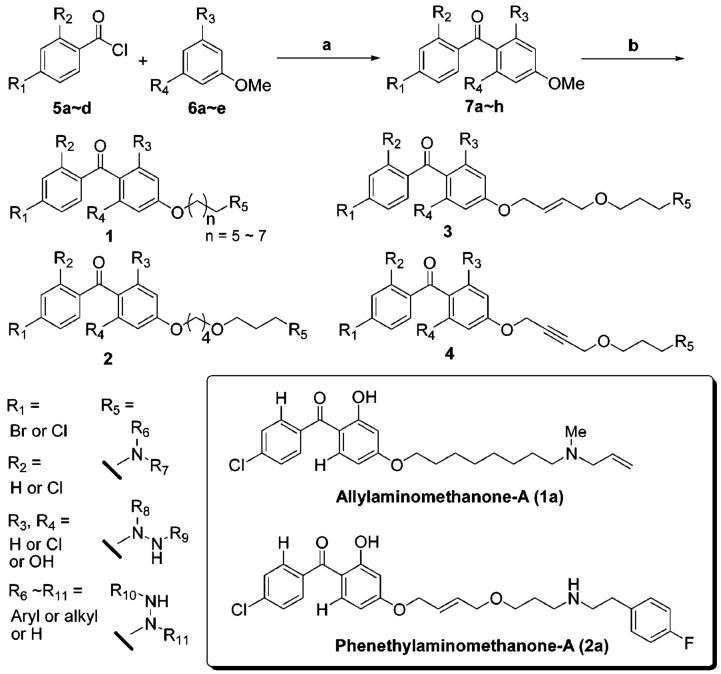
The MenA activity was characterized using membrane fractions prepared from M. tuberculosis as previously described.11 MenA is predicted to have five transmembrane segments, and there are highly conserved Asp residues that would be located in the inner-plasma membrane.12 The activity is absolutely dependent on the presence of the divalent cations such as Mg2+. Thus, it is likely that such divalent cations form ion pairs with Asp residues existing in the catalytic site of MenA. On the basis of the observation of this enzymatic activity and the structure of the MenA product, demethylmenaquinone (DMMK), we designed tertiary or secondary amine or hydrazine-containing DMMK mimics (1) in hope that the amine moiety would interact with Asp residue(s) directly or through the divalent cation(s) in the active site and (2) in which the chemically unstable 1,4-quinone system is replaced with the hydrophobicly substituted benzophenones. As illustrated in Scheme 1, the designed DMMK mimics were synthesized efficiently in four to six steps including (1) Friedel-Crafts acylation, (2) deprotection, (3) alkylation(s), (4) bromination, and (5) amination reactions.
Scheme 1.
Generation of a Library of Molecules in Solution10,a
a Reagents and conditions: (a) AlCl3, PhNO2 (75-90%); (b) (i) 48% HBr, AcOH (90%); (ii) 1,5-dibromopentane or 1,6-dibromohexane or 1,7-dibromoheptane or 1,8-dibromooctane, K2CO3, DMF (for 1) (80-95%); 1,4-dibromobutane, K2CO3, DMF; 1,3-propanediol, NaH, DMF; CBr4, PPh3, CH2Cl2 (for 2) (65%); 1,4-dibromobutene, K2CO3, DMF; 1,3-propanediol, NaH, DMF; CBr4, PPh3, CH2Cl2 (for 3) (65%); 1,4-dibromobutyne, K2CO3, DMF; 1,3-propanediol, NaH, DMF; CBr4, PPh3, zCH2Cl2 (for 4) (65%); (iii) R5 (primary or secondary amines or hydrazines), NaHCO3, DMF (50-98%); (iv) TFA, CH2Cl2 (for Boc-protected R5) (100%).
We have synthesized 100 molecules in solution, and the library of molecules was evaluated in enzymatic assays in vitro (IC50) against Mtb MenA11 and in mycobacterial growth assays (MIC). More than 18 molecules exhibited MenA IC50 and MIC values of less than 20 μM, and in all cases the MIC value was in good agreement with the IC50 value. From these preliminary screenings it was shown that the shorter length of linker (C5-C7 in 1) between the phenolic oxygen and the nitrogen atom decreased the ability to inhibit MenA and the efficacy of growth inhibition. In addition, the structure of amine or hydrazine significantly influences the activity; α-substituted amine or bulky tertiary amine containing molecules did not show MenA inhibitory activity at lower concentrations.10 Identification of the effective substitution pattern (R1, R2, R3, and R4) in benzophenone moiety requires extensive SAR studies; however, the hydroxy group on R3 (R3 = OH in 1 and 3) seems to be superior to the others (R3 = H or Cl) regardless of the structure of linker.13
Two molecules 1a and 2a, named allylaminomethanone-A and phenethylaminomethanone-A, respectively, showed MIC values of 1.5 and 12.5 μg/mL against Mtb (H37Rv, a common laboratory strain), respectively. We resynthesized 1a and 2a and determined MICs (μg/mL) against a variety of species and strains of Gram-positive (entries 1-19 in Table 1) and Gram-negative bacteria (entries 20-23 in Table 1). As summarized in Table 1, only Gram-positive bacterial growth was inhibited by 1a (for Mtb) and 2a (others), supporting the hypothesis that menaquinone synthesis is the target of these molecules.14 In addition, growth of drug-resistant Gram-positive organisms was sensitive to the MenA inhibitors (entries 2, 3, 14, 10-17), indicating that MenA is likely to be a valid drug target in Gram-positive pathogens involved in emerging diseases.
Table 1.
MICs of 1a, 2a, and Representative Antibacterial Agents (Clinically Used) for Gram-Positive Bacteria Including Mycobacterium spp
| MIC (μg/mL)a |
|||||||
|---|---|---|---|---|---|---|---|
| entry | species and strain | 1ab | 2ac | RFPd | INHd | VCMd | LZDd |
| 1 | M. tuberculosis H37Rv | 1.5 | 12.5 | 0.2 | 0.1 | ||
| 2 | M. tuberculosis H37Rv INHr | 1.5 | 0.2 | >25 | |||
| 3 | M. tuberculosis H37Rv RFPr | 1.5 | >25 | 0.05 | |||
| 4 | M. bovis BCG Tokyo | 3.1 | 0.1 | 0.1 | |||
| 5 | M. avium Flamingo | 6.3 | 3.1 | >25 | |||
| 6 | M. intracellulare ATCC15984 | 6.3 | 3.1 | 12.5 | |||
| 7 | M. aurum | 6.3 | 0.78 | 6.3 | |||
| 8 | M. fortuitum NIHJ1615 | 12.5 | >25 | 6.3 | |||
| 9 | M. smegmatis Takeo | 12.5 | >25 | 12.5 | |||
| 10 | MRSA high-resistance | 4 | 1 | 2 | |||
| 11 | VRSA 70 | 4 | 0.5 | 2 | |||
| 12 | MRSA 92-1191 | 4 | 1 | 2 | |||
| 13 | MRSA Mu50 | 8 | 1 | 2 | |||
| 14 | MRSA LDZ-resistance06 | 8 | 1 | 32 | |||
| 15 | S. aureus macrolide-resistance | 4 | 1 | 2 | |||
| 16 | E. faecalis NCTC12201 (VanA) | 8 | >128 | 2 | |||
| 17 | E. faecalis NCTC12203 (VanA) | 2 | >128 | 4 | |||
| 18 | S. aureus FDA209P | 4 | 1 | 2 | |||
| 19 | S. aureus Smith | 8 | 1 | 2 | |||
| 20 | E. coli NIHJ JC-2 | >128 | >128 | 128 | |||
| 21 | K. pneumoniae NCTN9632 | >128 | >128 | >128 | |||
| 22 | P. mirabilis IFO3849 | >128 | >128 | 128 | |||
| 23 | P. aeruginosa 46001 | >128 | >128 | >128 | |||
The agar plate dilution method was used (see Supporting Information).
MICs against Gram-positive bacteria were >12.5.
MICs of 2a against Mycobacterium spp. were >10-fold higher than those of 1a.
VCM: vancomycin. LZD: linezolid. RFP: rifampicin. INH: isoniazid.
In conclusion, we have shown, for the first time, that MenA inhibitors 1a and 2a inhibited growth of drug-resistant Mycobacterium spp. and other Gram-positive bacteria at low concentrations. The MenA inhibitors described here can be synthesized cost-effectively, and structural modifications to improve the inhibitory activity in vitro can be achieved in a time efficient manner. The results are expected to be of significance in terms of discovering new lead molecules that can be developed into new drugs to combat Gram-positive pathogens.
Acknowledgment
This paper is dedicated to Professor Yoshito Kishi (Harvard University) on the occasion of his 70th birthday. We thank the National Institutes of Health (NIAID Grants AI049151, AI018357, and AI06357) for generous financial support. We are grateful to Drs. Yamada and Hanaki (Kitasato Institute Research Center) and Dr. Matsumoto (Otsuka Pharmaceutical) for conducting MIC assays.
Footnotes
- MenA
- 1,4-dihydroxy-2-naphthoate prenyltransferase
- MDR
- multidrug-resistant
- MRSA
- methicillin-resistant Stapylococcus aureus
- MRSE
- methicillin-resistant Staphylococcus epidermidis
- Mtb
- Mycobacterium tuberculosis
- CoQ
- ubiquinone
- DHNA
- 1,4-dihydroxy-2-napthoate
- DMMK
- demethylmenaquinone
Supporting Information Available: Experimental procedures and results from characterization of compounds and MenA inhibitory assay. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Grenet K, Guillemot D, Jarlier V, Moreau B, Dubourdieu S, Ruimy R, Armand-Lefevre L, Brau P, Andremont A. Antibacterial resistance, Wayampis Amerindians, French Guyana. Emerging Infect. Dis. 2004;10:1150. doi: 10.3201/eid1006.031015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2)(a).Gaynes R, Edwards JR. Overview of nosocomial infections caused by Gram-negative bacilli. Healthcare Epideol. 2005;41:848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]; (b) Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, Cai M, Hanse NN, Per T, Ticehurst JR, Carroll K, Thomas DL, Nuermberger E, Bartlett JG. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus. Clini. Infect. Dis. 2005;40:100–1007. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]; (c) Schroeder MS. Clostridium difficile-associated diarrhea. Am. Fam. Physician. 2005;71:921. [PubMed] [Google Scholar]; (d) Haddadin AS, Fappiano SA, Lipsett PA. Methicillin resistant Staphylococcus aureus (MRSA) in the intensive care unit. Postgrad. Med. J. 2002;78:385–392. doi: 10.1136/pmj.78.921.385. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. Crit. Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]; (f) Martin MA. Methicillin-resistant Staphylococcus aureus: the persistent resistant nosocomial pathogen. Curr. Clin. Top. Infect. Dis. 1994;14:170–191. [PubMed] [Google Scholar]
- (3).Cohen J. New TB drug promises shorter, simpler treatment. Science. 2004;306:1872. doi: 10.1126/science.306.5703.1872. [DOI] [PubMed] [Google Scholar]
- (4).Pagune DJ, Gwynn MN, Holmes DJ, Pompliano DL. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. 2007;6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- (5).The peptide sequences of the menA and ubiA gene products were only 21% identity and 35% similarity. See the following:Suvana KD, Stevenson R, Meganathan R, Hudspeth MES. Menaquinone (vitamin K2) biosynthesis: localization and characterization of the menA Gene from Escherichia coli. J. Bacteriol. 1998;180:2782–2787. doi: 10.1128/jb.180.10.2782-2787.1998.
- (6)(a).Bentley R. Biosynthesis of vitamin-K and other natural naphthoquinones. Pure Appl. Chem. 1975;41:47–68. [Google Scholar]; (b) Bentley R, Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol. Rev. 1982;46:241–280. doi: 10.1128/mr.46.3.241-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Martin JL, McMillan FM. SAM (dependent) I AM: the S-adenosylmethionine-dependent methyltransferase fold. Curr. Opin. Struct. Biol. 2002;12:783–793. doi: 10.1016/s0959-440x(02)00391-3. [DOI] [PubMed] [Google Scholar]
- (7)(a).Truglio JJ, Theis K, Feng Y, Gajda R, Machutta C, Tong PJ, Kisher C. Crystal structure of Mycobacterium tuberculosis MenB, a key enzyme in vitamin K2 biosynthesis. J. Biol. Chem. 2003;24:42352–42360. doi: 10.1074/jbc.M307399200. [DOI] [PubMed] [Google Scholar]; (b) Collins MD, Jones D. Distribution of isoprenoid quinone structural types in bacterial and their taxomic implication. Microbiol. Rev. 1981;45:316–354. doi: 10.1128/mr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bishop DHL, Pandya KP, King HK. Ubiquinone and vitamin K in bacteria. Biochem. J. 1962;83:606–614. doi: 10.1042/bj0830606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Clearly, the electron transport chain is a central component in the production of ATP and the subsequent growth of bacteria.
- (9).MenA is a membrane-associated protein, and so far no crystal structure has been reported. Detailed mechanism of biosynthesis of DMMK catalyzed by MenA has not been reported.
- (10).For details, see Supporting Information.
- (11).Mtb utilizes nonaprenyldiphosphate (n = 8 in Figure 1) as an electrophile for DHNA in the synthesis of menaquinone. Conveniently, it was observed that farnesyl-PP (FPP) can be utilized as a substrate and we utilized [3H]-labeled FPP for MenA inhibitory assays.9 For localization and characterization of the menA gene from E. coli, see the following:Shineberg B, Young IG. Biosynthesis of bacterial menaquinones: the membrane-associated 1,4-dihydroxy-2-naphthoate octaprenyltransferase of Escherichia coli. Biochemistry. 1976;15:2754–2758. doi: 10.1021/bi00658a007.
- (12).Hirokawa T, Boon-Chieng S, Mitaku S. Classification and secondary structure prediction system for membrane proteins. Bio-informatics. 1998;14:378–379. doi: 10.1093/bioinformatics/14.4.378. [DOI] [PubMed] [Google Scholar]
- (13).Generation of small optimized libraries based on 1a and 2a is in progress.
- (14).The difference in sensitivities of 2a against Mycobacterium spp. is probably due to the compositional complexity of the mycobacterial cell envelope, which differentiates their drug sensitivity from that of most other prokaryotes. On the other hand, 1a may show lower permeability of the other Gram-positive cell wall than 2a.