Abstract
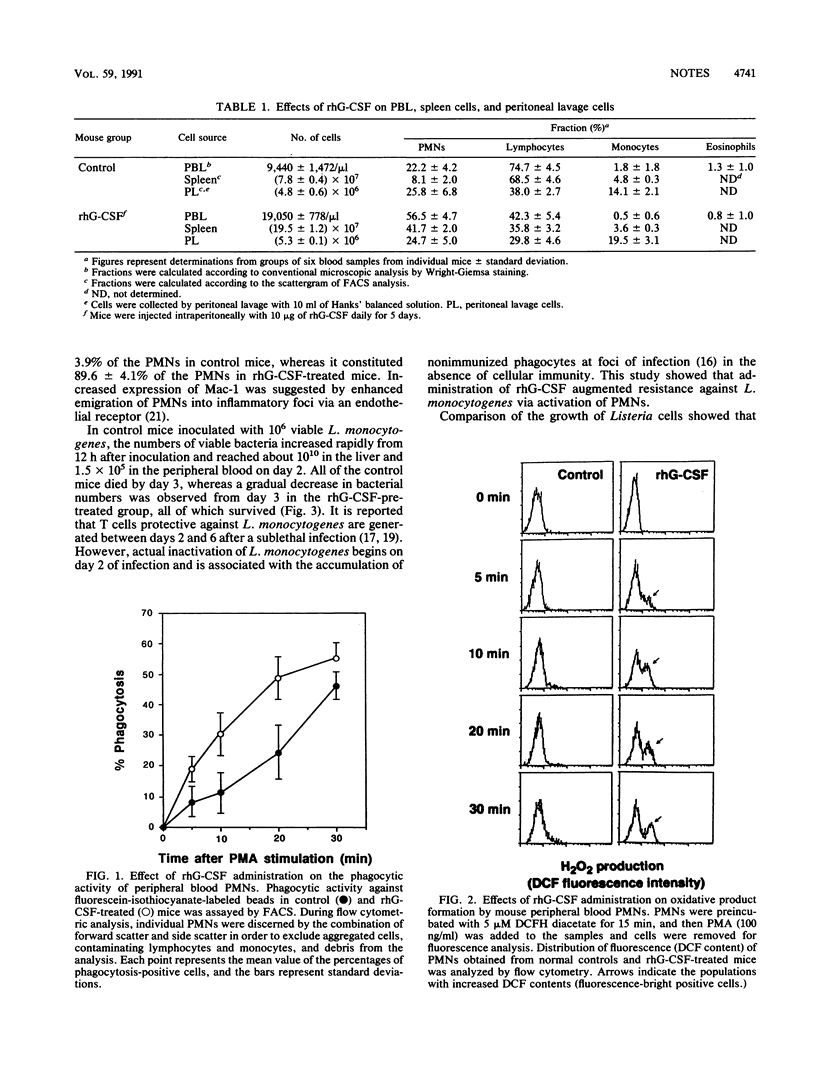
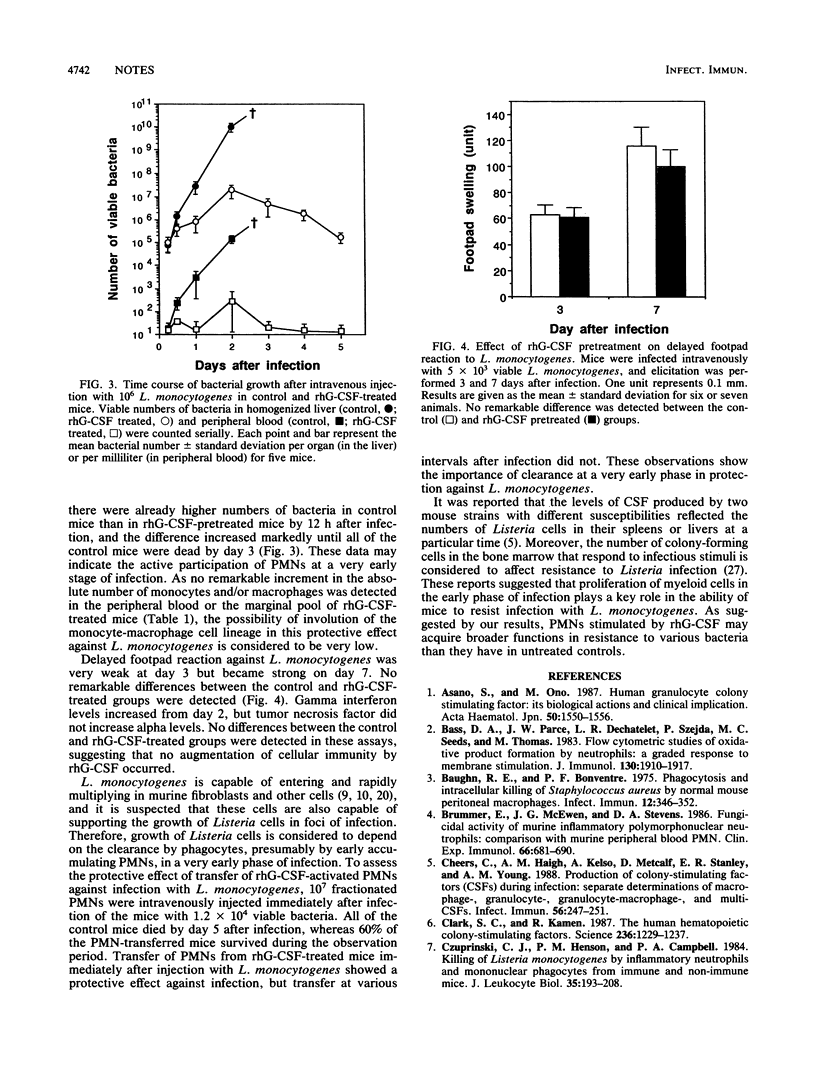
Phagocytosis, H2O2 production, Mac-1 expression, and in vivo elimination of Listeria monocytogenes were enhanced in granulocyte colony-stimulating factor (G-CSF)-treated mice. Transfer of polymorphonuclear leukocytes prolonged survival of mice infected with a lethal dose of L. monocytogenes. G-CSF augments the functions of polymorphonuclear leukocytes and thus plays a role in protection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano S., Ono M. Human granulocyte colony-stimulating factor: its biological actions and clinical implication. Nihon Ketsueki Gakkai Zasshi. 1987 Dec;50(8):1550–1556. [PubMed] [Google Scholar]
- Bass D. A., Parce J. W., Dechatelet L. R., Szejda P., Seeds M. C., Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983 Apr;130(4):1910–1917. [PubMed] [Google Scholar]
- Baughn R., Bonventre P. F. Phagocytosis and intracellular killing of Staphylococcus aureus by normal mouse peritoneal macrophages. Infect Immun. 1975 Aug;12(2):346–352. doi: 10.1128/iai.12.2.346-352.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., McEwen J. G., Stevens D. A. Fungicidal activity of murine inflammatory polymorphonuclear neutrophils: comparison with murine peripheral blood PMN. Clin Exp Immunol. 1986 Dec;66(3):681–690. [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Haigh A. M., Kelso A., Metcalf D., Stanley E. R., Young A. M. Production of colony-stimulating factors (CSFs) during infection: separate determinations of macrophage-, granulocyte-, granulocyte-macrophage-, and multi-CSFs. Infect Immun. 1988 Jan;56(1):247–251. doi: 10.1128/iai.56.1.247-251.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Czuprynski C. J., Henson P. M., Campbell P. A. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J Leukoc Biol. 1984 Feb;35(2):193–208. doi: 10.1002/jlb.35.2.193. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Tsuru S., Taniguchi M., Zinnaka Y., Nomoto K. Protective mechanisms against pulmonary infection with influenza virus. I. Relative contribution of polymorphonuclear leukocytes and of alveolar macrophages to protection during the early phase of intranasal infection. J Gen Virol. 1987 Feb;68(Pt 2):425–432. doi: 10.1099/0022-1317-68-2-425. [DOI] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M., Goebel W. Identification of an extracellular protein of Listeria monocytogenes possibly involved in intracellular uptake by mammalian cells. Infect Immun. 1989 Jan;57(1):55–61. doi: 10.1128/iai.57.1.55-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKI K., MACKANESS G. B. THE PASSIVE TRANSFER OF ACQUIRED RESISTANCE TO LISTERIA MONOCYTOGENES. J Exp Med. 1964 Jul 1;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science. 1985 Jul 5;229(4708):16–22. doi: 10.1126/science.2990035. [DOI] [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Nagata S., Tsuchiya M., Asano S., Kaziro Y., Yamazaki T., Yamamoto O., Hirata Y., Kubota N., Oheda M., Nomura H. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. 1986 Jan 30-Feb 5Nature. 319(6052):415–418. doi: 10.1038/319415a0. [DOI] [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Deissler J. F. Nature of "memory" in T-cell mediated antibacterial immunity: cellular parameters that distinguish between the active immune response and a state of "memory". Infect Immun. 1975 Oct;12(4):761–767. doi: 10.1128/iai.12.4.761-767.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Gordon S., North R. J. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J Exp Med. 1989 Jul 1;170(1):27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- Tatsukawa K., Mitsuyama M., Takeya K., Nomoto K. Differing contribution of polymorphonuclear cells and macrophages to protection of mice against Listeria monocytogenes and Pseudomonas aeruginosa. J Gen Microbiol. 1979 Nov;115(1):161–166. doi: 10.1099/00221287-115-1-161. [DOI] [PubMed] [Google Scholar]
- Tsuru S., Shinomiya N., Nomoto K. Depression of early protection against influenza virus infection by cyclophosphamide and its restoration by Y-19995 [2,4'-bis(1-methyl-2-dimethyl-aminoethoxyl)-3-benzoylpyridine dimaleate]. Nat Immun Cell Growth Regul. 1991;10(1):1–11. [PubMed] [Google Scholar]
- Wang J. M., Chen Z. G., Colella S., Bonilla M. A., Welte K., Bordignon C., Mantovani A. Chemotactic activity of recombinant human granulocyte colony-stimulating factor. Blood. 1988 Nov;72(5):1456–1460. [PubMed] [Google Scholar]
- Wood P. R., Spanidis V., Frangos K., Cheers C. The in vitro bactericidal activity of peritoneal and spleen cells from Listeria-resistant and -susceptible mouse strains. Cell Immunol. 1986 Apr 15;99(1):160–169. doi: 10.1016/0008-8749(86)90225-x. [DOI] [PubMed] [Google Scholar]
- Young A. M., Cheers C. Colony-forming cells and colony-stimulating activity during listeriosis in genetically resistant or susceptible mice. Cell Immunol. 1986 Feb;97(2):227–237. doi: 10.1016/0008-8749(86)90393-x. [DOI] [PubMed] [Google Scholar]
- Yuo A., Kitagawa S., Okabe T., Urabe A., Komatsu Y., Itoh S., Takaku F. Recombinant human granulocyte colony-stimulating factor repairs the abnormalities of neutrophils in patients with myelodysplastic syndromes and chronic myelogenous leukemia. Blood. 1987 Aug;70(2):404–411. [PubMed] [Google Scholar]



