Abstract
Tubulointerstitial fibrosis is the final common result of a variety of progressive injuries leading to chronic renal failure. Transforming growth factor-β (TGF-β) is reportedly upregulated in response to injurious stimuli such as unilateral ureteral obstruction (UUO), causing renal fibrosis associated with epithelial-mesenchymal transition (EMT) of the renal tubules and synthesis of extracellular matrix. We now show that mice lacking Smad3 (Smad3ex8/ex8), a key signaling intermediate downstream of the TGF-β receptors, are protected against tubulointerstitial fibrosis following UUO as evidenced by blocking of EMT and abrogation of monocyte influx and collagen accumulation. Culture of primary renal tubular epithelial cells from wild-type or Smad3-null mice confirms that the Smad3 pathway is essential for TGF-β1–induced EMT and autoinduction of TGF-β1. Moreover, mechanical stretch of the cultured epithelial cells, mimicking renal tubular distention due to accumulation of urine after UUO, induces EMT following Smad3-mediated upregulation of TGF-β1. Exogenous bone marrow monocytes accelerate EMT of the cultured epithelial cells and renal tubules in the obstructed kidney after UUO dependent on Smad3 signaling. Together the data demonstrate that the Smad3 pathway is central to the pathogenesis of interstitial fibrosis and suggest that inhibitors of this pathway may have clinical application in the treatment of obstructive nephropathy.
Introduction
Renal interstitial fibrosis is a progressive and potentially lethal disease caused by diverse clinical entities including urinary tract obstruction, chronic inflammation, and diabetes (1–3). Transforming growth factor-β (TGF-β) plays a pivotal role in chronic inflammatory changes of the interstitium and accumulation of extracellular matrix during renal fibrogenesis (4, 5). Emerging evidence suggests that TGF-β initiates the transition of renal tubular epithelial cells to myofibroblasts, the cellular source for extracellular matrix deposition, leading ultimately to an irreversible renal failure (6–9). To understand the mechanisms underlying the pathogenesis of renal fibrotic disorders, it is therefore essential to identify the molecular events involved in the induction of epithelial-mesenchymal transition (EMT) in this disease process.
In the past few years, the receptors and signal transduction pathways mediating the effects of TGF-β on cells have been identified, enabling the identification of the specific pathways involved in pathogenic events dependent on this cytokine. TGF-β type I and type II transmembrane receptor serine/threonine kinases transduce downstream signals via novel cytoplasmic latent transcription factors called Smad proteins. Smad2 and Smad3 are phosphorylated directly by the type I receptor kinase, after which they partner with Smad4 and translocate to the nucleus, where they act as transcriptional regulators of target genes, including those essential for apoptosis, differentiation, and growth inhibition (10–12). Unlike the targeted deletion of Smad2, which results in embryonic lethality, deletion of Smad3 results primarily in impaired mucosal immunity in mice, shortening their lifespan to 1–6 months (13). We have now utilized these mice (Smad3ex8/ex8) and their wild-type littermate controls to study the role of the Smad3 signaling pathway in the pathogenesis of fibrosis induced by unilateral ureteral obstruction (UUO), a model for renal tubulointerstitial fibrosis and obstructive nephropathy (14).
Methods
UUO.
Smad3-null (Smad3ex8/ex8) mice were generated as described (13) and were used at 6–8 weeks of age and 20–30 g in body weight. In mice sedated by general anesthesia, an incision was made in the right side of the back, and the right proximal ureter was exposed and double-ligated. Sham-operated mice had their ureter exposed but not ligated. All the experimental procedures were approved by Animal Care and Use Committee of Wakayama Medical University (Wakayama, Japan).
Primary culture of renal tubular epithelial cells.
Minced kidneys were washed with three changes of cold PBS containing 1 mM EDTA and were digested in 0.25% trypsin solution (Gibco BRL, Grand Island, New York, USA) in a shaking incubator at 37°C for 2 hours. Trypsin was neutralized with growth medium (DMEM and 10% FBS containing 100 unit/ml penicillin and 0.1 mg/ml streptomycin). The suspension was triturated by pipetting and was passed through a 100-μm cell strainer (Becton Dickinson Labware, Franklin Lakes, New Jersey, USA). The filtrate, consisting mostly of dispersed renal tubules, was plated onto culture dishes (Nalge Nunc International, Naperville, Illinois, USA) and two-well chamber slides (Nunc Lab-Tek II-CC2, Nalge Nunc International), and was incubated at 37°C in a CO2 incubator with medium changes every 2 days. Experiments were carried out in serum-free DMEM. EMT was induced by the addition of 10 ng/ml TGF-β1 (R&D Systems, Minneapolis, Minnesota, USA). Mouse monoclonal anti–TGF-β1, -β2, -β3 antibody (clone 1D11; R&D Systems) was used to neutralize TFG-β bioactivity at a concentration of 20 μg/ml, with mouse IgG (Sigma Aldrich, St. Louis, Missouri, USA) as a control.
Mechanical stretching.
Cells grown on culture plates with flexible bottoms coated with type I collagen (BioFlex, Flexcell International Corp., McKeesport, Pennsylvania, USA) were subjected to a mechanical strain of downward deformation by a computer-controlled vacuum using a Flexercell FX-2000 with alternate cycles of 5 seconds of stretch and 5 seconds of relaxation, at an elongation rate of 15% in a CO2 incubator at 37°C.
Bone marrow monocytes.
Mononuclear cells in the bone marrow were collected from tibias and femurs of 7-week-old mice and were cultivated for 7 days in growth medium containing 10 ng/ml of recombinant mouse macrophage colony-stimulating factor (R&D Systems) as described (15). Monocytes (5 × 104) suspended in 50 μl of DMEM were plated into primary culture of renal tubular epithelial cells in a two-well chamber slide preconditioned with 1 ml of serum-free DMEM for 24 hours. Coculture was continued for 48 hours. For transplantation of monocytes, the right kidney and proximal ureter were exposed after incision of the right side of the back in mice sedated by general anesthesia. Monocytes (2.5 × 105) suspended in 20 μl of DMEM were injected into the renal subcapsular space using a Hamilton syringe with a 26-gauge needle, and then the ureter was double-ligated. Mice were sacrificed at day 3 after operation.
Histology and immunofluorescence.
Histological sections were prepared from tissues fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, and were embedded in paraffin. Cryosections and bottom sheets of BioFlex were fixed in cold acetone and subjected to indirect immunofluorescence with anti–E-cadherin (clone: DECMA-1; Sigma Aldrich), anti–α-smooth muscle actin (anti–α-SMA) (clone: 1A4, NeoMarkers, Fremont, California, USA), anti–mouse type I collagen (Southern Biotechnology, Birmingham, Alabama, USA), and anti–mouse F4/80 (clone: A3-1; BMA, Augst, Switzerland). As secondary antibodies, FITC–anti-rat IgG (Sigma Aldrich), TRITC–anti-mouse IgG (Sigma Aldrich), and indocarbocyanine–anti-goat IgG (Sigma Aldrich) were used.
Immunoblot.
Cells and tissues were lysed in buffer containing 1% Nonidet P-40, 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM EDTA, and a 1:50 dilution of a protease inhibitor cocktail (P-2714; Sigma Aldrich). Proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, and blocked in 5% skim milk in PBS. After being incubated with primary antibodies against E-cadherin (clone 36; Transduction Laboratories, Lexington, Kentucky, USA), α-SMA (clone 1A4; Neomarkers, Fremont, California, USA) and actin (Sc-1616; Santa Cruz Biochemicals, Santa Cruz, California, USA), blots were reacted with peroxidase-conjugated goat anti-mouse IgG secondary antibody (Sigma Aldrich) and developed with ECL (Amersham Biosciences, Buckinghamshire, United Kingdom).
In situ hybridization.
Digoxigenin-11-UTP–labeled antisense riboprobes were prepared with an RNA-labeling kit (Roche Diagnostics Corp.-Boehringer Mannheim, Indianapolis, Indiana, USA) for in situ hybridization as described (16). The mouse α-SMA, Snail, and TGF-β1 RNA probes were transcribed from PCR products using the following primers: α-SMA (GenBank accession number N_M007392), 5′-CTGCTCTGCCTCTAGCACAC-3′ and 5′-TTAAGGGTAGCACATGTCTG-3′; Snail (XM_123964), 5′-ACACTGGTGAGAAGCCATTC-3′ and 5′-AGTTCTATGGCTCGAAGCAG-3′; TGF-β1 (M13177), 5′-CACGTGGAAATCAACGGGAT-3′ and 5′-GCGCACAATCATGTTGGACA-3′ from complete mouse mRNA. Sections were stained using a Ventana HX system (Ventana Medical Systems Inc., Tucson, Arizona, USA) according to the manufacturer’s instruction. After hybridization, sections were washed three times in 1 × SSC (0.15 M sodium chloride and 0.015 M sodium citrate, pH 7.0) high-stringency solution at 65°C and incubated with alkaline phosphatase–conjugated anti-digoxigenin Fab fragments (Roche Diagnostics Corp., Indianapolis, Indiana, USA). The color was developed in freshly prepared substrate solution of nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Digoxigenin detection kit; Roche Diagnostics Corp.).
Northern blot.
Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, California, USA). RNA (20 μg/lane) was separated by 1% agarose-formaldehyde gel electrophoresis and transferred to Hybond-HX nylon membranes (Amersham). Membranes were hybridized with cDNA probes for mouse Snail and TGF-β1 mRNA labeled with [32P]dCTP by random-primed DNA synthesis using the same primers as above (Rediprime II; Amersham Pharmacia Inc., Piscataway, New Jersey, USA). Filters were exposed to x-ray film at –80°C for 2–3 days. Band intensities were normalized to 28S and 18S ribosomal bands stained with ethidium bromide.
Immunoassay of TGF-β1.
Protein extracts from kidneys (6) and cell culture medium were used for the determination of active TGF-β1 with a Quantikine TGF-β1 assay kit (R&D Systems). Samples were acidified for the total TGF-β1 assay. Values were expressed as pg/mg protein for the protein extract or pg/cell number for cell culture medium.
Hydroxyproline assay.
Tissue samples were hydrolyzed in 6 N HCl (final concentration)for 12 hours at 110°C (50 mg/ml). The hydroxyproline content of supernatant solutions was assayed as described (17). Values were expressed as μg/mg tissue.
Statistics.
Results were expressed as the mean ± standard deviation. Student’s unpaired t test and an analysis of multiple variance by Scheffe’s method were used for statistical comparison. A P value of less than 0.05 was considered to indicate statistical significance.
Results
Renal architecture is preserved after UUO in mice lacking Smad3.
Two weeks after UUO, obstructed kidneys of wild-type mice were enlarged and exhibited dilated pelves and calyces, and a thin rim of remaining cortex, whereas an appreciable amount of the renal parenchyma was preserved in kidneys of Smad3-null littermates (Figure 1a). Obstructed kidneys of wild-type mice showed fibrotic changes, with dilated renal tubules accompanied by proliferation of fibroblastic cells and influx of inflammatory mononuclear cells (Figure 1b), whereas normal architecture was preserved in obstructed kidneys of Smad3-null mice (Figure 1c). Dual immunofluorescence showed a marked reduction of E-cadherin staining with concomitant expression of α-SMA in kidneys of wild-type mice at days 7 and 14 after UUO (Figure 1, d and e). At both of these time points, renal tubules of Smad3-null mice remained positive for E-cadherin, and α-SMA was restricted to vascular smooth muscle cells (Figure 1, f and g). Immunoblotting also showed a marked reduction of E-cadherin concomitant with an increase in α-SMA in wild-type mice after UUO, with no differences in sham-operated wild-type mice and Smad3-null mutants with UUO (Figure 1h). Snail, a potent repressor of transcription of the E-cadherin gene (18, 19), was expressed in kidneys of wild-type mice but not Smad3-null littermates at day 7 after UUO (Figure 1i). In situ hybridization showed that Snail mRNA was specifically localized to renal tubular epithelial cells of wild-type mice 7 days after UUO (Figure 2a), whereas α-SMA mRNA was detected in both renal tubular epithelial cells and fibroblastic cells adjacent to the renal tubules (Figure 2c). No hybridization to either the Snail or α-SMA mRNA probe was detected in obstructed kidneys from Smad3-null mice (Figure 2, b and d). These findings indicate that the transition of renal tubular epithelial cells to myofibroblasts is dependent on a Smad3-specific mechanism.
Figure 1.
Smad3-null mice maintain the renal architecture after UUO and have reversed EMT. (a) Obstructed kidneys from WT and Smad3-null (KO) mice at day 14 after UUO. (b and c) Hematoxylin and eosin staining of the obstructed kidneys at day 14 after UUO in WT (b) and KO (c) mice. Scale bars: 20 μm. (d–g) Dual immunofluorescence of E-cadherin (green) and α-SMA (red) in obstructed kidneys of WT (d and e) and KO (f and g) mice at day 7 (d and f) and day 14 (e and g) after UUO. DAPI (blue) was used for nuclear staining. Scale bars: 20 μm. (h) Immunoblot of E-cadherin (E-cad) and α-SMA with extracted proteins from kidneys of WT and KO mice with UUO and sham-operated WT mice (Sham). (i) Northern blot of Snail mRNA in kidneys of WT and KO mice with UUO and sham-operated counterparts (Sham).
Figure 2.
In situ hybridization of α-SMA, Snail, and TGF-β1. (a–d) De novo expression of Snail (a) and α-SMA (c) mRNA in the renal tubular epithelial cells of WT mice at day 7 after UUO. There are no positive signals for Snail (b) or α-SMA (d) mRNA in Smad3-null (KO) counterparts. (e and f) Signals for TGF-β1 mRNA in WT (e) and KO (f) mice at day 14 after UUO. Insets, negative controls reacted with sense probe. Counterstained in nuclear fast red solution. Scale bars: 20 μm. Similar results were obtained from three additional experiments.
Renal fibrosis induced by UUO is prevented in Smad3-null mice.
Two weeks after UUO, more type I collagen was deposited in obstructed kidneys of wild-type than in those of Smad3-null mice (Figure 3, a and b). Hydroxyproline content, a measure of total collagen, increased 2- to 3.5-fold in the obstructed kidneys of wild-type mice, whereas no changes were seen in kidneys of either Smad3-null littermates or sham-operated wild-type mice (Figure 3c). A greater number of monocytes, as identified by the immunofluorescence of F4/80 antigen, infiltrated into the interstitium of obstructed kidneys in wild-type compared with Smad3-null mice (Figure 3, d and e). Numbers of monocytes per unit area in obstructed kidneys of wild-type mice increased 6- to 10-fold after UUO for 3, 7, and 14 days, whereas no changes were seen in Smad3-null littermates or sham-operated wild-type mice (Figure 3f). Northern blot analysis of TGF-β1 mRNA also showed higher expression in obstructed kidneys of wild-type mice than in those of sham-operated counterparts, or Smad3-null mice with or without UUO (Figure 3g). In situ hybridization of TGF-β1 mRNA was enhanced in renal tubules and mononuclear cells, consisting mostly of monocytes, infiltrating the interstitium of the obstructed kidneys in wild-type mice compared with that of their Smad3-null counterparts (Figure 2, e and f). The concentrations of active and total TGF-β1 in extracts of obstructed kidneys of wild-type mice were three- to sixfold and two- to fourfold higher, respectively, than those of Smad3-null or sham-operated wild-type mice after UUO for 3, 7, and 14 days (Figure 3h). Together, these results show that none of the classical hallmarks of obstructive kidney disease seen in wild-type mice are found in mice lacking Smad3, suggesting that this pathway is essential in transducing the effects of the ureter blockage.
Figure 3.
Lack of Smad3 prevents renal fibrosis, monocyte influx, and TGF-β1 upregulation. (a and b) Immunofluorescence of type I collagen in obstructed kidneys of WT (a) and Smad3-null (KO) (b) mice at day 14 after UUO. (c) Hydroxyproline content in obstructed kidneys from WT and KO mice and sham-operated WT mice (Sham). (d and e) Immunofluorescence of F4/80 antigen, a mouse monocyte marker, in obstructed kidneys from WT (d) and KO (e) mice at day 14 after UUO. DAPI (blue) was used for nuclear staining. Scale bars: 20 μm. (f) Number of monocytes per unit area in obstructed kidneys from WT and KO mice with UUO and sham-operated WT mice (Sham). (g) Northern blot of TGF-β1 mRNA in kidneys from WT and KO mice with UUO and sham-operated counterparts (Sham). (h) Active and total TGF-β1 concentrations as determined by immunoassay in kidneys of WT and KO mice and sham-operated mice (Sham). Results are means ± standard deviation of four to five samples. *P < 0.01 compared with Sham or KO.
EMT requires TGF-β1/Smad3 signaling.
To ascertain whether these effects of the Smad3 pathway could be mediated by TGF-β, primary renal tubular epithelial cells were cultured from wild-type and Smad3-null mice. Experiments were conducted 5–7 days later, when greater than 95% of cells were E-cadherin–positive in regions of cell-cell adhesion (not shown). Treatment of wild-type epithelial cells with exogenous TGF-β1 resulted in a phenotypic change from cells exhibiting an epithelial-like cobblestone appearance to cells with a spindle-shaped, fibroblastic appearance (Figure 4, a and b), whereas TGF-β1-treated Smad3-null cells retained features of an epithelial monolayer (Figure 4, c and d). Marked reduction of E-cadherin and de novo expression of α-SMA were demonstrated by dual immunofluorescence in wild-type cells treated with TGF-β1 (Figure 4f). These changes were not seen in untreated wild-type cells and Smad3-null cells with or without TGF-β1 treatment (Figure 4, e, g, and h). Immunoblot analyses of E-cadherin and α-SMA in cell lysates from wild-type and Smad3-null epithelial cells in the absence or presence of TGF-β1 confirmed these findings (Figure 4i). Treatment of wild-type epithelial cells, but not Smad3-null cells, with TGF-β1 also resulted in de novo expression of Snail mRNA (Figure 4j), consistent with the data obtained in the in vivo model (Figure 2, a and b). These results indicate that the Smad3 pathway is essential for TGF-β1-induced EMT in the primary culture of renal tubular epithelial cells.
Figure 4.
Smad3-mediated EMT in cultured renal tubular epithelial cells. (a–d) Phase-contrast microscopy of epithelial cells from WT (a and b) and Smad3-null (KO) (c and d) mice in the absence (a and c) or presence (b and d) of TGF-β1 (10 ng/ml) for 24 hours. Scale bars: 100 μm. (e–h) Dual immunofluorescence of E-cadherin (green) and α-SMA (red) in epithelial cells from WT (e and f) and KO (g and h) mice in the absence (e and g) or presence (f and h) of TGF-β1 (10 ng/ml) for 24 hours. Scale bars: 20 μm. (i) Immunoblot of E-cadherin (E-cad) and α-SMA with extracted protein from epithelial cells of WT and KO mice in the absence (–) or presence (+) of TGF-β1 (10 ng/ml) for 24 hours. (j) Northern blot of Snail mRNA in the epithelial cells from WT and KO mice in the absence (–) or presence (+) of TGF-β1 (10 ng/ml) for 8 hours. Similar results were obtained from three additional experiments.
Autoinduction of TGF-β1 in primary culture of renal tubular epithelial cells.
The concentration of total TGF-β1 in the culture medium of renal tubular epithelial cells increased in a time-dependent manner up to 72 hours, with levels being significantly higher in the media of wild-type than Smad3-null cells (Figure 5a). To investigate whether these elevated levels of TGF-β1 could result from self-amplifying autocrine effects, TGF-β1 mRNA expression was determined in the presence or absence of exogenous TGF-β1. Addition of TGF-β1 to wild-type, but not to Smad3-null, epithelial cells enhanced expression of TGF-β1 mRNA compared with that of nontreated wild-type control cells (Figure 5b), suggesting that Smad3 signaling was essential to the autoinduction.
Figure 5.
Smad3-mediated autoinduction of TGF-β1 in cultured renal tubular epithelial cells. (a) Concentration of total TGF-β1 in culture medium of renal tubular epithelial cells from WT and Smad3-null (KO) mice. Results are means ± standard deviation of four to five samples. *P < 0.05 compared with KO. (b) Northern blot of TGF-β1 mRNA in epithelial cells from WT and KO mice in the absence (–) or presence (+) of TGF-β1 (10 ng/ml) for 24 hours. Cells without TGF-β1 were further treated with a neutralizing antibody against TGF-β (20 μg/ml) to exclude any effects of endogenous TGF-β1. The same amount of normal IgG was added to the medium of TGF-β1-treated cells. Results are means ± standard deviation of four samples. *P < 0.01 compared with WT (–), KO (–) or KO (+).
Stretch-induced upregulation of TGF-β1 and EMT.
An in vitro experimental model of mechanically stretched renal tubular epithelial cell culture was used to model the pathogenetic effects of renal tubular distention by urine in UUO (20). Cyclic stretching of cultured wild-type cells elicited EMT, as characterized by their phenotypic transition to α-SMA–expressing myofibroblasts with marked reduction of cell membrane–localized E-cadherin (Figure 6a). This stretch-induced EMT was abolished by a neutralizing antibody against TGF-β1 (Figure 6b). No phenotypic conversion was found in Smad3-null cells under any conditions (Figure 6, c and d). Mechanical stretch also induced de novo expression of Snail mRNA only in wild-type cells, and this was blocked by treatment with neutralizing anti–TGF-β1 coincident with effects on EMT (Figure 6e). Increased expression of TGF-β1 mRNA induced by mechanical stretching was also restricted to wild-type cells, and this was reversed by treatment with neutralizing anti–TGF-β1 (Figure 6f). Total TGF-β1 concentration in the culture medium was elevated more than twofold in stretched wild-type cells compared with nonstretched control cultures after either 24 or 48 hours of mechanical stretching. No significant changes were observed in similarly treated cultures of Smad3-null cells (Figure 6g). These results clearly show that production of TGF-β1 in this model is Smad3 dependent and, furthermore, that the TGF-β produced by renal epithelial cells in response to mechanical injury in vitro and, by implication, in response to UUO in vivo, is required for EMT.
Figure 6.
Epithelial-mesenchymal transition and TGF-β1 upregulation in an environment of mechanical stretch. (a–d) Dual immunofluorescence of E-cadherin (green) and α-SMA (red) in renal tubular epithelial cells derived from WT (a and b) and Smad3-null (KO) mice (c and d) stretched for 24 hours in the absence (a and c) or presence (b and d) of neutralizing anti–TGF-β1 (20 μg/ml). Scale bars: 20 μm. (e) Northern blot of Snail mRNA in the epithelial cells either stretched for 24 hours or nonstretched, in the absence or presence of neutralizing anti–TGF-β1. Similar results were obtained from additional two experiments. (f) Northern blot of TGF-β1 mRNA in primary culture of the epithelial cells either stretched for 24 hours or nonstretched, in the absence or presence of a neutralizing anti–TGF-β1 antibody. Results are means ± standard deviation of five samples. *P < 0.01 compared with other experimental groups. (g) Total TGF-β1 concentration in culture medium of epithelial cells either stretched or nonstretched. Results are means ± standard deviation of five samples. *P < 0.05 compared with nonstretched counterparts.
Acceleration of EMT by exogenous monocytes.
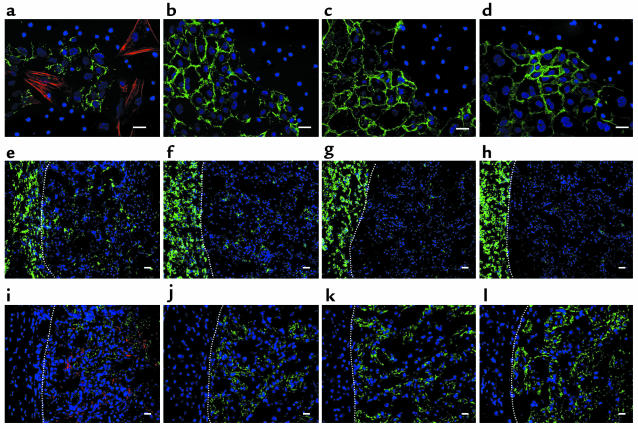
As monocyte influx appeared to play an important role in EMT during UUO, we further investigated a direct interaction of bone marrow monocytes with renal tubular epithelial cells. Primary cultures of wild-type epithelial cells, when cocultured with wild-type, but not Smad3-null, monocytes for 48 hours, showed a fibroblastic phenotype, as characterized by de novo expression of α-SMA with a marked reduction in E-cadherin (Figure 7, a and b). Smad3-null epithelial cells showed no phenotypic change in coculture with monocytes, regardless of their genotype (Figure 7, c and d).
Figure 7.
Role of exogenous monocytes in EMT of renal tubular epithelial cells. (a–d) Dual immunofluorescence of E-cadherin (green) and α-SMA (red) in renal tubular epithelial cells and bone marrow monocytes cocultured for 48 hours. (a) WT epithelial cells and WT monocytes. (b) WT epithelial cells and Smad3-null (KO) monocytes. (c) KO epithelial cells and WT monocytes. (d) KO epithelial cells and KO monocytes. (e–l) Three days after transplantation of monocytes into the subcapsular space of the kidney with UUO. Dotted lines indicate the border between the subcapsular space (left) and the renal cortex (right). (e–h) Immunofluorescence of F4/80 antigen (green). (i–l) Dual immunofluorescence of E-cadherin (green) and α-SMA (red). (e and i) Transplantation of WT monocytes into WT kidneys. (f and j) Transplantation of KO monocytes into WT kidneys. (g and k) Transplantation of WT monocytes into KO kidneys. (h and l) Transplantation of KO monocytes into KO kidneys. DAPI (blue) was used for nuclear staining. Scale bars: 20 μm. Similar results were obtained from four additional experiments.
To examine the effect of the Smad3 genotype of monocytes on the response to UUO in vivo, monocytes were injected into the renal subcapsular space just prior to ligation of the ureter. Wild-type mice transplanted with wild-type monocytes showed a higher number of monocytes infiltrating in the renal cortex (Figure 7e) than did mice transplanted with Smad3-null monocytes (Figure 7f), suggesting that exogenous wild-type, but not Smad3-null, monocytes exhibited increased chemotaxis toward the renal cortex where the level of TGF-β1 is already elevated at day 3 after UUO (Figure 3h). No influx of transplanted wild-type (Figure 7g) or Smad3-null monocytes (Figure 7h) was observed in Smad3-null kidneys, consistent with the lack of elevation of TGF-β in the renal cortex in these kidneys (Figure 3h). Dual immunofluorescence showed de novo expression of α-SMA with a marked reduction in E-cadherin in wild-type renal cortices transplanted with wild-type monocytes (Figure 7i). Wild-type mice transplanted with Smad3-null monocytes showed a lesser degree of reduction in E-cadherin expression (Figure 7j), which was essentially similar to the early phenotypic change seen in renal tubules of wild-type mice at day 3 after UUO (not shown); α-SMA was undetectable. Expression of E-cadherin was retained in Smad3-null kidneys transplanted with either wild-type or Smad3-null monocytes (Figure 7, k and l). These findings suggest that exogenous monocytes accelerate the EMT of obstructed kidneys and require Smad3 both for chemotaxis and probably for Smad3-dependent expression of TGF-β1.
Discussion
EMT of renal tubular epithelial cells has been described in both animal models and TGF-β1–treated cells in culture (7, 8, 21). Here we demonstrate that Smad3, a signaling intermediate downstream of TGF-β and activin receptors, is essential both for TGF-β1–induced EMT of cultured renal tubular epithelial cells and for EMT following UUO in vivo. In both of these systems, expression of all markers of EMT is blocked in the absence of Smad3, thus blocking the formation of fibrogenic myofibroblasts from epithelial precursors.
Experiments utilizing mutated forms of the TGF-β type I receptor unable to bind and activate Smad proteins have clearly shown that the Smad pathway is necessary but not sufficient for induction of EMT by TGF-β (22, 23), and that other pathways involving phosphatidylinositol 3-kinase, Rho-A and p38MAPK pathways are probably also required (24–26). Although these experiments do not differentiate between Smad2 and Smad3, we have recently shown that EMT of lens epithelial cells in response to injury in vivo is completely blocked in the absence of Smad3 (S. Saika, et al., manuscript submitted for publication). Interestingly, in this regard the TGF-β–dependent EMT of cardiac endothelial cells required for the formation of endocardial cushions in the atrioventricular canal of the developing heart is not affected in the Smad3-null mice. This EMT requires expression of the type III TGF-β receptor and may utilize signaling pathways different from those involved in mediating injury-induced EMT (27, 28).
Although Smad2 and Smad3 are each activated by the TGF-β and activin receptors, they have very different effects on gene transcription (29). Somewhat surprisingly, studies in mouse embryo fibroblasts showed that deletion of Smad3 did not affect endogenous levels of Smad2 or its phosphorylation, and vice versa (29). Thus, EMT of renal epithelial cells following UUO is probably independent of Smad2. Smad3 is critical in mediating the effects of TGF-β on the elaboration of extracellular matrix components, including the synthesis of collagens by fibroblasts (30), and its loss affords protection from radiation-induced fibrosis (31) and bleomycin-induced pulmonary fibrosis (32), presumably by interrupting the pathways necessary for matrix production by fibroblasts. Here we show that Smad3 plays an even more essential role in fibrosis initiated by EMT, as it is also required for generation of the fibrogenic myofibroblasts from epithelial precursors.
The Snail family of zinc-finger transcription factors are strong repressors of transcription of the E-cadherin gene and are implicated in both physiological and pathological EMT (18, 19, 33, 34). Recent studies in mouse embryo fibroblasts have identified Snail as an immediate-early gene target of the TGF-β1/Smad3 pathway (E.P. Bottinger, personal communication). Our data, which show that expression of Snail is blocked by neutralizing antibodies against TGF-β in cultured cells in vitro and that EMT is blocked in any condition that interferes with expression of Snail, including loss of Smad3, suggest that it is a critical early-response gene in the TGF-β–driven EMT resulting from UUO.
All the data obtained after UUO in vivo and from mechanical stress–induced EMT of renal tubular epithelial cells in vitro suggest that EMT is initiated by TGF-β1 produced by the renal tubular cells. Especially convincing are the data showing that a neutralizing antibody against TGF-β1 blocked both the increase in TGF-β1 mRNA and the subsequent EMT of mechanically stressed renal epithelial cells in culture, demonstrating that TGF-β1, and not mechanical force per se, initiates the EMT of the stretched cells. Moreover, the absence of TGF-β1 induction in Smad3-null mice implicates this pathway in injury-induced elaboration of TGF-β1 and amplification through a positive-feedback autoinductive loop, similar to that previously reported in monocytes and fibroblasts (29, 35). This process may be initiated by activation by mechanical stress of latent forms of TGF-β1 secreted constitutively from renal tubular epithelial cells and sequestered by the matrix. In support of this, endothelial and vascular smooth muscle cells reportedly secrete a higher amount of tissue plasminogen activator (t-PA) in response to shear stress (36, 37). Thus renal tubular epithelial cells facing high-pressure backflow of urine by UUO may also facilitate activation of latent TGF-β1 through any of many pathways described, including proteolytic activation by generation of plasmin from plasminogen by t-PA (38, 39), or nonproteolytic mechanisms involving thrombospondin-1 (40) or αVβ6 integrin (41, 42).
TGF-β is one of the most potent cytokines known for chemotaxis of monocytes (43, 44). The significantly reduced levels of monocytes in Smad3-null kidneys following UUO implicates both endogenous TGF-β and the Smad3 pathway in the influx of inflammatory cells in this injury model. As Smad3-null monocytes also show impaired autoinduction of TGF-β1, the reduced inflammatory influx probably contributes secondarily to the reduced levels of TGF-β1 following UUO. Exogenous wild-type but not Smad3-null monocytes, either cocultured with renal tubular epithelial cells in vitro or transplanted into the obstructed kidney in the UUO model in vivo, facilitated EMT, providing evidence that the monocyte influx in UUO contributes to the pathogenesis of the developing fibrosis. In summary, the present results demonstrate that selective ablation of the Smad3 signaling pathway blocks EMT of renal tubular epithelial cells and subsequent pathologic accumulation of matrix proteins while presumably preserving other Smad3-independent TGF-β1 signaling arms. This provides a therapeutic rationale for the development of putative small molecular weight inhibitors of Smad3 that may have fewer side effects than either anti-ligand or anti-receptor approaches, which block all downstream signaling (45, 46). Our data suggest that selective inhibitors of the Smad3 pathway may prove highly effective in a wide range of fibrotic disorders, including not only obstructive nephropathy and chronic interstitial nephritis but also pulmonary and hepatic fibrosis.
Acknowledgments
This study was supported by a Research Grant on Priority Areas from Wakayama Medical University (to A. Ooshima, Y. Muragaki, and S. Saika). We thank Mario Anzano for his help with the Smad3-null mice.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: epithelial-mesenchymal transition (EMT); unilateral ureteral obstruction (UUO); α-smooth muscle actin (α-SMA); tissue plasminogen activator (t-PA).
References
- 1.Eddy AA. Molecular insights into renal interstitial fibrosis. J. Am. Soc. Nephrol. 1996;7:2495–2508. doi: 10.1681/ASN.V7122495. [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N. Eng. J. Med. 1998;339:1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- 3.Stahl PJ, Felsen D. Transforming growth factor-β, basement membrane, and epithelial-mesenchymal transdifferentiation: implications for fibrosis in kidney disease. Am. J. Pathol. 2001;159:1187–1192. doi: 10.1016/s0002-9440(10)62503-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor β in human disease. N. Eng. J. Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 5.Border WA, Noble NA. TGF-β in kidney fibrosis: a target for gene therapy. Kidney Int. 1997;51:1388–1396. doi: 10.1038/ki.1997.190. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am. J. Pathol. 2001;159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeisberg M, Maeshima Y, Mosterman B, Kalluri R. Renal fibrosis: extracellular matrix microenvironment regulates migratory behavior of activated tubular epithelial cells. Am. J. Pathol. 2002;160:2001–2008. doi: 10.1016/S0002-9440(10)61150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwano M, et al. Evidence that fibroblasts derived from epithelium during tissue fibrosis. J. Clin. Invest. 2002;100:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li JH, et al. Smad7 inhibits fibrotic effect of TGF-β on renal tubular epithelial cells by blocking Smad2 activation. J. Am. Soc. Nephrol. 2002;13:1464–1472. doi: 10.1097/01.asn.0000014252.37680.e4. [DOI] [PubMed] [Google Scholar]
- 10.Massagué J, Wotton D. Transcriptional control by the TGF-β/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J. Cell. Physiol. 2002;191:1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- 12.Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-β responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am. J. Physiol. Renal Physiol. 2002;283:F861–F875. doi: 10.1152/ajprenal.00362.2001. [DOI] [PubMed] [Google Scholar]
- 15.Feldman GM, et al. STAT5A-deficient mice demonstrate a defect in granulocyte-macrophage colony-stimulating factor-induced proliferation and gene expression. Blood. 1997;90:1768–1776. [PubMed] [Google Scholar]
- 16.Gong Y, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 17.Kivirikko KI, Laitinen O, Prockop DJ. Modifications of a specific assay for hydroxyproline in urine. Anal. Biochem. 1967;19:249–255. doi: 10.1016/0003-2697(67)90160-1. [DOI] [PubMed] [Google Scholar]
- 18.Nieto MA. The Snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 19.Cano A, et al. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000;2:76–82. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 20.Miyajima A, et al. Interaction of nitric oxide and transforming growth factor-β1 induced by angiotensin II and mechanical stretch in rat renal tubular epithelial cells. J. Urol. 2000;164:1729–1734. [PubMed] [Google Scholar]
- 21.Yang J, Dai C, Liu Y. Hepatocyte growth factor gene therapy and angiogensin II blockade synergistically attenuate renal interstitial fibrosis in mice. J. Am. Soc. Nephrol. 2002;13:2464–2477. doi: 10.1097/01.asn.0000031827.16102.c1. [DOI] [PubMed] [Google Scholar]
- 22.Itoh S, et al. Elucidation of Smad requirement in transforming growth factor-β type I receptor-induced responses. J. Biol. Chem. 2003;278:3751–3761. doi: 10.1074/jbc.M208258200. [DOI] [PubMed] [Google Scholar]
- 23.Yu L, Hebert MC, Zhang YE. TGF-β receptor-activated p38 MAP kinase mediates Smad-independent TGF-β responses. EMBO J. 2002;21:3749–3759. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor β-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- 25.Bhowmick NA, et al. Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin β1 signaling is necessary for transforming growth factor-β activation of p38MAPK and epithelial plasticity. J. Biol. Chem. 2001;276:46707–46713. doi: 10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- 27.Brown CB, Boyer AS, Runyan RB, Barnett JV. Requirement of type III TGF-β receptor for endocardial cell transformation in the heart. Science. 1999;283:2080–2082. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- 28.Boyer AS, Runyan RB. TGFβ Type III and TGFβ Type II receptors have distinct activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev. Dyn. 2001;221:454–459. doi: 10.1002/dvdy.1154. [DOI] [PubMed] [Google Scholar]
- 29.Piek E, et al. Functional characterization of transforming growth factor β signaling in Smad2- and Smad3-deficient fibroblasts. J. Biol. Chem. 2001;276:19945–19953. doi: 10.1074/jbc.M102382200. [DOI] [PubMed] [Google Scholar]
- 30.Verrecchia F, Mauviel A. Transforming growth factor-β signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J. Invest. Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 31.Flanders KC, et al. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am. J. Pathol. 2002;160:1057–1068. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao J, et al. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L585–L593. doi: 10.1152/ajplung.00151.2001. [DOI] [PubMed] [Google Scholar]
- 33.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat. (Basel). 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 34.Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. The mouse Snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol. Cell Biol. 2001;21:8184–8188. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashcroft GS, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 36.Diamond SL, et al. Tissue plasminogen activator messenger RNA levels increase in cultured human endothelial cells exposed to laminar shear stress. J. Cell Physiol. 1990;143:364–371. doi: 10.1002/jcp.1041430222. [DOI] [PubMed] [Google Scholar]
- 37.Papadaki M, et al. Differential regulation of protease activated receptor-1 and tissue plasminogen activator expression by shear stress in vascular smooth muscle cells. Circ. Res. 1998;16:1027–1034. doi: 10.1161/01.res.83.10.1027. [DOI] [PubMed] [Google Scholar]
- 38.Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor β1 by plasmin. J. Cell Biol. 1990;110:1361–1367. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato Y, Tsuboi R, Lyons R, Moses H, Rifkin DB. Characterization of the activation of latent TGF-β by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J. Cell Biol. 1990;111:757–763. doi: 10.1083/jcb.111.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crawford SE, et al. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell. 1998;26:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 41.Munger JS, et al. The Integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 42.Morris DG, et al. Loss of integrin αvβ6-mediated TGF-β activation causes Mmp12-dependent emphysema. Nature. 2003;422:169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- 43.Wahl SM, et al. Transforming growth factor type β induces monocyte chemotaxis and growth factor production. Proc. Natl. Acad. Sci. U. S. A. 1987;84:5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiseman DM, Polverini PJ, Kamp DW, Leibovich SJ. Transforming growth factor-β (TGF β) is chemotactic for human monocytes and induces their expression of angiogenic activity. Biochem. Biophys. Res. Commun. 1988;15:793–800. doi: 10.1016/s0006-291x(88)80319-x. [DOI] [PubMed] [Google Scholar]
- 45.Cosgrove D, et al. Integrin α1β1 and transforming growth factor-β1 play distinct roles in alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am. J. Pathol. 2000;157:1649–1659. doi: 10.1016/s0002-9440(10)64802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters H, Noble NA, Border WA. Transforming growth factor-β in human glomerular injury. Curr. Opin. Nephrol. Hypertens. 1997;6:389–393. doi: 10.1097/00041552-199707000-00014. [DOI] [PubMed] [Google Scholar]