Abstract
Ever more unexpected roles for the LDL receptor gene family in a variety of cellular signaling pathways continue to emerge. Three recent studies now add another function to this collection. By interacting with active tissue-type plasminogen activator, LDL receptor–related protein appears to control permeability of the blood-brain barrier, vascular tone, and the expression of MMPs. All of these parameters impact upon postischemic infarct size following stroke. These novel findings are discussed in the context of known mechanisms of signaling by the LDL receptor family.
The LDL receptor gene family constitutes a class of structurally closely related cell surface receptors that fulfill diverse biological functions in different organs, tissues, and cell types. The role that is most commonly associated with this evolutionarily ancient gene family is the endocytosis and removal of LDL, the main carrier of cholesterol in the circulation of humans, by the liver (1). Indeed, the regulation of cholesterol homeostasis through this mechanism remains the sole known function of the family’s namesake, the LDL receptor. Yet, over the last few years, considerable evidence has accumulated at an increasingly rapid pace that shows that the members of the LDL receptor gene family also have fundamental functions in transmitting or modulating signals between specialized cells in many, if not all, multicellular organisms.
Two recent papers, the first by Nassar and colleagues in Blood (2) and the second by Yepes and colleagues in this issue of the JCI (3), now report on a novel role of the LDL receptor–related protein (LRP) and one of its ligands, the proteolytically active serine proteinase tissue-type plasminogen activator (tPA), as regulators of vascular tone and permeability of the blood-brain barrier (BBB). Using neuroserpin, a plasminogen activator inhibitor that is abundant in the brain, combined with a series of knockout mice lacking tPA, urokinase-type plasminogen activator (uPA), plasminogen, or MMP-9, Yepes et al. now show that an increase in perivascular tPA, as a consequence of experimentally induced cerebral ischemia, results in an opening of the BBB, and that this permeability increase is independent of plasminogen, uPA, and MMP-9. Injection of tPA, but not uPA, into the cerebrospinal fluid increased vascular permeability (as determined by Evans Blue extravasation) even in the absence of ischemia. However, functional blockade of LRP by anti-LRP antibodies or inhibition with the receptor-associated protein, a universal blocker of ligand binding to LRP, abolished the vasoactive effect of tPA. A similar direct effect of LRP on matrix degradation–independent vasoactivity by tPA was also observed by Nassar et al. These findings are complemented by another recent report (4) that showed upregulation of active MMP-9 expression by cerebral endothelial cells in response to in vitro and in vivo tPA exposure. This increase was also dependent on LRP.
Taken together, these three studies suggest an important role for LRP in the brain, and more specifically at the BBB, where LRP controls vasoactivity, permeability, and postischemic lesion formation in response to an active proteinase (tPA). This is, of course, of considerable clinical importance, since intracerebral hemorrhages induced or aggravated by tPA are a serious potential complication of its therapeutic use as a thrombolytic agent. In the following, I will briefly review our current understanding of relevant signaling pathways in which members of the LDL receptor family are involved, and I will comment on potential mechanisms by which tPA may act through LRP upon the cerebral vasculature.
Mechanisms of signaling by the LDL receptor family
Definitive biochemical and genetic evidence for a direct role of the LDL receptor gene family in intercellular signaling events came from the analysis of knockout mice that were deficient in apoE receptor-2 (Apoer2, also known as Lrp8) and the VLDL receptor (Vldlr). Both receptors are structurally almost identical to the LDL receptor, yet the parts they play in lipoprotein transport, as far as we know, are small compared with their signaling role. They function together in a partially redundant fashion in regulating neuronal migration and positioning during embryonic brain development (5). Ligation of Apoer2 and/or Vldlr by their ligand Reelin activates nonreceptor tyrosine kinases of the Src family (6) and initiates further kinase cascades within the cell that regulate microtubule stability, nuclear distribution, and possibly vesicular transport (7).
LRP, on the other hand, in its role as a hepatic chylomicron remnant receptor, actively participates in cholesterol and lipid transport in conjunction with the LDL receptor. However, LRP is much larger (approximately 600 kDa) and structurally more complex, enabling it to serve numerous unrelated functions (8). It is also known as the α2-macroglobulin receptor for its role in the removal of many extracellular proteases and proteinase complexes, including plasminogen activators and their inhibitor plasminogen activator inhibitor-1, or as CD91 for its role in the removal of apoptotic cells and debris.
Although the insights into the roles of Apoer2 and Vldlr have established a strong basis for pivotal functions of LDL receptor family members in the nervous system, mounting data also point toward regulatory functions in the vascular wall (and other tissues) that are, at first glance, unrelated to cholesterol transport. For instance, both LRP (9) and Vldlr (10) have been shown to bind tissue factor pathway inhibitor (TFPI), an inhibitor of factor Xa, factor VIIa, and tissue factor–initiated blood coagulation. In addition, TFPI binding to the Vldlr, which is expressed at much higher levels than LRP on the luminal surface of the endothelium, was shown to mediate the antiproliferative effects of TFPI on endothelial cells, although the precise molecular mechanisms that mediate this effect remain currently unknown (10).
LRP as a signal modulator in VSMCs
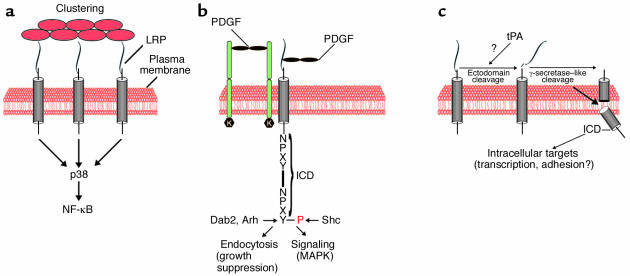
Increasing evidence also suggests that LRP is directly involved in cellular signal transduction, either as a coreceptor (11) or potentially by activating cellular signaling cascades through receptor ligation (12, 13) in a manner similar to that of Apoer2 and Vldlr. LRP complexes with PDGFRβ as well as its ligand, PDGF-BB (14), and is instrumental in regulating the activity of this mitogenic tyrosine kinase receptor (11). Loss of LRP expression in VSMCs results in smooth muscle cell proliferation and greatly enhanced susceptibility to atherosclerosis. Tyrosine phosphorylation of the LRP intracellular domain (ICD) occurs in response to PDGF signaling on a tyrosine residue (14) that regulates the ability of the LRP to interact with adapter proteins that are involved either in the regulation of endocytosis (such as Arh and Dab2) (15, 16) or in the coupling of the receptor to MAPK pathways (such as Shc) (17). Thus, LRP might act as a molecular switch that toggles between growth suppression in the unphosphorylated state by binding to the endocytosis-promoting adapter proteins Dab2 and Arh, and mitogenic signaling through Shc (Figure 1).
Figure 1.
Potential mechanisms of signaling by LRP. (a) Signaling through homotypic clustering. This mode of signaling has been reported to result in the activation of stress-activated protein kinase (SAPK, also known as p38). It involves clustering of LRP through, for example, binding of calreticulin to extracellular protein complexes or apoptotic debris (red circles) and may thereby function in the regulation of inflammation (13). Activation of PI3K and Erk through calreticulin ligation and potential roles in cell adhesion have also been reported (21). (b) Heterocomplex formation of LRP with the membrane tyrosine kinase receptor PDGFRβ modulates PDGFR signaling and controls the proliferation of VSMCs (11, 14). Phosphorylation (P) of the LRP ICD may function as a molecular switch that directs LRP toward endocytosis or mitogenic signaling. (c) Proteolytic processing of LRP by tPA or a tPA-dependent mechanism, followed by release of the ICD by intramembranous cleavage, analogous to the processing of Notch, may activate intracellular mechanisms, e.g., transcriptional regulation, translocation to sites of cellular adhesion, cytoskeletal rearrangements, etc.
Potential mechanisms of signaling by tPA and LRP at the BBB
Another mechanism by which LRP might transduce extracellular signals to the cell, and which has particular appeal in light of the findings by Yepes et al. (3), Nassar et al. (2), and Wang et al. (4), involves the proteolytic cleavage and release of its ICD by γ-secretase (18), the same proteinase that performs the ultimate processing step of the amyloid precursor protein (APP), which leads to the release of the APP ICD. Prior proteolytic cleavage of the extracellular domain of APP, LRP, and a range of other cell surface receptors that undergo similar processing events is a requirement for the subsequent intramembranous processing that results in the release of the ICD into the cytoplasm. A conceivable mechanism by which enzymatically active tPA might modulate vascular tone and permeability in the brain could thus involve the regulated cleavage of the LRP extracellular domain. Although in the study by Yepes et al. (3) the effect of tPA on vascular permeability was independent of MMP-9 or plasminogen, enzymatic activity of tPA was required for both increased vascular permeability (3) and tPA-induced vasoconstriction (2). This suggests that tPA is either directly or indirectly involved in a proteolytic activation step that results in the cleavage of the LRP extracellular domain or, since LRP is also required, activates another cell surface protein that interacts with LRP. Conceivably, the latter mechanism might involve the family of proteinase-activated receptors (PARs) to which the thrombin receptor belongs, although association of these G protein–coupled receptors with LRP has so far not been demonstrated.
By contrast, there is a precedent for a transmembrane receptor that undergoes regulated intramembranous proteolysis and that regulates the integrity of cerebral blood vessels. Mutations in this receptor, Notch 3, cause the devastating disease cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) in humans (19). If LRP were to function in a similar manner, the release of the ICD initiated by tPA could function through the regulation of, for instance, MMP-9 gene transcription (20), but also rapidly and directly through relocalization of the ICD scaffold and attached cytoplasmic proteins to other subcellular compartments where they may regulate cell adhesion. Indeed, a role for LRP in regulating cell adhesion (21) and migration (22) through signaling-dependent mechanisms has recently been demonstrated.
Clearly, much work remains on the road toward a comprehensive understanding of the complex biological roles and signaling functions of the LDL receptor gene family. The present studies by Yepes et al. (3), Nassar et al. (2), and Wang et al. (4) now provide us with another model system in which these functions can be investigated. Genetically altered mice with targeted mutations in LDL receptor family–dependent metabolic and signaling pathways will likely play an increasingly important role in these endeavors.
Acknowledgments
The author is supported by grants from the NIH, the Alzheimer’s Association, the Perot Family Foundation, and the Alexander von Humboldt Foundation.
Footnotes
See the related article beginning on page 1533.
Conflict of interest: The author has declared that no conflict of interest exists.
Nonstandard abbreviations used: LDL receptor–related protein (LRP); tissue-type plasminogen activator (tPA); blood-brain barrier (BBB); urokinase-type plasminogen activator (uPA); apoE receptor-2 (Apoer2); VLDL receptor (Vldlr); tissue factor pathway inhibitor (TFPI); intracellular domain (ICD); amyloid precursor protein (APP).
References
- 1.Goldstein, J.L., Hobbs, H.H., and Brown, M.S. 2001. Familial hypercholesterolemia. In The metabolic and molecular bases of inherited disease. C.R. Scriver et al., editors. McGraw-Hill. New York, New York, USA. 2863–2913.
- 2.Nassar, T., et al. 2003. The in vitro and in vivo effect of tPA and PAI-1 on blood vessel tone. Blood. doi:1182/blood-2003-05-1685. [DOI] [PubMed]
- 3.Yepes M, et al. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor–related protein. J. Clin. Invest. 2003;112:1533–1540. doi:10.1172/JCI200319212. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, et al. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat. Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 5.Tissir F, Goffinet AM. Reelin and brain development. Nat. Rev. Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- 6.Arnaud L, Ballif BA, Forster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr. Biol. 2003;13:9–17. doi: 10.1016/s0960-9822(02)01397-0. [DOI] [PubMed] [Google Scholar]
- 7.Bock HH, et al. Phosphatidylinositol 3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J. Biol. Chem. 2003;278:38772–38779. doi: 10.1074/jbc.M306416200. [DOI] [PubMed] [Google Scholar]
- 8.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J. Clin. Invest. 2001;108:779–784. doi:10.1172/JCI200113992. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narita M, et al. Two receptor systems are involved in the plasma clearance of tissue factor pathway inhibitor in vivo. J. Biol. Chem. 1995;270:24800–24804. doi: 10.1074/jbc.270.42.24800. [DOI] [PubMed] [Google Scholar]
- 10.Hembrough TA, Ruiz JF, Papathanassiu AE, Green SJ, Strickland DK. Tissue factor pathway inhibitor inhibits endothelial cell proliferation via association with the very low density lipoprotein receptor. J. Biol. Chem. 2001;276:12241–12248. doi: 10.1074/jbc.M010395200. [DOI] [PubMed] [Google Scholar]
- 11.Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 12.Lutz C, et al. Evidence of functional modulation of the MEKK/JNK/cJun signaling cascade by the low density lipoprotein receptor-related protein (LRP) J. Biol. Chem. 2002;277:43143–43151. doi: 10.1074/jbc.M204426200. [DOI] [PubMed] [Google Scholar]
- 13.Gardai SJ, et al. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 14.Loukinova E, et al. Platelet-derived growth factor (PDGF)-induced tyrosine phosphorylation of the low density lipoprotein receptor-related protein (LRP). Evidence for integrated co-receptor function between LRP and the PDGF. J. Biol. Chem. 2002;277:15499–15506. doi: 10.1074/jbc.M200427200. [DOI] [PubMed] [Google Scholar]
- 15.He G, et al. ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin, and AP-2. J. Biol. Chem. 2002;277:44044–44049. doi: 10.1074/jbc.M208539200. [DOI] [PubMed] [Google Scholar]
- 16.Mishra SK, et al. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 2002;21:4915–4926. doi: 10.1093/emboj/cdf487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes H, Larsen B, Tyers M, van Der Geer P. Tyrosine-phosphorylated low density lipoprotein receptor-related protein 1 (Lrp1) associates with the adaptor protein SHC in SRC-transformed cells. J. Biol. Chem. 2001;276:19119–19125. doi: 10.1074/jbc.M011437200. [DOI] [PubMed] [Google Scholar]
- 18.May P, Reddy YK, Herz J. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J. Biol. Chem. 2002;277:18736–18743. doi: 10.1074/jbc.M201979200. [DOI] [PubMed] [Google Scholar]
- 19.Hassan A, Markus HS. Genetics and ischaemic stroke. Brain. 2000;123:1784–1812. doi: 10.1093/brain/123.9.1784. [DOI] [PubMed] [Google Scholar]
- 20.Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 21.Orr AW, et al. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J. Cell Biol. 2003;161:1179–1189. doi: 10.1083/jcb.200302069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Z, et al. Regulation of Rac1 activation by the low density lipoprotein receptor-related protein. J. Cell Biol. 2002;159:1061–1070. doi: 10.1083/jcb.200207070. [DOI] [PMC free article] [PubMed] [Google Scholar]