Abstract
Unequivocal direct observations have established that the bacteria that cause device-related and other chronic infections grow in matrix-enclosed biofilms. The diagnostic and therapeutic strategies that have served us so well in the partial eradication of acute epidemic bacterial diseases have not yielded accurate data or favorable outcomes when applied to these biofilm diseases. We discuss the potential benefits of the application of the new methods and concepts developed by biofilm science and engineering to the clinical management of infectious diseases.
Introduction
Clinicians who deal with device-related and other chronic bacterial infections have gradually defined a new category of infectious disease that differs radically from the acute epidemic bacterial diseases that predominated until the middle of the last century (1). The happy fact that acute epidemic diseases are largely in the past is a consequence of progress in microbiology, and can be directly attributed to the vaccines and antibiotics that were developed as we came to understand planktonic bacteria. The free-floating bacteria that caused diphtheria and cholera were accurately modeled in test tube cultures, their pathogenic mechanisms (e.g., toxins) became epitopes for vaccines, and antibiotics that killed them were derived from nature or from directed synthesis. These specialized pathogens caused disease in perfectly healthy individuals, they ran their courses quickly, and they retreated to different animal populations or to natural reservoirs when the population under attack became immune.
As we began to gain control over epidemic diseases, another type of disease came to the fore. These diseases are much less aggressive than acute infections, they often persist for months or years, and they progress through periods of quiescence that alternate with periods of acute exacerbation. When the sophisticated tools of microbiology were brought to bear on these chronic diseases, many anomalies emerged. The pathogens were common environmental organisms, with which individuals had daily contact and against which they often had adequate immunity, and their pathogenic mechanisms were often diffuse and poorly defined. When they were grown in conventional lab cultures, these environmental organisms appeared to be sensitive to conventional antibiotics, but these same antibiotics failed to resolve the bacterial infections, although they gave some relief during acute exacerbations. Even more puzzling was the observation that, in many cases, it was not possible at all to recover any bacteria by traditional culture mechanisms. This led many investigators to posit that these chronic disease states were sterile inflammatory conditions that persisted after the eradication of all microorganisms. However, the application of molecular diagnostics demonstrated unequivocally that bacteria were both present and metabolically active, even when no bacteria were recovered by plating. In some cases (e.g., otitis media, cholesteatoma, tonsilitis), affected individuals were cured by surgical treatment or by growth-related anatomical changes, while many other victims were relegated to intermittent antibiotic therapy for the remainder of their lives (cystic fibrosis [CF], prostatitis). When we looked at the infecting bacteria as they grew in affected tissues, we noted that they actually lived in matrix-enclosed communities that closely resembled the biofilms that are the predominant form of growth of bacteria in industrial and environmental ecosystems. The simple fact that the organisms that cause device-related and other chronic infections grow in biofilms (1) goes some distance toward explaining the perceived anomalies of these diseases, and offers a measure of hope that they can eventually be controlled.
Chronic bacterial disease: a clinical entity.
When the model used to analyze a natural process is incorrect, our attempts to understand and manipulate the process will fail, many honest and conscientious people will be frustrated, and the reputations of whole research groups will be damaged. In the case of chronic bacterial diseases, diagnostic microbiology labs reported that cultures of Pseudomonas aeruginosa from CF patients were sensitive to antibiotics (e.g., cloxacillin), but pulmonary clinicians saw little improvement when these antibiotics were used. The sera of CF patients contained very large amounts of specific antibodies against Pseudomonas, but the disease persisted, and the use of anti-Pseudomonas vaccines resulted in the deaths of some patients. Middle ear specimens from children with chronic otitis media with effusion (COME) yielded negative bacterial cultures, so that a host-sustained inflammatory etiology was suspected, but the factors driving the inflammation could not be identified and serology did not confirm the persistent involvement of viruses. Patients with raging febrile prostate infections yielded expressed prostatic secretion (EPS) samples that produced cultures negative for bacteria, and material recovered from osteomyelitis debridations with frank pus yielded only a few colonies of skin and environmental organisms. All was shadows and fog, and the reputations of the microbiology units of many hospitals plummeted from the high levels they had attained earlier.
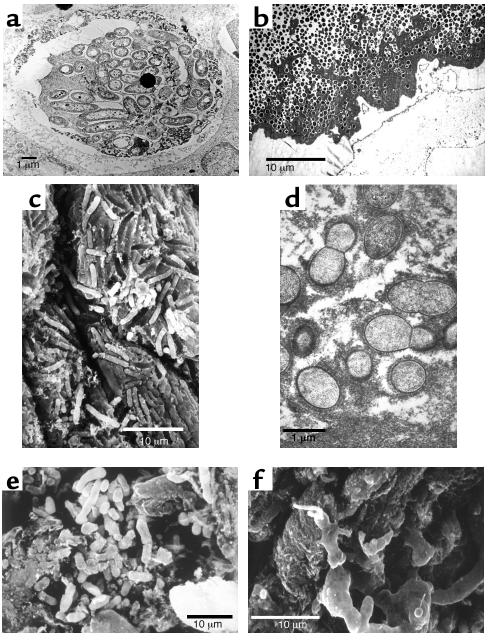
When our research group was unable to imagine why bacteria associated with chronic infections would grow as planktonic cells when biofilms predominated in virtually all industrial and environmental ecosystems, we undertook a series of simple morphological examinations. The bacteria in a very large number of device-related infections were seen to grow in biofilms (2–4), and these infections were recalcitrant to antibiotic therapy and insensitive to host defense mechanisms. The cells of P. aeruginosa in the sputum and in the lungs (postmortem) of CF patients were seen to grow in biofilms in which the cells were surrounded by very large expanses of matrix material (Figure 1a) (5). The cells of the pathogens that caused osteomyelitis in patients and in lab animals were seen to grow in enormous biofilms in which millions of bacterial cells (Figure 1b) were embedded in thick matrix material (6). Cells of Proteus vulgaris that caused chronic pyelonephritis, with complications involving the formation of “staghorn” calculi, were seen to grow in biofilms whose matrix had been “petrified” by crystalline struvite (Figure 1c). Vegetations in endocarditis caused by Candida species were composed of yeast and hyphal elements embedded in matrix material, and vegetations in experimental and clinical native-valve endocarditis were composed of streptococcal cells in equally extensive fibrous matrices (Figure 1d). The prostate of a patient whose EPS samples had yielded no bacteria was seen to be colonized by bacterial cells (Figure 1e) whose matrix material could be visualized especially well when it was reacted with the patient’s serum to yield the classic quellung reaction (Figure 1f). In COME luxuriant biofilms were demonstrated to be growing directly on the mucosal surface, and vital dye imaging showed that the embedded bacteria were all viable in spite of antibiotic treatment and loss of culturability. In some infections the biofilms were composed almost exclusively of bacterial cells and matrix material, while the microbial communities in other infections also contained fragmented platelets and host molecules (e.g., fibrin), so that the bacteria were widely dispersed. However, biofilms were found in all of the chronic infections examined in the 12 years during which this morphological series was pursued.
Figure 1.
Electron micrographs of pathogenic bacterial biofilms from a variety of bacterial infections. (a) Transmission Electron Micrograph (TEM) of a section of lung tissue taken (postmortem) from a CF patient. The matrix-enclosed micro-colony of P. aeruginosa cells is surrounded by a prominent electron-dense “crust” of material that reacted very strongly with antibodies directed against IgG. Image published with permission from Infection and Immunity (59). (b) TEM of a section from the affected bone of a patient with very long-term osteomyelitis that had been treated with antibiotics (for four decades) and several debridations. Note the large number of Gram-positive cells (S. aureus was cultured) and the dehydrated remnants of the fibrous matrix of this massive biofilm. (c) Scanning electron micrograph (SEM) of a struvite crystal from the hilus of the kidney of a patient with acute pyleonephritis, who was affected by “staghorn” calculi. Cells of the infecting agent (P. vulgaris) have formed a biofilm whose matrix has become infused with struvite to produce a “petrified” biofilm. (d) TEM of a section from a vegetation formed on the endocardium of a rabbit in an animal model of native-valve endocarditis. Cells of the infecting agent (viridans group streptococci) are seen to have formed this macroscopic biofilm and to have produced very large amounts of fibrous matrix material. (e) SEM of tissue from a culture-negative prostatitis, showing the presence of rod-shaped bacterial cells. (f) SEM of tissue from the prostatitis patient in e, which had been reacted with the patient’s serum, so that the matrix material of this well-developed biofilm was protected from dehydration, and is shown at its full extent.
Morphological data are unequivocal in that it is highly unlikely that bacteria do not exist in a tissue in which we have found the distinctive features of prokaryotic cells, and these cells are integrated into their matrices and into the structure of the tissue itself. However, morphological data tell us little about the species of bacteria that are present in these chronic infections, or about their viability and their phenotypic pattern of gene expression at the time that the sample was obtained. These data are provided by modern techniques for the analysis of nucleic acids, and the Center for Genomic Sciences (Pittsburgh, Pennsylvania, USA) has established that living bacteria are present in otitis media and in otorrhea with effusion (7). Curtis Nickel’s group has used nucleic acid analysis to establish that bacteria are present in EPS samples from prostatitis patients and from some individuals without overt symptoms (8). Similar analysis of sputum from CF patients has shown that cells of P. aeruginosa are present and that they express a phenotype different from that seen in planktonic cells in culture. Finally, Pradeep Singh and colleagues have analyzed the ratio between two types of acyl homoserine lactone (AHL) signals to show that the cells of P. aeruginosa in the CF lung do indeed grow in the biofilm phenotype (9). At some time in recent history the conceptual balance tipped in favor of a biofilm etiology, at a different time in the case of each chronic disease, and the general notion that device-related and other chronic bacterial infections are caused by biofilms is now widely accepted (1).
Biofilm science as it applies to chronic infectious diseases.
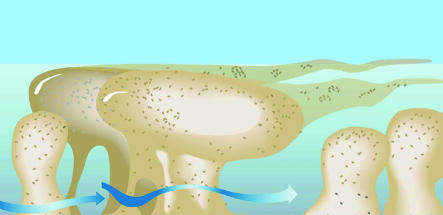
Biofilm science and biofilm engineering have been active fields of study since these sessile communities were first described and named in 1978 (10). Biofilms were first visualized as matrix-enclosed aggregates of bacteria, very similar to the planktonic cells of the species concerned, that were simply immobilized on surfaces or at interfaces in the ecosystems in which they were known to predominate. Later, direct examination of metabolic processes in natural biofilms showed that these sessile populations are much more active than their planktonic counterparts (11). Direct structural examination of biofilms showed that their component micro-colonies (Figure 2), which are composed of cells (±15% by volume) embedded in matrix material (±85% by volume), are bisected by ramifying water channels that carry bulk fluid into the community by convective flow (12). Direct measurement of several parameters by the use of microelectrodes established that biofilms are structurally and metabolically heterogeneous and that aerobic and anaerobic processes occur simultaneously in different parts of the multicellular community. The structural sophistication of biofilms suggested that these communities must be regulated by signals analogous to the hormones and pheromones that regulate multicellular eukaryotic communities, and the first of these regulatory signals were identified in 1998 (13). As we began to study the patterns of gene expression in biofilms, we found that these patterns produce a distinct biofilm phenotype, which differs very profoundly from that of the biofilms’ planktonic counterparts (14).
Figure 2.
Diagrammatic representation of the tower- and mushroom-like micro-colonies that constitute the structural elements of biofilms. Sessile cells constitute only approximately 15% of the volume of their matrix-enclosed communities, so the micro-colonies are viscoelastic and deformable in high shear. Well-developed water channels conduct water in convective flow and deliver nutrients to most parts of the community. Figure is reproduced with permission from the American Society for Microbiology (60).
Biofilms as self-assembling multicellular communities.
When planktonic bacteria encounter a surface or an interface, they adhere to that interface in a reversible fashion while they “explore” the locale to ascertain whether it offers nutrient or other advantages. If the “decision” favors permanent settlement, the adherent cells upregulate the genes (15, 16) involved in matrix production (within as few as 12 minutes), and the process of biofilm formation begins. Kolter et al. (17) and others have described biofilm formation as a developmental sequence that varies to some extent between species but generally results in the formation of a mature community of tower- and mushroom-shaped micro-colonies (Figure 2). Both biofilm formation and biofilm detachment are under the control of chemical signals of the same type that regulates quorum sensing (18, 19), and these regulatory molecules guide the formation of slime-enclosed micro-colonies and water channels. The cells are remarkably evenly distributed in the biofilm matrix, suggesting that some matrix component may dictate their precise location (20); Stoodley et al. have shown that the community as a whole has material properties similar to those of a viscous fluid. The micro-colonies are deformable, they oscillate in high-shear systems, and they break and detach as biofilm fragments if their tensile strength is exceeded by the shear forces (21). Biofilms also show “creeping” activity, in high shear, and whole biofilms can be seen moving across surfaces, with the development of transitory waves and areas of enhanced detachment. These mechanical properties of biofilms are documented in video sequences that are available on the Center for Biofilm Engineering website (http://www.erc.montana.edu). Biofilms form in many high-shear environments, like the vegetations that form on native heart valves, so these material properties are germane and can be used to predict when and where fragments will detach and where they will end up in a flowing system.
Biofilms as protected sessile communities.
Stoodley et al. (20) have made the point that microbial biofilms constitute the most “defensive” life strategy that can be adopted by prokaryotic cells. In very hostile environments, in which many locations are too hot or too acid or too dry, the stationary mode of growth is inherently defensive, because bacterial cells are not swept into areas where they will be killed. These stationary sessile communities, which seem to have predominated in primitive earth, were attacked by bacteriophages and by amoebae, and their collective growth in matrix-enclosed micro-colonies gave complete protection from these predators. One of the most important protections afforded by the biofilm mode of growth is protection from drying and from ultraviolet light. These sessile communities are especially favored, and very visible, in the intermittently wet splash zones of freshwater and marine ecosystems, where these communities allow prokaryotic cells to survive daylong exposures to drying and intense sunlight and to revive when they are rehydrated. Experiments conducted in military defense establishments have shown that planktonic cells of lab-adapted strains of bacteria survive for very short lengths of time when they are released from high-flying airplanes, whereas cells in biofilm fragments survive long enough to reach the ground. In their 2002 review, Stoodley et al. (20) made the intriguing suggestion that bacteria may have developed their biofilm phenotype early in the evolutionary process, when survival in a hostile environment was a sine qua non. They suggest that the planktonic phenotype, with its genetically “expensive” and very sophisticated chemotactic and motility mechanisms, may have developed later, for the purposes of dissemination and the colonization of new habitats.
The biofilm as a distinct, signal-controlled phenotype.
The inherent resistance of biofilm bacteria to antibiotics (22) and to virtually all antibacterial agents was an early indication that sessile cells differ radically from their planktonic counterparts. When it was established that this resistance was not the result of diffusion limitation (23) but was predicated on some change in cellular characteristics (24), the research community missed the central point and wandered clueless for more than a decade. Then various groups began to use the tools of modern molecular biology, like mRNA analysis by gene arrays (25–27) and proteomic analysis of gene products (28–30), and they showed that cells in biofilms express a radically different set of genes. This virtual “bombshell” of the concept of a distinct biofilm phenotype has changed most of the intellectual constructs in the biofilm field. The genes expressed in a biofilm differ from those expressed in the corresponding planktonic cell more than they do from the genes expressed in a spore or in the biofilm cells of another species (28). There is no single biofilm phenotype, but gene expression in sessile communities goes through a whole spectrum of changes as the community matures (20), and the planktonic phenotype begins to emerge as the biofilm begins to shed mobile cells. Now that we know that bacteria adopt a radically different phenotype when they adhere to a surface or interface and initiate biofilm formation, we understand such mysteries as the resistance of biofilms to antibiotics in terms of the expression of different sets of genes. Many other characteristics of sessile bacteria can now be explained in the same way, and matrix-assisted laser desorption-ionization time-of-flight (MALDITOF) and gene chip analyses of the genes expressed in biofilms are already painting a picture of considerable variety and specialization in these multicellular communities. In the microbiological community, the notions are taking shape that biofilms predominate in most ecosystems and that these sessile cells express genes we have never seen expressed and reach levels of interaction and community behavior that we have never imagined. Now that the phenotypes of planktonic and biofilm cells have been shown to be so profoundly different, pure cultures of planktonic bacteria seem to be a very poor model for the study of these organisms, which actually grow in mixed-species sessile communities in natural and medical systems (31).
The simple observations that microbial biofilms are composed of structured micro-colonies and that water channels are created and maintained suggested that some type of cell-cell signal system must be operative in these multicellular communities. We speculated that these signals would resemble the hormones that dictate the structure of more complex eukaryotic organisms and communities, and we visualized a type of “embryology” of biofilms. We demonstrated that the quorum-sensing molecules that had been discovered in planktonic bacteria (18) also control biofilm development (13) and many other bacterial behaviors, and we now speculate that these signal systems may have evolved to control biofilms, especially in the light organ of the squid. The AHL signals of Gram-negative bacteria and the two-component peptide signals of Gram-positive bacteria (32) have been shown to affect biofilm formation, and the newly discovered autoinducer II signal (33) appears to have a similar effect. Analysis of signal-negative mutants suggests that all aspects of bacterial behavior are regulated by an interactive network of signals, with large inputs from environmental factors, so that biofilms can now be seen as integrated communities that are very reactive to their environments. This demonstration of cross-talk among bacteria of the same and of different species presents us with the Rosetta stone of the bacterial language that controls biofilm formation, species interactions, growth rates, toxin production, invasive properties, and many other behaviors of great interest to humankind. We estimate that dozens of new signal systems will be discovered in the next decade, and that control measures based on signal manipulation will largely replace bactericidal agents in human attempts to control bacteria (34). This new strategy has gained some impetus from the observation that many plants use natural analogues of bacterial signals to inhibit (35) or to encourage bacterial colonization and biofilm formation on their surfaces. The pharmaceutical industry, which has been bedeviled by the emergence of resistance to all of their most carefully crafted antibiotics, is intrigued by the fact that resistance to these natural antibiofilm signal analogues has not yet been recorded.
A re-examination of chronic bacterial diseases using biofilm concepts.
In view of the unequivocal demonstration that the bacteria that cause device-related and other chronic bacterial infections grow in biofilms, it seems useful to survey this large number of infections for new insights in terms of this new microbiological concept.
What new biofilm-specific methods tell us about biofilm bacteria.
One of the most time-honored of microbiological conventions is the detection of bacteria by recovery methods, which usually involve mechanical removal of the organisms (often by swabbing) and their propagation in liquid or on solid media. We recently conducted a survey (36) of the bacterial colonization of the vaginas of 3,000 human subjects using standard swabbing and culture (plating) techniques, and concluded (as most similar studies have) that 10.8% of individuals carried Staphylococcus aureus. We then examined subsets of these individuals, using PCR to identify S. aureus by its DNA and using FISH probes to identify cells of this species by their 16S RNA content, and we found that 100% of these individuals carried this organism. We then examined whether individuals who yielded positive data in swab and plate tests carried more S. aureus as detected by PCR and FISH, and found there was no correlation (36). If certain bacteria are present on a tissue or an inert surface, the swab may or may not pick them up, they may be present in huge aggregates of hundreds of cells that will yield only one colony on plating, and they may be expressing the set of genes that constitute the biofilm phenotype and be unable to grow in the culture conditions provided. Swabbing and plating are techniques that date from the middle 1800s, but they are still used to screen key medical personnel for nasal carriage of S. aureus as well as to detect bacteria in suspected infections and to identify the pathogens concerned. It may be time for us to adopt more modern and accurate methods.
The biofilm concept of chronic infections offers at least a partial explanation for a phenomenon that has troubled clinicians for some time, that blood cultures from patients who show many signs of overt bacterial infection are often negative. If we remember that the infecting organisms exist both as biofilms and as individual planktonic cells that trigger most of the overt symptoms of infection, we must expect the planktonic cells to be killed by circulating antibiotics and activated phagocytes. Because the planktonic cells are killed and the biofilm cells are not released, except as multicellular matrix-enclosed biofilm fragments, no colonies develop on the plates, and negative reports are returned. The same problem occurs in middle ear (37) and prostate (8) infections, in that the reservoirs of biofilms in the tissues shed few fragments, and the planktonic cells that are detached from these sessile communities are killed by antibiotics and phagocytes before they can be recovered and grown. Until these biofilm bacteria were detected by molecular methods such as PCR and FISH probing, their bacterial etiology was in question and bizarre viral and/or immunological etiologies were being proposed.
We have found bacterial biofilms in a series of orthopedic implant patients who had been diagnosed as having “aseptic loosening” of hip prostheses (G. Maale, personal communication), because no bacteria had ever been cultured from blood, from tissues, or from the prostheses themselves. What had previously been seen as a mechanical problem became an infection problem, and replacement of the prostheses has been accomplished very successfully with the use of aggressive perioperative antibiotic therapy. When early detection of biofilm infections is particularly important, as in the case of vascular grafts that may fail catastrophically if they develop biofilm infections, new ELISA tests to detect antibodies against biofilm-specific epitopes common to all staphylococcal species are proving to be particularly useful. Incipient biofilms stimulate antibodies against biofilm within 10 days of the initial colonization of these vascular grafts, but staphylococcal cells are virtually never recovered by conventional culture techniques.
When bacteria are isolated from patients with biofilm diseases, they are plated to produce single colonies, and material from these single colonies is used to inoculate liquid media for their subsequent propagation. When cells of P. aeruginosa are first cultured in liquid media, they form macroscopic biofilms on the surfaces of the culture vessel, especially at the air-water interface. When planktonic cells are withdrawn from the culture to inoculate subsequent liquid media, the biofilms are left behind, and the next culture shows less “scum” formation on surfaces. After cultures have been passaged several times in liquid media, we have all seen that the cells of P. aeruginosa grow as an evenly turbid suspension in the liquid medium, and thus we have transformed a biofilm-forming pathogen into a planktonic lab-adapted strain. These lab-adapted cultures are really not good models for the study of diseases that have been shown unequivocally to be caused by bacteria growing in biofilms (5).
One of the most spectacular cases in which modern methods have been used to resolve long-standing anomalies is in the area of the resistance of biofilms to host defenses. Ward et al. (38) showed that preformed biofilms could be introduced into the peritonea of rabbits that had both bactericidal and opsonizing antibodies as well as normal phagocytic cells, and that the sessile communities persisted for weeks and even months. At first, this anomaly was explained in terms of exclusion of both antibodies and phagocytes by the biofilm matrix (39), but more recently Leid et al. (40) have shown that activated polymorphonuclear neutrophils (PMNs) are attracted to biofilms and may penetrate these sessile communities. Figure 3 shows PMNs that have entered into the water channels of a biofilm (see movie at http://www.erc.montana.edu), and have penetrated 5–10 μm into individual micro-colonies. However, although the membranes of these phagocytes remain intact, they seem to be “paralyzed” in that they have not internalized any bacteria and that bacteria that are only 3–5 μm from these normally very aggressive leucocytes are alive (Figure 3, green) in this viability stain. This resistance to normal host defense factors may have been acquired when bacteria formed biofilms in the primitive earth (20) for defense against bacteriophages and against free-living amoebae.
Figure 3.
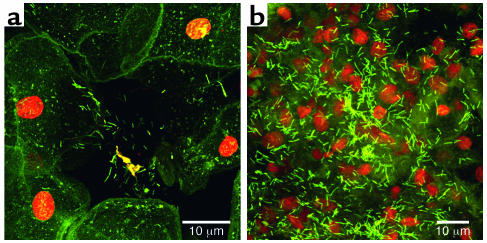
Confocal scanning-laser micrograph showing the invasion of a biofilm by PMNs. The PMNs (large red nuclei) have entered the biofilm via the open water channels and have invaded short distances (1–5 μm) into the biofilm. The bacterial cells have been stained with the live/dead BacLight stain (BacLight Bacterial Viability kit; Molecular Probes, Eugene, Oregon, USA) and living bacterial cells (green) are seen in very close proximity (<1 μm) to PMNs. We conclude that PMNs invade biofilms but are virtually inactive in killing sessile cells and resolving biofilm infections. Reproduced with permission from Infection and Immunity (40).
The potential effect of the biofilm concept on the management of chronic infections.
If we (the scientific microbiology community and the clinical infectious disease community) visualize the bacteria that cause device-related and other chronic infections as planktonic cells swimming or floating at or near the surfaces of the affected biomaterials and tissues, we will persist in the control strategies that have failed us so often in the past. If we grasp the concept that these bacteria are embedded in matrix material, that they have adopted a distinct biofilm phenotype, and that they have formed interactive communities, then we can bring all of the power of biofilm science to bear on chronic infections. If we acknowledge that the surface layers of the skin are colonized by living biofilms of S. epidermidis, even after the bacteria and fungi on the skin surface have been killed by surgical preparations, we will not allow devices to touch the cut edges of the skin when they are installed. If we realize that all vascular catheters are heavily colonized by biofilms if they have been in place for more than 1 week, we will not replace them over wire catheter replacement guides (J wires), because the wire will displace biofilm fragments and the new catheter will be inoculated as it slides over the wire. When we visualize macroscopic bacterial biofilms (vegetations) on natural or mechanical heart valves, we will realize that these large micro-colonies will form living bacterial emboli in the nearest capillary beds if they are detached by surgical interventions. We will treat acute exacerbations of device-related infections with antibiotics, and we will expect to see the resolution of many overt symptoms, but we will not expect to kill all the sessile cells in the biofilm on the device. If we realize that we are confronted by classic biofilm infections, we will probably remove devices (with their adherent biofilms) sooner rather than later, and we will use aggressive antibiotic therapy to prevent the recolonization of the replacement devices.
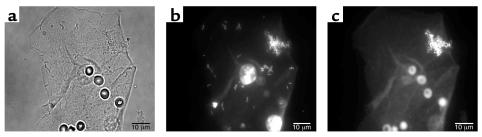
When we acknowledge that all device-related and other chronic bacterial infections are caused by bacteria living in biofilms, we will use biofilm-specific methods in diagnosis and research-based therapy. Because biofilm science has discredited “swab and plate” census methods, we will use direct microscopic methods to map and speciate the natural bacterial populations of human tissues, including the middle ears of children and the prostate glands of older men. These direct methods usually begin with the simple visualization of the tissue surface (Figure 4a) in situ or ex situ; the adherent bacteria can usually be resolved by simple phase-contrast microscopy. Then all of the colonizing bacteria can be stained with fluorescein or with the eubacterial domain (known as EUB 338) 16S RNA probe (Figure 4b), and living bacteria can be differentiated from dead cells by the use of the BacLight live/dead stain. After this overall census, we can identify and locate the cells of species of interest (Figure 4c) using specific 16S RNA probes in a FISH reaction that causes only the cells of the species for which the probes were designed to fluoresce.
Figure 4.
Fluorescence micrographs of epithelial cells recovered from the vaginas of human volunteers. (a) A single epithelial cell with highly refractile blood cells (the volunteer was menstruating) and colonized by rod-shaped and coccoid bacteria. (b) A similar cell reacted with the fluorescent eubacterial domain (EU 338) probe, whose base sequence reacts with the 16S RNA of all eubacteria, including both lactobacilli and staphylococci. (c) The same cell as in a reacted with a fluorescent probe that reacts only with the 16S RNA of S. aureus. This organism was found in the vaginal flora of all volunteers tested, and this result was confirmed by PCR. Figures reproduced with permission from the Journal of Infectious Diseases (36).
If we know what natural and pathogenic bacterial populations are present on human tissues in many organ systems and we can determine the species identity and viability of these colonizing organisms, we can place diagnosis and treatment on a solid logical base. If we can see cells of P. aeruginosa growing in biofilms in the lungs of CF patients and we can detect very high titers of antibodies against Pseudomonas in the sera of these patients, we will be unlikely to continue with unsuitable immunization experiments (41). If we can see bacteria growing in biofilms in the middle ears of all children while we note symptoms of infection in only a subset that does not differ in the nature or extent of colonization, we will probably examine the host response to this colonization. If we examine the host response to this and other microbial colonization of tissues, we may be led to manipulate the Th1 and Th2 immune responses, instead of trying (unsuccessfully) to use antibiotics to kill all of the biofilm bacteria on a naturally colonized tissue surface.
When we examine the proteome of cells in biofilms (14) or analyze the biofilm phenotype by using DNA arrays (26), we find that sessile cells express many genes that are never expressed in planktonic cells, and vice versa. Now that we know that the inherent antibiotic resistance of biofilms does not result from diffusion limitation, and that all presently available antibiotics were selected for their ability to kill planktonic cells, we are looking for new biofilm-specific antibiotic targets. Ideally, these new agents will kill planktonic and sessile cells with equal efficacy, and their effects may be enhanced by the specific blockage of the fmtC gene, which mediates antibiotic resistance in biofilm cells of all species of Staphylococcus. Because biofilm science has discovered that many aspects of biofilm behavior are controlled by cell-cell signals, specific signal analogues have been developed to shut off toxin production and to block biofilm formation and “lock” bacteria in the planktonic phenotype. One of these biofilm-control signal analogues (the RNAIII-inhibiting peptide analogue of the RNAIII-activating peptide signal) has been shown to block biofilm formation by all species of Staphylococcus and thus facilitate the killing of cells of these species even in the presence of a biomaterial (34). Biofilm engineering has also contributed new technologies of potential interest in the control of biofilm infections, in that biofilms have been shown to be much more susceptible to conventional antibiotics in direct current electric fields (42) or when treated with ultrasonic radiation (43). As biofilm science and engineering continue their exponential growth and biofilm problems in industry are solved, these concepts and technologies will be made available to medicine as long as we hold fast to the biofilm concept of chronic infection.
The role of biofilm fragments in the initiation of infections.
Because the majority of bacteria in virtually all natural ecosystems grow in biofilms (44), microbial challenge to humans often comes from this source. We know that natural aquatic biofilms recruit and retain pathogens, such as Salmonella and Escherichia coli, and that immunocompetent humans can be infected from these sources. Immunocompromised individuals may be especially susceptible to the nontubercular Mycobacterium avium complex organisms that are a major component of many natural aquatic biofilms. Horizontal gene transfer is facilitated in biofilms (45), and G. Ehrlich and colleagues have recently suggested (46, 47) that these sessile communities may play a major role in the pathogenicity of bacterial species (e.g., Vibrio cholerae) that alternate between human hosts and natural reservoirs. This group has offered evidence that species of bacteria are composed of multiple strains, each of which contains a unique distribution of contingency genes from a population-based supragenome that is much larger than the genome of any single pathogen. During periods of stress, bacteria upregulate autocompetence and autotransformation systems within biofilms to promote the reassortment of genes that will result in the creation of some strains that may have a selective advantage under prevailing environmental conditions. They further suggest that natural marine and freshwater biofilms constitute a staging area in which the small DNA sequences can be cobbled together to recreate the pathogenicity “islands” necessary for the organisms’ attack on human populations. It is clear that all examinations of communicable enteric diseases must use biofilm models and that investigators of these systems should subscribe to the biofilm concept.
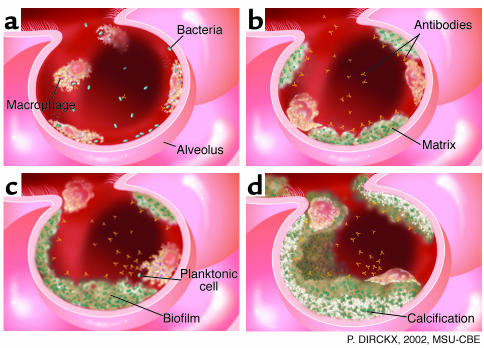
Although the digestive system and the integument may be colonized by bacteria and fungi from environmental biofilms, the human organ system that is by far the most susceptible to attack from environmental biofilms is the pulmonary system. The trachea and the lungs are well designed to resist colonization by planktonic bacteria, and animal experiments have shown the clearance of as many as 1 × 106 bacterial cells in as little as 20 minutes, provided the challenging cells are single and unaggregated (48). Experiments in the same animal species using the same bacterial species (5) have shown that the lungs of normal animals are not able to clear bacteria that are introduced in the form of biofilm fragments or of cells enclosed in artificial matrices (e.g., as agar beads). When biofilm fragments or agar beads containing bacteria are introduced into the lung, these aggregates resist phagocytosis by resident phagocytes and killing by both innate and acquired immune factors, and they persist for weeks and even months (48). Figure 1a illustrates the mode of growth of the micro-colonies that comprise the Pseudomonas biofilm in the lung in animal models of CF, and Figure 5 shows how this matrix-enclosed population persists despite the immune reactions of the host. Woods and his colleagues proposed that the lungs may be colonized by the detachment of biofilm fragments from the oropharynx, which becomes overgrown by P. aeruginosa during periods of stress (49), and that these fragments cannot be cleared by pulmonary defense mechanisms. These data from animal experiments appear to have been confirmed by clinical examination of CF patients, and they raise the very grim specter of the inevitable colonization of the lungs with biofilm fragments when endotracheal tubes become colonized by mixed-species biofilms. Examination of endotracheal tubes that have been used for assisted ventilation has shown massive aggregations of mixed-species biofilms (Figure 6); simple detachment of fragments could lead to chronic colonization of the lungs. In studies of intubated patients in intensive care units, we noted that the biofilms on endotracheal tubes often contained bacteria from the digestive tract when nasogastric tubes were also in use, and that these organisms were often found in the lungs of patients who had died after assisted ventilation. It is well documented that biofilm fragments are aspirated into the lung, and that these matrix-enclosed organisms cannot be cleared by pulmonary defense mechanisms and develop into micro-colonies that persist for months and may give rise to disseminated infection.
Figure 5.
Diagrammatic representation of the defense strategies of the lung. (a) The surface of the alveolar epithelium is “patrolled” by PMNs and macrophages, which phagocytose incoming planktonic bacteria quickly and easily. (b) The alveolar phagocytes are unable to engulf bacteria in matrix-enclosed biofilm fragments, even when these invaders are reacted with specific antibodies. (c) Biofilm fragments grow and burgeon in the colonized lung, and release occasional planktonic cells that react with antibodies and are phagocytosed. (d) The mature biofilm reaches a “standoff” with the immune system, and parts of the microbial community become calcified to form a long-term pulmonary nidus.
Figure 6.
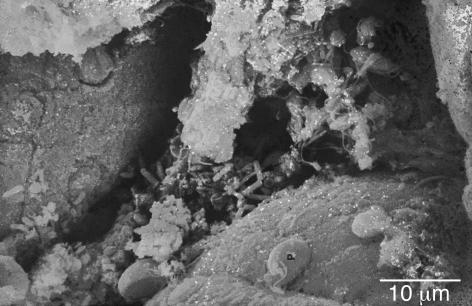
SEM of a large multispecies biofilm aggregate that formed on the lumenal surface of an endotracheal tube used to ventilate a patient in a Systems Failure Intensive Care Unit. These uvula-shaped aggregates have a rubbery consistency, and they routinely break off of these surfaces and are aspirated into patients’ lungs.
If the pulmonary system is in fact susceptible to colonization by aspirated biofilm fragments that cannot be cleared and may act as foci for chronic and/or acute bacterial infection, then other environmental organisms should be able to invade mammalian lungs. For this reason, it is germane to examine a trio of environmental bacteria that are “card-carrying” members of natural ecosystems and that share with P. aeruginosa the invidious ability to colonize mammalian lungs and cause serious diseases in immunocompetent mammals. Pasteurella haemolytica is a component of the normal oropharyngeal flora of cattle that proliferates when the animals are shipped to feedlot operations, and the aspiration of fragments of these biofilms into the lungs of these stressed animals results in respiratory infections that kill as many as 2% of these calves (50, 51). Legionella pneumophila are the predominant inhabitants of warm littoral zones in freshwater lakes, where they grow in association with green algae (Fisheria sp.) and avoid predation by free-living amoebae by forming biofilms and secreting antiphagocytic factors. As humans devised air conditioners and elaborate domestic hot water systems, L. pneumophila found an alternate home in hotels and hospitals, and emerged to kill several dozen elderly gentlemen in a notorious hotel in Philadelphia, as well as many other people in various hospitals and office buildings. An organism that lives in warm water, forms biofilms, and resists phagocytosis by amoeboid enemies survives very well in the condensate trays of air conditioners or in human lungs (52). The remarkable thing is not the pathogenicity of an environmental organism with no previous history of attacking humans, but the chilling realization that thousands of people must have died of legionellosis before the disease was defined by the Centers for Disease Control. If an individual dies of idiopathic pneumonia, this death causes very little interest, but the cause of this death may have been the mobilization of an acute respiratory infection from niduses of biofilm infection that were acquired by the aspiration of biofilm fragments. The sources of these fragments may be environmental, as in the case of cooling towers that cause seroconversion to L. pneumophila in people working downwind of the towers, or they may be very focal, as in the case of dental hygienists who breath aerosols for long periods of time every working day.
Perhaps the best example of a pulmonary disease that is caused by biofilms is meloidosis, which affects people in Southeast Asia and Australia, and caused pulmonary infections in US soldiers in Vietnam. The causative pathogen is Burkholderia pseudomallei, which is a natural component of the freshwater ecosystems in the area; humans make contact with biofilms formed by these organisms when they work in rice paddies or swim in local rivers (53). Rates of seroconversion indicate that more than 80% of the people in northeast Thailand have been exposed to this pathogen, presumably by aspiration of fragments of its exuberant biofilms, and we have visualized a population with multiple micro-colonies in their lungs (53). When we set up animal experiments by introducing agar beads containing these organisms into the lower left lobes of the lungs of guinea pigs, we induced an asymptomatic chronic infection that persisted for months (54). However, when we stressed these chronically infected animals with steroid injections (54), planktonic bacteria were released from the biofilm micro-colonies, and the animals rapidly succumbed to the resultant acute infections and bacteremias. Several hundreds of people die of acute meloidosis in northeast Thailand when they are stressed by seasonal starvation cycles, so the response to stress seen in the animal model appears to operate in human populations. These data add to the Damoclean image of the pulmonary health of modern humans, because bacteria from biofilm niduses acquired by the aspiration of biofilm fragments from many sources may be mobilized in times of stress and may cause acute pneumonias. So, many among us may carry the seeds of fatal pneumonia (the “old man’s friend”) in their lungs as they enjoy their air-conditioned offices and their homes with spas and hot tubs.
Biofilms may also be involved in the dissemination of disease within the body of an infected individual. When we speak of the hematogenous spread of infection, we must now specify whether the infectious units are planktonic cells or biofilm fragments, because these entities differ radically in important properties such as their antibiotic resistance and their adhesion characteristics. Planktonic cells are shed from virtually all mature biofilms, they are generally susceptible to antibiotics, and they adhere to certain tissues and to inert surfaces with considerable avidity. For this reason it is logical to use prophylactic antibiotic therapy to prevent the colonization of recently installed medical devices by planktonic bacteria introduced into the bloodstream by routine tooth brushing or by dental manipulations. On the other hand, many of the cells that detach from biofilms growing on native heart valves (resulting in endocarditis) or vascular catheters are in the form of matrix-enclosed biofilm fragments (21) that are very resistant to antibiotics, and they usually circulate until they “jam” in a capillary bed. For this reason, low-dose antibiotic therapy does not prevent the dissemination of bacteria in these biofilm diseases, and the best way of preventing this process is very aggressive high-dose treatment of native valve endocarditis and rapid removal of colonized vascular devices. We have developed animal models of dissemination in biofilm diseases, and we have been amazed at how well sheep lungs can tolerate the small hemorrhages that result from hundreds of biofilm fragments lodging in capillary beds. However, it is clear that one or two large biofilm fragments can cause profound damage if they detach and find their way to critical loci in the lungs or in the brain.
The role of commensal biofilms in protection from infection.
One of the conceptual areas in which microbial ecology can assist medical practitioners most effectively and immediately is the area of natural microbial biofilms on the surfaces of normal human tissues. These tissues are among the most attractive surfaces in nature because they are homeostatic and rich in simple nutrients, and bacteria have adapted to virtually all types of mammalian tissues with considerable success. Perhaps the least welcoming tissue is dry skin, but cells of S. epidermidis grow in prolific biofilms between the squamous cells of the outer three to ten layers of this stratified epithelium and colonize hair follicles and sebaceous glands very successfully. Secretory epithelia like those of the eye, the vagina, and the mouth attract many species of bacteria. The eye limits colonization by producing antibacterial factors (surfactants and defensins); the vagina develops an exclusive acid-dominated environment favoring Lactobacilli (Figure 4, a–c, and Figure 7); and the mouth is colonized by a remarkable series of multispecies biofilms on different surfaces. Mucus-secreting tissues, like those of the trachea and the intestine, are covered by “mucus blankets,” and this viscous material is often moved across the surface of the tissues by such forces as ciliary beating or by peristalsis. Bacteria have great difficulty in accessing mucus-covered tissues, especially if the mucus blanket is 200–250 μm in thickness and is moving at a considerable speed across the tissue surface. For this reason, commensal organisms often use “hold fast” mechanisms and flagella that operate well in viscous fluids just to stay in the tissue surface ecosystem, and successful pathogens often find ways to disrupt the mucus blanket and gain access to the tissue surface. The outer surfaces of “mucus blankets” constitute a rich, if somewhat ephemeral, bacterial habitat, and many species proliferate on these surfaces (Figure 8).
Figure 7.
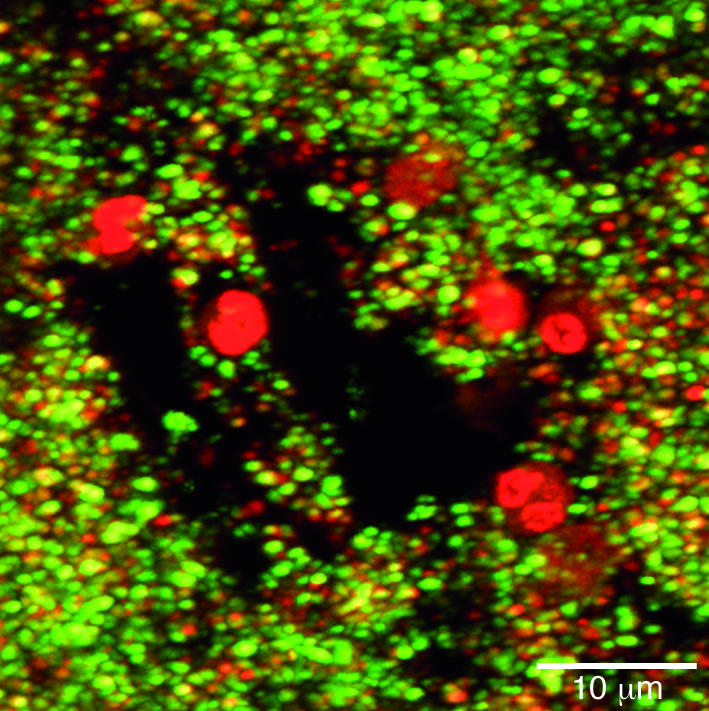
Confocal scanning-laser micrographs of tissue recovered from the vaginal epithelium of volunteers and stained with fluorescein. (a) The orange nuclei and green cytoplasm of the human cells are clearly seen, as are the bacterial cells in a biofilm aggregate partly detached from human cell surfaces. (b) The green rod-shaped cells of the vaginal biofilms are seen, with the lighter green matrix material that surrounds them, in aggregates at least 30 μm “tall.” The extent and thickness of the vaginal biofilm are indicated by the fact that the orange tissue nuclei appear to be “buried” by this beneficial microbial population.
Figure 8.
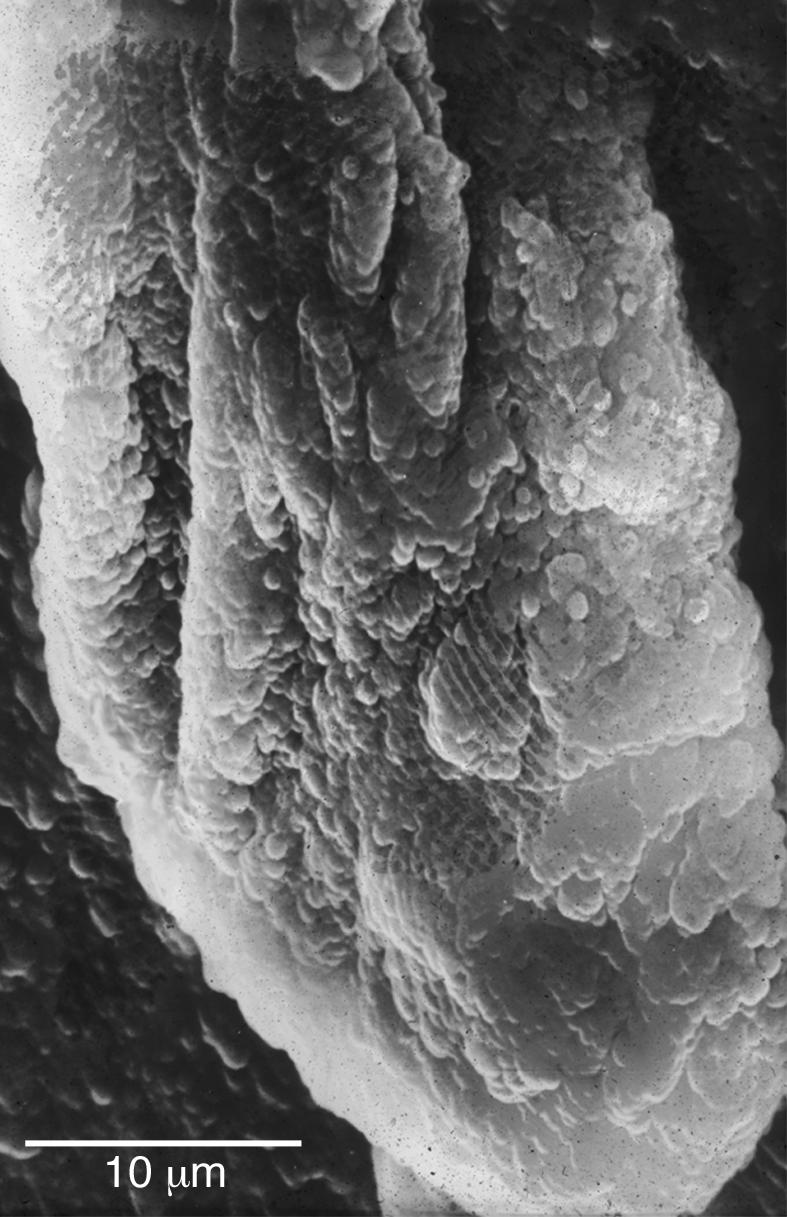
SEM of the lumenal surface of the intestine of a mouse. The dehydration-condensed residue of the intestinal biofilm is seen to occupy much of the tissue surface, and to be composed of a rich mixture of bacterial and protozoan species. A large Giardia cell is seen to be attached to the surface (P) of the intestinal epithelium, while a detached cell of the same species shows its well-developed sucker structure, and the microvillar surface of opposite side shows the scars of previous protozoan attachment. Attachment plays a large role in the microbial ecology of the intestine, because the intestinal mucus exerts powerful shear forces that tend to remove loosely attached organisms.
Many organ systems in the human body must, in order to fulfill their normal functions, make contact with the bacteria-laden environment at their distal extremes, while maintaining strict asepsis in their proximal “core” organs. A simple example is the biliary system, which must deliver bile to the intestine while maintaining a bacteria-free situation in the liver, which is extraordinarily sensitive to bacterial infection (55). In extensive microbiological studies of the biliary system in the cat, we discovered that the common bile duct is not colonized by gut bacteria, but that these organisms enter the duct if the valve-like sphincter of Oddi is compromised or if a biliary stent is inserted (55). Planktonic bacteria make periodic excursions from the gut to the gall bladder, but in the absence of an inert surface on which to form a biofilm, they cannot colonize the tissues in the presence of bile salts, and they are washed back to the intestine. We speculated that most cholangitis results from vascular challenge, not from traffic in the bile duct, and we showed that the introduction of as few as 1 × 105 cells into the posterior vena cava was sufficient to overwhelm the defenses of the liver and cause acute hepatic disease (56). A more complex example of an organ system that must maintain strict sterility in core organs, while allowing microbiological traffic in more distal organs, is the female reproductive tract. The peritoneum, the ovaries, and the fallopian tubes must be protected from bacterial colonization, and the fallopian tubes are particularly sensitive to scarring (with resultant sterility) if they are exposed to bacteria. In contrast, the vagina and the uterus are exposed to bacteria from environmental and intestinal sources, and they must accommodate the passage of sperm while providing a sterile environment for the implantation of the egg and the development of the fetus. The fact that this system has functioned so well, even in primitive mammals and in humans without modern housing and bathing facilities, is evidence for the efficacy of its microbiological “design.”
The partnership between the vaginal tissues and species-specific strains of Lactobacillus is exemplary, in that the lactic acid–rich environment of this organ is credited with enabling internal fertilization in the evolution of mammals from amphibians. It seems impossible that these lactic acid–producing organisms dominate the surfaces of these tissues by first access, and we have proposed that they are attracted to this niche by animal-bacterial signals like those that dictate the colonization of the digestive tissue of newborn ruminants (57). We have shown that mammalian sperm cotransport bacteria when they traverse the vagina and enter the uterus, and we speculate that the acidic environment of the vagina may slow growth and biofilm formation in non-Lactobacillus species. Direct examination of the surfaces of the endometrium in human uteri removed because of fibroid development has shown the presence of bacterial biofilms that cover as much as one third of the surface of this organ. Moreover, in people using many types of intrauterine contraceptive devices (IUDs), these plastic and metal devices were very extensively colonized by very thick bacterial biofilms (58). The use of IUDs did not make people permanently sterile, and normal uteri are colonized by bacterial biofilms, so we conclude that neither the biofilms on the IUD nor those on the endometrium regularly shed planktonic cells that infect the fallopian tubes. We therefore speculate that the host defenses of the endometrium must be sufficiently efficient to kill or engulf all planktonic cells that are released from these biofilms before they can infect “upstream” organs.
The methods available to modern microbial ecologists (Figures 3, 4, and 7) allow these intrepid scientists to map the microbial colonization of tissues within organ systems, to identify and locate all species of interest, and to assess viability and biofilm formation. A full knowledge of the extent and nature of bacterial biofilms on these tissues will allow us to determine the role that they play in normal tissue functions and in resistance to disease. Happily, these methods can also be used to determine the invidious effects of broad-spectrum antibiotic therapy and of other common medical procedures, and we may be able to avoid damage to natural biofilm populations, on which we depend for much of our protection from infectious disease.
Epilogue
The bacteria that colonize the tissues of the human body are no different from those that colonize the surfaces in all other aquatic ecosystems, in that they form biofilms in preference to growing as planktonic cells. If the tissue in question is exposed to the environment and is “designed” to be colonized by bacteria, these commensal biofilm populations may be beneficial, in that they preclude colonization by pathogens. If biofilms form on the surfaces of tissues or of natural (e.g., teeth) or artificial and extraneous inert materials in the body, they may grow slowly and be well controlled, in that the planktonic cells that are released may be killed by normal defense mechanisms. In these cases, the colonizing biofilms may be essentially nonpathogenic but capable of causing inflammation and bacterial dissemination if they become more extensive or if the host becomes compromised. Once the balance between colonization and infection has been tipped in favor of overt infection, biofilms constitute a peculiar problem that characterizes 65% of infections treated by physicians in the developed world (1). Although antibiotic therapy and activated host defenses can kill derived planktonic cells and often obviate symptoms, they cannot kill the biofilm cells that constitute the niduses of these chronic infections. For this reason, colonized medical devices must often be removed in order to effect a cure, and patients with devices and tissues that cannot be removed must reconcile themselves to intermittent antibiotic therapy for the remainder of their lives. The most immediate hope in this dismal prognosis is the pace at which biofilm science discovers new agents that preclude biofilm formation and “lock” potential pathogens in the planktonic mode of growth, in which they can be killed by antibiotics and host defense factors.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: cystic fibrosis (CF); chronic otitis media with effusion (COME); expressed prostatic secretion (EPS); acyl homoserine lactone (AHL); polymorphonuclear neutrophil (PMN); intrauterine contraceptive device (IUD).
References
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Marrie TJ, Nelligan J, Costerton JW. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation. 1982;66:1339–1341. doi: 10.1161/01.cir.66.6.1339. [DOI] [PubMed] [Google Scholar]
- 3.Marrie TJ, Costerton JW. Scanning and transmission electron microscopy of in situ bacterial colonization of intravenous and intraarterial catheters. J. Clin. Microbiol. 1984;19:687–693. doi: 10.1128/jcm.19.5.687-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury AE, Lam K, Ellis B, Costerton JW. Prevention and control of bacterial infections associated with medical devices. ASAIO Transactions. 1992;38:M174–M178. doi: 10.1097/00002480-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Lam J, Chan R, Lam K, Costerton JW. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect. Immun. 1980;28:546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambe DW, Jr, Ferguson KP, Mayberry-Carson KJ, Tober-Meyer B, Costerton JW. Foreign-body-associated experimental osteomyelitis induced with Bacteroides fragilis and Staphylococcus epidermidis in rabbits. Clin. Ortho. 1991;266:285–294. [PubMed] [Google Scholar]
- 7.Rayner MG, et al. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA. 1998;279:296–299. doi: 10.1001/jama.279.4.296. [DOI] [PubMed] [Google Scholar]
- 8.Nickel JC, Costerton JW, McLean RJC, Olson M. Bacterial biofilms: Influence on the pathogenesis, diagnosis and treatment of urinary-tract infections. J. Antimicrob. Chemother. 1994;33:31–41. doi: 10.1093/jac/33.suppl_a.31. [DOI] [PubMed] [Google Scholar]
- 9.Singh PK, et al. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- 10.Costerton JW, Geesey GG, Cheng GK. How bacteria stick. Sci. Am. 1978;238:86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- 11.Wyndham RC, Costerton JW. Heterotrophic potentials and hydrocarbon degradation potentials of sediment microorganisms within the Athabasca oil sands deposit. Appl. Environ. Microbiol. 1981;41:783–790. doi: 10.1128/aem.41.3.783-790.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Beer D, Stoodley P, Lewandowski Z. Liquid flow in heterogeneous biofilms. Biotechnol. Bioeng. 1994;44:636–641. doi: 10.1002/bit.260440510. [DOI] [PubMed] [Google Scholar]
- 13.Davies DG, et al. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 14.Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 2002;184:1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies DG, Chakrabarty AM, Geesey GG. Exopolysaccharide production in biofilms: Substratum activation of alginate gene expression by. Pseudomonas aeruginosa. Appl. Envir. Microbiol. 1993;59:1181–1186. doi: 10.1128/aem.59.4.1181-1186.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies DG, Geesey GG. Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl. Environ. Microbiol. 1995;61:860–867. doi: 10.1128/aem.61.3.860-867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Toole GA, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 18.Fuqua WC, Winans EP, Greenberg EP. Quorum sensing in bacteria: The Lux R-Lux I family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuqua WC, Greenberg EP. Listening in on bacteria: acyl-homoserine lactone signaling. Nat. Rev. Mol. Cell Biol. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 20.Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- 21.Stoodley P, et al. Growth and detachment of cell clusters from mature mixed species biofilms. Appl. Environ. Microbiol. 2001;67:5608–5613. doi: 10.1128/AEM.67.12.5608-5613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nickel JC, Ruseska I, Wright JB, Costerton JW. Tobramycin resistance of cells of Pseudomonas aeruginosa growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 1985;27:619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suci PA, Mittelman MW, Yu FP, Geesey GG. Investigation of ciprofloxacin penetration into Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 1994;38:2125–2133. doi: 10.1128/aac.38.9.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart PS. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob. Agents Chemother. 1996;40:2517–2522. doi: 10.1128/aac.40.11.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoolnik GK, et al. Whole genome DNA microarray expression analysis of biofilm development by Vibrio cholerae O1 E1 Tor. Methods Enzymol. 2001;336:3–18. doi: 10.1016/s0076-6879(01)36573-4. [DOI] [PubMed] [Google Scholar]
- 26.Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH. Microarray analysis of Pseudomonas aeruginosa quorum-sensing reulons: effects of growth phase and environment. J. Bacteriol. 2003;185:2080–2095. doi: 10.1128/JB.185.7.2080-2095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shirtliff, M.E., Leid, J.G., and Costerton, J.W. 2003. Basic science in musculoskeletal infections. In Musculoskeletal Infections. J.T. Mader and J.H. Calhoun, editors. Marcel Dekker Inc. New York, New York, USA. 1–61.
- 28.Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 2002;184:1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremoulet F, Duche O, Namane A, Martinie B, Labadie JC. A proteomic study of Escherichia coli O157:H7 NCTC 12900 cultivated in biofilm or in planktonic growth mode. FEMS Microbiol. Lett. 2002;215:7–14. doi: 10.1111/j.1574-6968.2002.tb11363.x. [DOI] [PubMed] [Google Scholar]
- 30.Miller BS, Diaz-Torres MR. Proteome analysis of biofilms: growth of Bacillus subtilis on solid medium as model. Methods Enzymol. 1999;310:433–441. doi: 10.1016/s0076-6879(99)10034-x. [DOI] [PubMed] [Google Scholar]
- 31.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Ann. Rev. Micro. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 32.Novick RP, et al. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xavier KB, Bassler BL. LuxS quorum sensing: more than just a numbers game. Curr. Opin. Microbiol. 2003;6:191–197. doi: 10.1016/s1369-5274(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 34.Balaban N, et al. Use of the quorum-sensing inhibitor RNAIII-inhibiting peptide to prevent biofilm formation in vivo by drug-resistant Staphylococcus epidermidis. J. Infect. Dis. 2003;187:625–630. doi: 10.1086/345879. [DOI] [PubMed] [Google Scholar]
- 35.de Nys R, et al. Broad spectrum effects of secondary metabolites from the red alga Delisea pulchra in antifouling assays. Biofouling. 1995;8:259–271. [Google Scholar]
- 36.Veeh RH, et al. Detection of Staphylococcus aureus biofilm on tampons and menses components. J. Infect. Dis. 2003;188:519–530. doi: 10.1086/377001. [DOI] [PubMed] [Google Scholar]
- 37.Post JC. Direct evidence of bacterial biofilms in otitis media. Laryngoscope. 2001;111:2083–2094. doi: 10.1097/00005537-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Ward KH, Olson ME, Lam K, Costerton JW. Mechanism of persistent infection associated with peritoneal implants. J. Med. Micro. 1992;36:406–413. doi: 10.1099/00222615-36-6-406. [DOI] [PubMed] [Google Scholar]
- 39.Jensen ET, Kharazmi A, Lam K, Costerton JW, Hoiby N. Human polymorphonuclear leukocyte response to Pseudomonas aeruginosa grown in biofilms. Infect. Immun. 1990;58:2383–2385. doi: 10.1128/iai.58.7.2383-2385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leid JG, Shirtliff ME, Costerton JW, Stoodley P. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect. Immun. 2002;70:6339–6345. doi: 10.1128/IAI.70.11.6339-6345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costerton, J.W., and Anwar, H. 1994. Pseudomonas aeruginosa: The microbe and pathogen. In Pseudomonas aeruginosa infections and treatment. A.L. Baltch and R.P. Smith, editors. Marcel Dekker Inc. New York, New York, USA. 1–20.
- 42.Costerton JW, Ellis B, Lam K, Johnson F, Khoury AE. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob. Agents Chemother. 1994;38:2803–2809. doi: 10.1128/aac.38.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rediske AM, Hymas WC, Wilkinson R, Pitt WG. Ultrasonic enhancement of antibiotic action on several species of bacteria. J. Gen. Appl. Microbiol. 1998;44:283–288. doi: 10.2323/jgam.44.283. [DOI] [PubMed] [Google Scholar]
- 44.Costerton JW, et al. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 45.Ghigo J-M. Natural conjugative plasmids induce biofilm development. Nature. 2001;412:442–445. doi: 10.1038/35086581. [DOI] [PubMed] [Google Scholar]
- 46.Shen, K., Wang, X., Post, J.C., and Ehrlich, G.D. 2003. Molecular and translational research approaches for the study of bacterial pathogenesis in otitis media. In Evidence-based otitis media. 2nd edition. R. Rosenfeld and C.D. Bluestone, editors. B.C. Decker Inc. Hamilton, Ontario, Canada. 91–119.
- 47.Ehrlich, G.D., Hu, Z.E., and Post, J.C. Role of biofilms in infectious diseases. ASM Press. Washington, DC, USA. In press.
- 48.Woods DE, Bass JA, Johanson WG., Jr Role of adherence in the pathogenesis of Pseudomonas aeruginosa lung infection in cystic fibrosis patients. Infect. Immun. 1980;30:694–701. doi: 10.1128/iai.30.3.694-699.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woods DE, Straus DC, Johanson WG, Bass JA. Role of fibronectin in the prevention of the adherence of Pseudomonas aeruginosa to buccal cells. J. Infect. Dis. 1981;143:784–790. doi: 10.1093/infdis/143.6.784. [DOI] [PubMed] [Google Scholar]
- 50.Morck DW, et al. Electron microscopic description of glycocalyx and fimbriae on the surface of Pasturella haemolytica. Can. J. Vet. Res. 1987;51:83–88. [PMC free article] [PubMed] [Google Scholar]
- 51.Morck DW, et al. A guinea pig model of bovine pneumonic Pasteurellosis. Can. J. Vet. Res. 1990;54:139–145. [PMC free article] [PubMed] [Google Scholar]
- 52.Wright JB, Athar MA, van Olm TM, Wootliff JS, Costerton JW. Atypical legionellosis: Isolation of Legionella pneumophila serogroup 1 from a patient with aspiration pneumonia. J. Hosp. Inf. 1989;13:187–190. doi: 10.1016/0195-6701(89)90026-1. [DOI] [PubMed] [Google Scholar]
- 53.Vorachit M, Lam K, Jayanetra P, Costerton JW. Electron microscopy study of the mode of growth of Pseudomonas pseudomallei in vitro and in vivo. J. Trop. Med. Hyg. 1995;98:379–391. [PubMed] [Google Scholar]
- 54.Vorachit M, Lam K, Jayanetra P, Costerton JW. The study of the pathogenicity of Burkholderia pseudomallei–a guinea pig model. J. Infect. Dis. Antimicrob. Agents. 1995;12:115–121. [Google Scholar]
- 55.Sung JY, et al. Ascending infection of the biliary tract after surgical sphincterotomy and biliary stenting. J. Gastroenterol. Hepatol. 1992;7:240–245. doi: 10.1111/j.1440-1746.1992.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 56.Sung JY, et al. Bacterial invasion of the biliary system by way of the portal-venous system. Hepatology. 1991;14:313–317. [PubMed] [Google Scholar]
- 57.Cheng, K.-J., and Costerton, J.W. 1981. Adherent rumen bacteria: Their role in the digestion of plant material, urea, and epithelial cells. In Digestive physiology and metabolism in ruminants. Y. Ruchebusch and P. Thivend, editors. MTP Press. Lancaster, United Kingdom. 227–250.
- 58.Marrie TJ, Costerton JW. A scanning and transmission electron microscopy study of the surfaces of intrauterine contraceptive devices. Am. J. Obstet. Gynecol. 1983;146:384–394. doi: 10.1016/0002-9378(83)90818-9. [DOI] [PubMed] [Google Scholar]
- 59.Lam JS, et al. Immunogenicity of Pseudomonas aeruginosa outer membrane antigens examined by crossed immunoelectrophoresis. Infect. Immun. 1983;42:88–89. doi: 10.1128/iai.42.1.88-98.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Costerton, J.W., and Stewart, P.S. 2000. Biofilms and device-related infections. In Persistent bacterial infections. J.P. Nataro, M.J. Blaser, and S. Cunningham-Rundels, editors. American Society for Microbiology. Washington, DC, USA. 423–437.