Abstract
Objective To review outcomes in randomised controlled trials comparing hydralazine against other antihypertensives for severe hypertension in pregnancy.
Study design Meta-analysis of randomised controlled trials (published between 1966 and September 2002) of short acting antihypertensives for severe hypertension in pregnancy. Independent data abstraction by two reviewers. Data were entered into RevMan software for analysis (fixed effects model, relative risk and 95% confidence interval); in a secondary analysis, risk difference was also calculated.
Results Of 21 trials (893 women), eight compared hydralazine with nifedipine and five with labetalol. Hydralazine was associated with a trend towards less persistent severe hypertension than labetalol (relative risk 0.29 (95% confidence interval 0.08 to 1.04); two trials), but more severe hypertension than nifedipine or isradipine (1.41 (0.95 to 2.09); four trials); there was significant heterogeneity in outcome between trials and differences in methodological quality. Hydralazine was associated with more maternal hypotension (3.29 (1.50 to 7.23); 13 trials); more caesarean sections (1.30 (1.08 to 1.59); 14 trials); more placental abruption (4.17 (1.19 to 14.28); five trials); more maternal oliguria (4.00 (1.22 to 12.50); three trials); more adverse effects on fetal heart rate (2.04 (1.32 to 3.16); 12 trials); and more low Apgar scores at one minute (2.70 (1.27 to 5.88); three trials). For all but Apgar scores, analysis by risk difference showed heterogeneity between trials. Hydralazine was associated with more maternal side effects (1.50 (1.16 to 1.94); 12 trials) and with less neonatal bradycardia than labetalol (risk difference -0.24 (-0.42 to -0.06); three trials).
Conclusions The results are not robust enough to guide clinical practice, but they do not support use of hydralazine as first line for treatment of severe hypertension in pregnancy. Adequately powered clinical trials are needed, with a comparison of labetalol and nifedipine showing the most promise.
Introduction
In both the United States and the United Kingdom, reports into maternal mortality have consistently shown the excess maternal mortality associated with the hypertensive disorders of pregnancy, particularly the severe hypertension of pre-eclampsia.1-6 In the most recent triennium in the UK series (1997-9),6 maternal mortality from hypertensive disease was most commonly attributed to intracerebral haemorrhage. There is general consensus that maternal risk is decreased by antihypertensive treatment that acutely lowers very high blood pressure.1,7,8 Recognition of this specific risk has meant that the control of acutely raised blood pressure has become central for women with severe hypertension, particularly that of preeclampsia.6
Three short acting antihypertensive agents—hydralazine, labetalol, and short acting (sublingual or orally administered) nifedipine—are commonly used to control acute, very high blood pressure in women with severe hypertension in pregnancy, who may require emergency caesarean section and often receive magnesium sulphate.1 All three agents have their proponents and detractors.
For many years, hydralazine has been the recommended antihypertensive of first choice for severe hypertension in pregnancy.1,7,8 Its side effects (such as headache, nausea, and vomiting) are common and mimic symptoms of deteriorating pre-eclampsia. Although a precipitous hypotensive overshoot may occur with any antihypertensive agent used to treat the severe hypertension of pre-eclampsia,9-13 a meta-analysis of clinical trials showed that maternal hypotension may be more common with parenteral hydralazine, which was also associated with an excess of caesarean sections, placental abruptions, and low Apgar scores (< 7) at five minutes.14
Short acting nifedipine has the clinical advantage of being able to be given as required by midwives or nurses in the absence of a doctor. However, uncertainty exists about how safe short acting calcium channel blockers are for the mother.15 When used for treating hypertension in patients with coronary artery disease or diabetes, these agents have been associated with excess cardiovascular morbidity and mortality.16,17 Two case reports of transient neuromuscular weakness in patients taking nifedipine and magnesium sulphate have caused concern about concomitant use of these agents.18,19 The withdrawal of short acting nifedipine from some markets has been lamented by many experts in the field of pregnancy hypertension.20
Labetalol has been used extensively in pregnancy and has a favourable side effect profile. However, specific concern has been raised about the risk of neonatal bradycardia with parenteral labetalol.21
This meta-analysis of randomised controlled trials for treatment of moderate to severe hypertension in pregnancy aimed to compare the effects of short acting antihypertensive agents (in comparison to parenteral hydralazine) on perinatal, maternal, and neonatal outcomes, particularly maternal hypotension.
Methods
We updated our previous literature review (1966-97)14 by searching Medline (1997-September 2002), the journal Hypertension in Pregnancy (hand-searched), conference proceedings, bibliographies (including those of relevant publications in the Cochrane Database of Systematic Reviews), and textbooks. We looked for articles addressing the treatment of severe hypertension in pregnancy with short acting antihypertensive agents, comparing them with parenteral hydralazine.
For the Medline search we used (and exploded) {"antihypertensive agent", "bed rest", "plasma volume", "plasma substitute" or "hospitalization"} AND {"pregnancy", "pregnancy complications", "maternal mortality", "perinatology", "neonatology", "infant, newborn, diseases", "infant mortality", or "infant"}.
Criteria for inclusion were moderate to severe hypertension in pregnancy (regardless of type), randomised controlled trial, hydralazine compared with another short acting antihypertensive (generally via parenteral administration), and relevant clinical outcomes addressing maternal, perinatal, or paediatric benefit or risk. Articles in any language were included. Abstracts without accompanying articles were included if they met the above criteria. We contacted authors for missing information or clarification, when necessary. Data were abstracted independently by two reviewers (LAM and CC, PvD, or EJW), and discrepancies were resolved by discussion.
The severity of hypertension was defined according to mean diastolic blood pressure at enrolment: mild (90-99 mm Hg), moderate (100-109 mm Hg), or severe (≥ 110 mm Hg). The type of hypertension was defined according to national high blood pressure education programme (NHBPEP) standards.7
Some trials enrolled mixed populations of women with either pre-existing hypertension or gestational hypertension with or without proteinuria; we used the term "mixed" hypertension in such instances. Otherwise, we used pre-eclampsia when all trial participants had pregnancy induced hypertension with proteinuria at enrolment, and pregnancy induced hypertension when women both with and without proteinuria were enrolled.
Data from trials of single drugs were accepted for maternal haemodynamic outcomes and stillbirth, and for neonatal outcomes if the antihypertensive could be expected to be in the maternal-fetal bloodstream at delivery and could affect the health of the neonate. In the case of duplicate publications, the most recent and complete data were included in the analysis.
Outcome definitions that were not standardised were documented at data abstraction and considered as potential sources of variation in outcome between studies. Maternal outcomes were persistent severe hypertension, need for additional antihypertensive therapy, maternal hypotension, caesarean section, placental abruption, maternal mortality or morbidity (eclampsia, intracerebral haemorrhage, HELLP (haemolysis, raised liver enzymes, low platelets) syndrome, pulmonary oedema, oliguria, and disseminated intravascular coagulation), and maternal side effects (overall and those thought to indicate deteriorating maternal pre-eclampsia: headache, visual symptoms, epigastric pain, and nausea or vomiting). Perinatal outcomes were adverse effects on fetal heart rate, stillbirth, Apgar scores at one minute and five minutes, neonatal death, neonatal bradycardia, tachycardia, hypotension, hypothermia, hypoglycaemia, admission to neonatal intensive care unit, respiratory distress syndrome, intraventricular haemorrhage, and necrotising enterocolitis.
We used Cochrane review manager software (Revman version 4.0.1; Oxford, UK) for quantitative analyses. We determined heterogeneity between trials by examining the forest plot (of relative risk for each trial, and with the χ2 statistic, using P < 0.10) to reflect statistically significant heterogeneity.22 A P value < 0.10 was considered significant given that χ2 is known not to be sensitive to heterogeneity between trials.22,23 When heterogeneity between trials was found, we examined differences in study design (for example, method of randomisation), characteristics of participants (for example, type of pregnancy hypertension), intervention (for example, drug and dosage), and outcome definitions (for example, the diastolic blood pressure at which additional antihypertensive therapy was prescribed). The summary statistic was relative risk (and 95% confidence interval), a relative effect measure appropriate for use when summarising evidence.22 In addition, we calculated risk difference, as recommended by the neonatal review group of the Cochrane Collaboration.24 Risk difference is a measure of absolute effect and is sensitive to between trial differences in absolute event rates. In the calculation of risk difference, all trials (even those without reported events in either arm of the trial) contribute to the summary statistic. Data were entered by subgroup according to the type of antihypertensive that was compared with hydralazine. The fixed effects model was used, on the assumption that any between trial differences in outcome were due to random variation, so trials were weighted on the basis of precision. For outcomes with significant differences between groups, the median event rate and its range were also presented.
Results
We identified 11 new trials in 16 publications (from 1991 to 2002) that met the inclusion criteria.25-40 Therefore, this study includes 21 trials (1085 women), including the 10 trials41-50 in the previous meta-analysis. 14 Table 1 shows selected characteristics of included trials. About half (12/21 trials) enrolled mixed populations of women with pregnancy hypertension; hypertension was usually severe (16/21 trials). In two trials, single doses were given,27,39 and in three trials patients were switched to oral antihypertensives when blood pressure had been stabilised.28,43,46 Most commonly, hydralazine was compared with standard doses of other antihypertensives: nifedipine (eight trials); labetalol (five trials); ketanserin (four trials); urapidil (two trials); epoprostenol (one trial); or isradipine (one trial with three publications).
Table 1.
Randomised controlled trials for management of severe hypertension in pregnancy
| Trial | No of women | Type of hypertension* | Severity of hypertension | Antihypertensive compared with hydralazine | Route (hydralazine/other antihypertensive)† | Method of randomisation | Blinding to outcome |
|---|---|---|---|---|---|---|---|
| Aali and Nejad36 | 126 | Pre-eclampsia | Severe | Nifedipine | Intravenous bolus/sublingual | Not known | Partial (for blood pressure only) |
| Ashe et al41 | 20 | Mixed | Severe | Labetalol | Intravenous infusion | Not known | Not known |
| Bhorat et al42 | 34 | Pregnancy induced hypertension | Severe | Labetalol | Intravenous bolus/infusion | Not known | No |
| Bolte et al25,26 | 44 | Mixed | Severe | Ketanserin | Intravenous infusion/intravenous bolus→infusion | Adequate | No |
| Bolte et al37 | 66 | Not stated | Severe | Ketanserin | "Intravenous" | Not known | Not known |
| Duggan et al39 | 9 | Mixed | Severe | Nifedipine | Intravenous+oral placebo/oral+intravenous placebo | Not known | Yes |
| Fenakel et al43 | 49 | Pregnancy induced hypertension | Severe | Nifedipine | Intravenous bolus/sublingual ‡ | Inadequate | No |
| Garden et al44 | 6 | Mixed | Severe | Labetalol | Intravenous infusion | Not known | Not known |
| Harper and Murnaghan27 | 30 | Mixed | Moderate | Labetalol | Intravenous bolus | Adequate | No |
| Howarth et al45 | 33 | Mixed | Moderate to severe | Urapidil | Intravenous bolus | Adequate | No |
| Jegasothy and Paranthaman46 | 200 | Mixed | Severe | Nifedipine | Intravenous/sublingual ‡ | Inadequate | No |
| Kwawukume and Ghosh28 | 98 | Pre-eclampsia | Severe | Nifedipine | Intravenous/sublingual ‡ | Inadequate | No |
| Mabie et al47 | 60 | Mixed | Severe | Labetalol | Intravenous bolus | Adequate | No |
| Maharaj et al29-31 | 40 | Pregnancy induced hypertension | Severe | Isradepine | Intravenous bolus/infusion | Adequate | Not known |
| Martins-Costa et al48 | 37 | Pregnancy induced hypertension | Severe | Nifedipine | Intravenous/oral | Adequate | Yes |
| Moodley and Gouws32 | 47 | Mixed | Severe | Epoprostenol | Intravenous infusion | Adequate | Not known |
| Rodriguez40 | 27 | Pre-eclampsia | Severe | Nifedipine | Intravenous,/intramuscular or sublingual | Adequate | No |
| Rossouw et al49 | 20 | Mixed | Moderate to severe | Ketanserin | Intravenous bolus | Not known | Yes |
| Seabe et al50 | 33 | Pregnancy induced hypertension | Severe | Nifedipine | Intravenous bolus/oral | Adequate | No |
| Steyn and Odendaal33 | 80 | Mixed | Mod | Ketanserin | Intravenous bolus | Adequate | Yes |
| Wacker et al34,35 | 26 | Pre-eclampsia | Mod | Urapidil | Intravenous bolus | Adequate | No |
Mixed=pre-existing or gestational hypertension (with or without proteinuria); pregnancy induced hypertension=with or without proteinuria.
When route of drug administration was the same for both groups, only one route is stated.
Switched to oral therapy when hypertension had been controlled with short acting agents.
Most trials were small, with a median of 37 women enrolled (range 6-200). Half (11/21) described adequate methods of randomisation, but seven publications did not describe the method at all. Assessment of outcome was blinded in four trials, and for some outcomes in one other trial. The quality of the methods had no discernible impact on outcome.
Table 2 presents the maternal and perinatal outcomes in trials that compared hydralazine with other antihypertensives. If the results of the trial that compared hydralazine and epoprostenol are excluded,32 the results of outcomes to which this trial contributed (persistent severe hypertension, caesarean section, maternal side effects, perinatal mortality, and respiratory distress syndrome) are not changed.
Table 2.
Maternal and perinatal outcomes in trials comparing hydralazine with other antihypertensives for severe hypertension of pregnancy
|
No of trials
|
No of women
|
Heterogeneity
|
Heterogeneity
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Relative risk (95% CI) | χ2 | df | P value | Risk difference (95% CI) | χ2 | df | P value | ||
| Maternal outcomes | ||||||||||
| Persistent severe hypertension | 14 | 729 | 1.08 (0.78 to 1.49) | 28.09 | 9 | 0.0009 * | 0.01 (−0.04 to 0.06) | 44.36 | 13 | <0.0001 * |
| Additional drugs for blood pressure | 10 | 564 | 1.32 (0.83 to 2.13) | 14.06 | 6 | 0.029 * | 0.03 (−0.02,0.08) | 22.92 | 9 | 0.006 * |
| Maternal hypotension | 13 | 687 | 3.29 (1.50 to 7.23) * | 3.22 | 6 | 0.78 | 0.04 (0.01 to 0.08) * | 40.66 | 12 | 0.0001 * |
| Eclampsia | 8 | 311 | 0.75 (0.20 to 2.86) | 1.40 | 3 | 0.70 | −0.01 (−0.05 to 0.04) | 2.03 | 7 | 0.96 |
| HELLP syndrome | 2 | 142 | 2.33 (0.83 to 6.67) | 3.70 | 1 | 0.05 * | 0.08 (0.00 to 0.17)) | 18.87 | 1 | <0.0001 * |
| Placental abruption | 5 | 203 | 4.17 (1.19 to 14.28) * | 1.29 | 4 | 0.86 | 0.08 (0.01 to 0.15) * | 7.9 | 4 | 0.095 * |
| Caesarean section | 14 | 650 | 1.30 (1.08 to 1.59) * | 12.19 | 11 | 0.35 | 0.08 (0.02 to 0.13) * | 25.67 | 13 | 0.02 * |
| Intracerebral haemorrhage | 1 | 44 | 3.03 (0.13 to 100) | 0 | 0 | NA | 0.05 (−0.08 to 0.17) | 0 | 0 | NA |
| Pulmonary oedema | 3 | 161 | 4.00 (0.65 to 25.00) | 1.09 | 1 | 0.30 | 0.05 (−0.01 to 0.12) | 7.05 | 2 | 0.03 * |
| Oliguria | 3 | 105 | 4.00 (1.22 to 12.50) * | 0.10 | 2 | 0.95 | 0.17 (0.05 to 0.29) * | 7.44 | 2 | 0.02 * |
| Disseminated intravascular coagulation | 1 | 44 | 0.33 (0.01 to 7.69) | 0 | 0 | NA | −0.05 (−0.17 to 0.08) | 0 | 0 | NA |
| Maternal death | 9 | 471 | 3.33 (0.52 to 20.00) | 0 | 2 | 1.00 | 0.01 (−0.02 to 0.04) | 2.11 | 8 | 0.98 |
| Maternal side effects | ||||||||||
| Any | 12 | 494 | 1.50 (1.16 to 1.94) * | 27.51 | 11 | 0.004 * | 0.12 (0.05 to 0.19) * | 51.38 | 11 | <0.0001 * |
| Headache | 11 | 528 | 1.61 (1.06 to 2.38) * | 14.34 | 10 | 0.16 | 0.07 (0.01 to 0.13) * | 29.15 | 10 | 0.001 * |
| Visual symptoms | 1 | 44 | 9.09 (0.51 to 100) | 0 | 0 | NA | 0.18 (0.00 to 0.36) | 0 | 0 | NA |
| Nausea or vomiting | 6 | 210 | 2.22 (0.94 to 5.26) | 4.17 | 4 | 0.38 | 0.08 (0.00 to 0.16) | 12.61 | 5 | 0.03 * |
| Epigastric pain | 1 | 44 | 0.67 (0.12 to 3.57) | 0 | 0 | NA | −0.05 (−0.23 to 0.14) | 0 | 0 | NA |
| Flushing | 3 | 119 | 0.31 (0.12 to 0.79) * | 8.08 | 2 | 0.02 * | −0.20 (−0.32 to −0.08) * | 30.42 | 2 | <0.0001 * |
| Palpitations | 5 | 132 | 3.57 (1.72 to 7.69) * | 3.11 | 4 | 0.54 | 0.28 (0.15 to 0.41) * | 15.06 | 4 | 0.005 * |
| Tachycardia >110 beats/min | 5 | 305 | 5.56 (2.38 to 12.5) * | 4.42 | 4 | 0.35 | 0.18 (0.11 to 0.25) * | 11.96 | 4 | 0.02 * |
| Dizziness | 5 | 153 | 1.82 (0.53 to 6.25) | 3.35 | 3 | 0.34 | 0.04 (−0.04 to 0.12) | 5.72 | 4 | 0.22 |
| Bronchospasm | 1 | 12 | 0.33 (0.17 to 6.67) | 0 | 0 | NA | −0.17 (−0.59 to 0.25) | 0 | 0 | NA |
| Drugs changed because of side effects | 7 | 328 | 2.44 (0.38 to 14.28) | 0.03 | 1 | 0.86 | 0.01 (−0.02 to 0.05) | 1.65 | 6 | 0.95 |
| Effects on fetus | ||||||||||
| Adverse effects on fetal heart rate | 12 | 601 | 2.04 (1.32 to 3.16) * | 13.60 | 8 | 0.09 | 0.07 (0.03 to 0.12) * | 45.97 | 12 | <0.0001 * |
| Perinatal outcomes | ||||||||||
| Perinatal death | 17 | 744 | 1.43 (0.77 to 2.63) | 4.21 | 12 | 0.98 | 0.02 (−0.02 to 0.05) | 7.25 | 16 | 0.97 |
| Stillbirth | 17 | 744 | 2.00 (0.85 to 4.76) | 0.66 | 5 | 0.99 | 0.02 (−0.01 to 0.05) | 4.61 | 16 | 1.00 |
| Neonatal death | 17 | 729 | 1.00 (0.43 to 2.38) | 3.74 | 8 | 0.88 | 0.00 (−0.03 to 0.03) | 5.47 | 16 | 0.99 |
| 1-minute Apgar <7 | 3 | 52 | 2.70 (1.27 to 5.88) * | 4.03 | 2 | 0.13 | 0.36 (0.13 to 0.59) * | 4.48 | 2 | 0.11 |
| 5-minute Apgar <7 | 6 | 271 | 1.23 (0.69 to 2.22) | 3.74 | 5 | 0.59 | 0.03 (−0.05 to 0.11) | 5.85 | 5 | 0.32 |
| Admission to neonatal intensive care unit | 1 | 98 | 1.18 (0.59 to 2.38) | 0 | 0 | NA | 0.04 (−0.13 to 0.21) | 0 | 0 | NA |
| Neonatal bradycardia | 3 | 50 | 0.16 (0.02 to 1.11) | 0.01 | 1 | 0.91 | −0.24 (−0.42 to −0.06) * | 15.43 | 2 | 0.0004 * |
| Neonatal hypotension | 1 | 19 | 5.88 (0.28 to 100) | 0 | 0 | NA | 0.17 (−0.20 to 0.53) | 0 | 0 | NA |
| Neonatal hypothermia | 1 | 25 | Not estimable | 0.00 (−0.16 to 0.16) | 0 | 0 | NA | |||
| Neonatal hypoglycaemia | 3 | 64 | 0.88 (0.14 to 5.26) | 0.84 | (1) | 0.36 | −0.01 (−0.13 to 0.10) | 0.94 | 2 | 0.63 |
| Respiratory distress syndrome | 6 | 250 | 1.56 (0.78 to 3.13) | 2.68 | (5) | 0.75 | 0.05 (−0.03 to 0.12) | 3.72 | 5 | 0.59 |
| Intraventricular haemorrhage | 2 | 72 | 4.17 (0.47 to 33.33) | 0.11 | (1) | 0.74 | 0.07 (−0.05 to 0.18) | 0.75 | 1 | 0.39 |
| Necrotising enterocolitis | 1 | 53 | 2.86 (0.12 to 100) | 0 | (0) | NA | 0.04 (−0.06 to 0.14) | 0 | 0 | NA |
NA=not applicable; HELLP=haemolysis to elevated liver enzymes to low platelets
Significant at the P<0.05 level, and discussed in the text.
Maternal outcomes
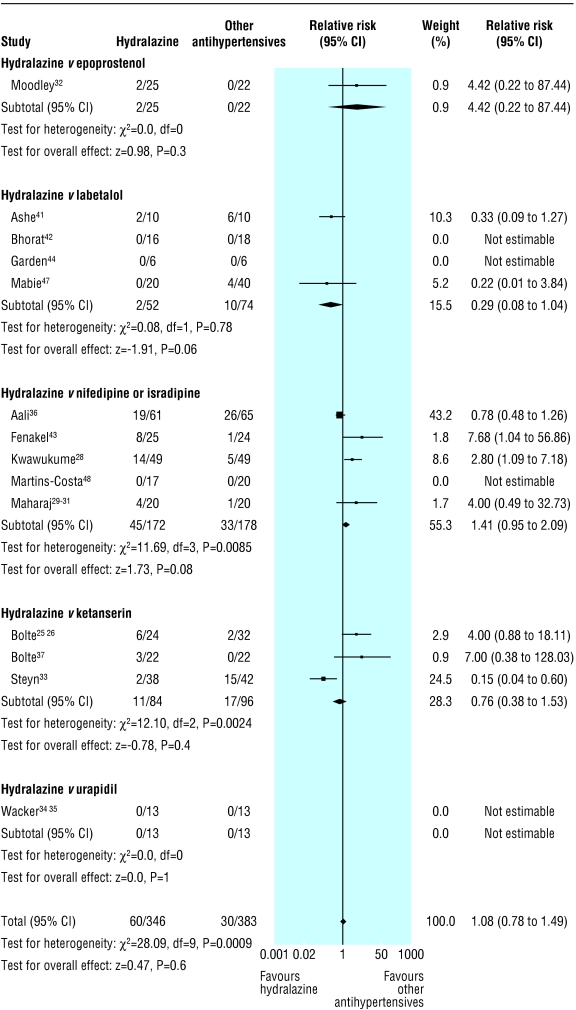
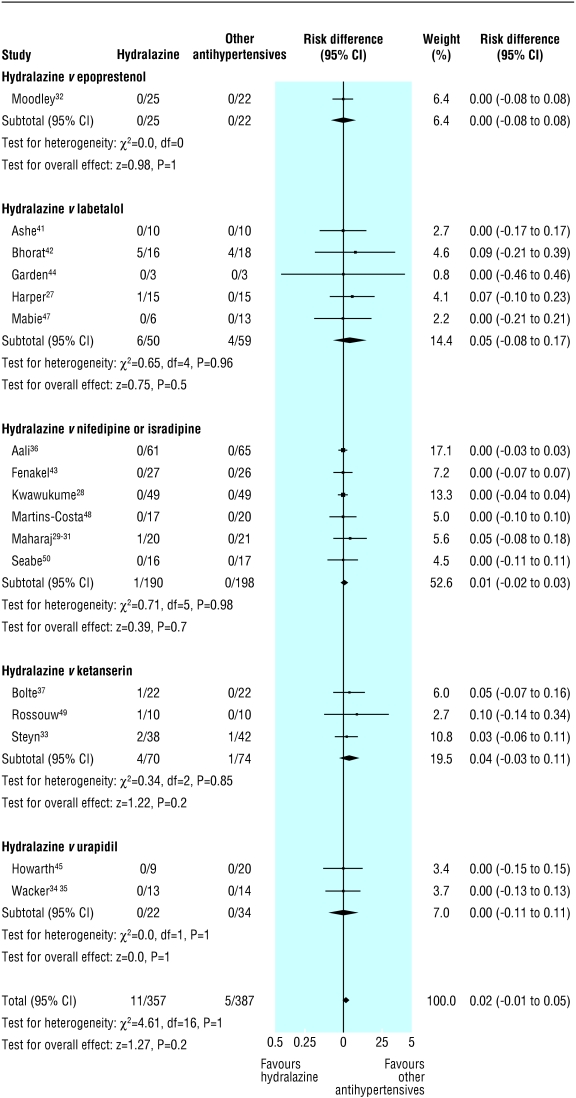
Persistent severe hypertension was variably defined as diastolic blood pressure ≥ 90 mm Hg,33 ≥ 95 mm Hg,29-31 ≥ 100 mm Hg,41,42,44,47-49 or ≥ 110 mm Hg25,26,28,43; mean arterial blood pressure ≥ 120 mm Hg45; and failure to achieve a drop in systolic/diastolic blood pressure of 30/15 mm Hg.32 Hydralazine did not differ from other antihypertensives in impact on persistent severe hypertension or on use of additional antihypertensives (table 2). However, the results differed by more than could be expected by chance alone, with the heterogeneity explained largely by the type of the other antihypertensive. Hydralazine was associated with a trend towards lower rates of persistent severe hypertension (median event rate 0% (range 0-20%) v labetalol (5% (0-60%)); relative risk 0.29 (0.08 to 1.04); two trials; χ2 = 0.08, df = 1, P = 0.78; risk difference -0.11 (-0.21 to -0.02); four trials; χ2 = 6.91, df = 3, P = 0.08; fig 1) and was not associated with use of additional antihypertensives (5% (0-10)% for hydralazine v 5% (0-10)% for labetalol; relative risk 1.00 (0.07 to 13.87); one trial; χ2 = 0, df = 0; risk difference 0 (-0.12 to 0.12); two trials; χ2 = 0, df = 1, P = 1.00). Hydralazine was associated with a trend towards more persistent severe hypertension (29% (0-32%) compared with nifedipine or isradipine (5% (0-40%); relative risk 1.41 (0.95 to 2.09); four trials; χ2 = 11.69, df = 3, P = 0.009; risk difference 0.08 (-0.01 to 0.16); five trials; χ2 = 12.36, df = 4, P = 0.02; fig 1) and with use of additional antihypertensives (13% (0-32%) for hydralazine v (5% (0-24%)) nifedipine only; relative risk 2.13 (1.20 to 3.85); four trials; χ2 = 5.24, df = 3, P = 0.15; risk difference 0.08 (0.02 to 0.14); five trials; χ2 = 12.32, df = 4, P = 0.02), but there was still significant heterogeneity between trials within this subgroup. In the three trials with nifedipine or isradipine in which hydralazine was associated with more severe hypertension, the methods of allocation concealment were either clearly inadequate28,43 or unstated,29-31 but other characteristics of the trials did not differ.
Fig 1.
Persistent severe maternal hypertension in trials that compared hydralazine with other antihypertensives
In comparison with ketanserin, hydralazine was not associated with a consistent effect on maternal blood pressure (fig 1); this effect was partially explained by the doses of hydralazine used. A low dose hydralazine infusion (1 mg/h intravenously, increased by 1 mg/h every hour to a maximum of 10 mg/h) was associated with a trend towards more persistent severe hypertension than ketanserin (5 mg intravenous bolus, then 4 mg/h intravenously).25,26 Higher dose bolus hydralazine (5 mg intravenously every 20 min) was associated with less persistent severe hypertension than ketanserin (10 mg intravenously every 20 min).33
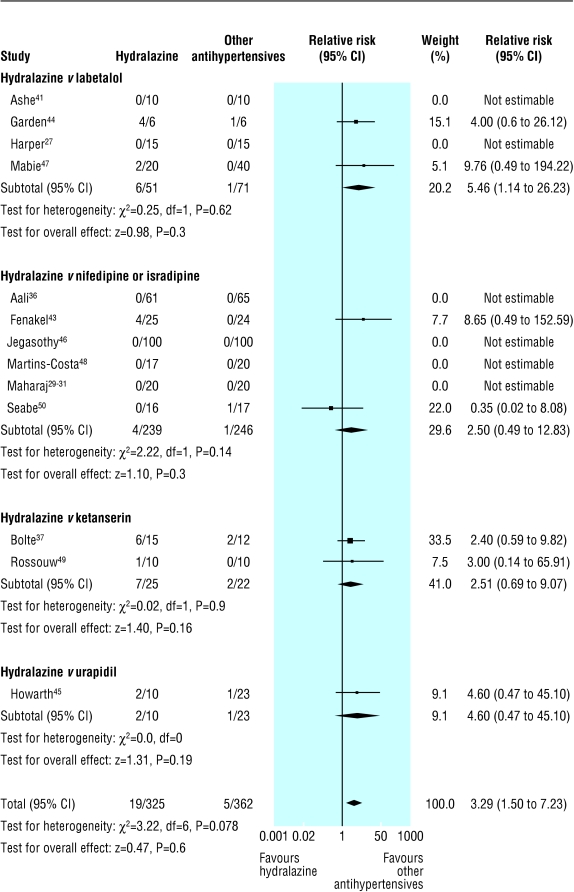
Hydralazine was associated with more maternal hypotension than other antihypertensives (0% (0-67%) v 0% (0-17%); table 2, fig 2). Calculations of risk difference showed significant heterogeneity between trials, which was largely absent when subgroups of other antihypertensive agents were examined: hydralazine v labetalol (risk difference 0.10 (0 to 0.20); four trials; χ2 = 6.46, df = 3, P = 0.09); hydralazine v nifedipine or isradipine (0.01 (-0.01 to 0.04); six trials; χ2 = 6.58, df = 5, P = 0.25); hydralazine v urapidil (0.16 (-0.11 to 0.42); one trial); and hydralazine v ketanserin (0.18 (-0.04 to 0.39); two trials; χ2 = 0.51, df = 1, P = 0.47). In the hydralazine v labetalol subgroup in which there was still heterogeneity, the incidence of maternal hypotension with hydralazine ranged from 0% (in 10 patients) to 67% (in 4 of 6 patients); the rate of 67% occurred in the very small trial by Garden et al,44 in which hydralazine was given in higher dosage (initially 10 mg/h, by intravenous infusion) than in other trials.
Fig 2.
Maternal hypotension in trials that compared hydralazine with other antihypertensives
Several maternal outcomes occurred more often with hydralazine than with other antihypertensives: caesarean section (67% (8-100%) v 59% (5-100%) for other antihypertensives); placental abruption (18% (3-20%) v 0% (0-2%)); and maternal oliguria (17% (4-41%) v 0% (0-9%)) (table 2); however, the risk difference analysis showed heterogeneity between trials. Groups did not differ in other measures of maternal morbidity—eclampsia, intracerebral haemorrhage, HELLP syndrome, pulmonary oedema, disseminated intravascular coagulation, or mortality. However, the two trials that reported HELLP syndrome as an outcome differed by more than could be expected by chance alone25,26,28: comparing hydralazine with nifedipine, Kwawukume and Ghosh reported no raised liver enzymes,28 but in a comparison of hydralazine and ketanserin Bolte et al reported a significantly higher incidence (45% v 9%) of HELLP syndrome (using Sibai's definition in the hydralazine group51).25,26
In summary, hydralazine was associated with more persistent severe hypertension than nifedipine or isradipine, and more of the following outcomes when compared with all antihypertensives: maternal hypotension, placental abruption, caesarean section, and maternal oliguria. However, absolute event rates ranged widely within trials, and outcomes showed significant heterogeneity when risk difference was used as the summary statistic.
Maternal side effects
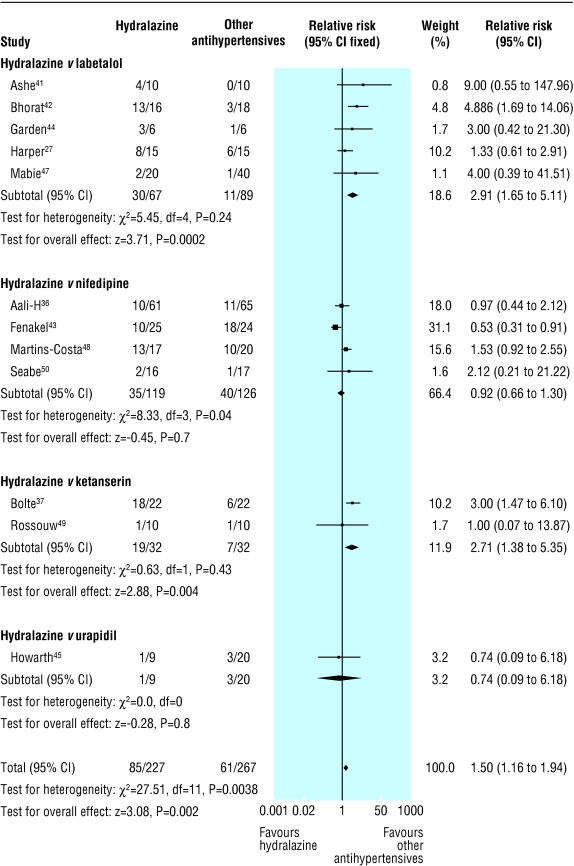
Hydralazine was associated with more maternal side effects (of any sort) and headache than other antihypertensives (40% (10-82%) v 17% (0-75%) and 29% (0-67%) v 0% (0-20%), respectively; table 2). For any maternal side effects, the significant heterogeneity between trials was confined to the nifedipine subgroup (fig 3). In particular, the trial by Fenakel et al found that hydralazine was associated with fewer side effects than nifedipine.43 The dose of hydralazine was higher than in the other three trials, and the dose of nifedipine was the same. However, the duration of treatment was longer than in other trials (days to weeks, rather than hours to days) because women were changed to oral antihypertensive therapy.
Fig 3.
Any maternal side effect reported in trials that compared hydralazine with other antihypertensives
Hydralazine was associated with more palpitations than other antihypertensives (18% (11-81%) v 0% (0-17%); table 2). Three of the five trials compared hydralazine with labetalol, and within this subgroup the effect was significant (relative risk 5.26 (2.00 to 14.28); three trials; χ2 = 0.29, df = 2, P = 0.87; risk difference 0.48 (0.30 to 0.67); three trials; χ2 = 4.79, df = 2, P = 0.09).
Hydralazine was also associated with more maternal tachycardia than other antihypertensives (24% (10-67%) v 0% (0-6%); table 2). Three of the five trials were comparisons of hydralazine against nifedipine, and within this subgroup the results were significant (relative risk 5.56 (2.17 to 14.29); three trials; χ2 = 4.10, df = 2, P = 0.13; risk difference 0.18 (0.11 to 0.25); three trials; χ2 = 11.96, df = 4, P = 0.02). Hydralazine was associated with less flushing than nifedipine (0-12.5% v 0-58%; only comparisons with nifedipine reported flushing); however, there was heterogeneity between trials. More flushing was reported in the trial by Fenakel et al, which treated women for longer than other trials.43 Groups did not differ in visual symptoms, nausea or vomiting, epigastric pain, dizziness, or bronchospasm (table 2).
Despite the high prevalence of side effects (in 85 of 227 patients given hydralazine and 61 of 257 patients given other antihypertensives), few women changed drugs because they experienced side effects (3 of 161 changing from hydralazine, 1 of 167 changing from other antihypertensives); the proportion did not differ between groups.
In summary, hydralazine was associated with more maternal side effects than labetalol or ketanserin, and more headache, palpitations, and maternal tachycardia than other antihypertensives. Whether hydralazine was associated with more side effects than nifedipine was unclear. For all outcomes, absolute event rates ranged widely within trials and significant heterogeneity was seen when risk difference was used as the summary statistic.
Adverse effects on fetal heart rate
Adverse effects on fetal heart rate were defined as "acute fetal distress"39,43,45,49; need for caesarean section due to fetal distress28 or a decelerative fetal heart rate pattern33; "deterioration in the cardiotocographic tracings"48; abnormal fetal heart rate patterns in the six hours after treatment34,35; "abnormal" fetal heart rate in labour47; fetal heart rate decelerations29-31; late decelerations during continuous fetal heart rate monitoring41; or "CTG abnormalities."36 Hydralazine was associated with more adverse effects on fetal heart rate than other antihypertensives (11% (0-56%) v 0% (0-50%)), with the significant heterogeneity isolated to the hydralazine v labetalol subgroup (fig 4). The doses of hydralazine and labetalol were lower in the trial of Ashe et al (hydralazine 3.7 mg/h given intravenously v labetalol 20 mg/h given intravenously with increases every 30 min)41 and higher in the trial of Mabie et al (hydralazine 5 mg given intravenously every 10 min v labetalol 20 mg given intravenously, then 30 mg given intravenously every 10 min)47; otherwise, the differences remained unexplained, although both trials were small and the 95% confidence intervals overlapped substantially.
Fig 4.
Adverse effects on fetal heart rate (FHR) in trials that compared hydralazine with other antihypertensives
Perinatal outcomes
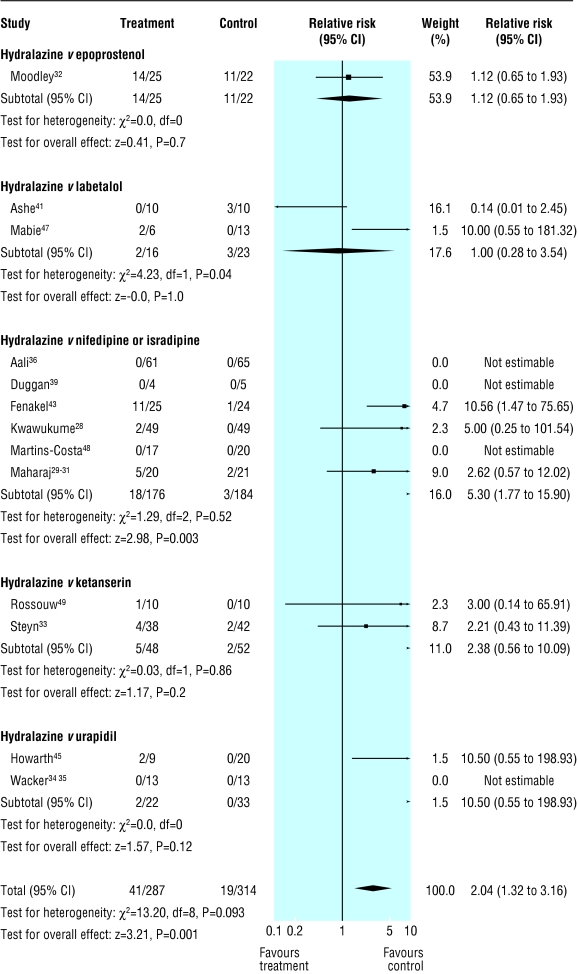
Hydralazine was associated with more low Apgar scores at one minute than other antihypertensives (67% (38-83%) v 15% (14-67%); table 2), but the incidence of low Apgar scores at five minutes did not differ between groups. Hydralazine was associated with less neonatal bradycardia than labetalol (0% (0-0%) v 21% (0-100%)), but the results differed more than could be expected by chance alone, as we reported earlier.14 Few trials reported other perinatal outcomes, and these outcomes (perinatal mortality; admission to neonatal intensive care unit; neonatal hypotension, hypothermia or hypoglycaemia; or complications of prematurity: respiratory distress syndrome, intraventricular haemorrhage or necrotising enterocolitis) did not differ between groups. However, figure 5 shows a statistical trend towards more stillbirths with hydralazine than with other antihypertensives (0% (0% to 31%) v 0% (0% to 22%)).
Fig 5.
Stillbirth in trials that compared hydralazine with other antihypertensives
In summary, hydralazine was associated with more low Apgar scores at one minute and a trend towards an increase in stillbirth compared with other antihypertensives. Hydralazine was associated with less neonatal bradycardia than labetalol.
Discussion
This meta-analysis of randomised controlled trials for the treatment of severe hypertension in pregnancy shows that hydralazine was associated with some poorer maternal and perinatal outcomes than other antihypertensives, particularly labetalol and nifedipine. Hydralazine was found to be a less effective antihypertensive than nifedipine or isradipine, and did not clearly differ from labetalol. In comparison with all other antihypertensives, hydralazine was associated with more of several adverse outcomes: maternal hypotension, placental abruption, adverse effects on fetal heart rate, caesarean section, maternal oliguria, stillbirth (statistical trend only), and low Apgar score at one minute. Hydralazine was associated with less neonatal bradycardia than labetalol, but no trials since our previous meta-analysis reported this outcome.
Hydralazine was more poorly tolerated than other antihypertensives. More maternal side effects were seen than with labetalol or ketanserin. More headaches (raising the issue of imminent eclampsia), palpitations, and maternal tachycardia were seen than with other antihypertensives, with the exception of nifedipine; in trials that showed these side effects, outcomes differed more than could be expected by chance alone, possibly because of differences in design of the trials.
Use of summary statistics
How the consistency of the results differed according to the summary statistic used is worth comment. We used relative risk as the primary summary statistic for this meta-analysis and used risk difference in a secondary analysis. All outcomes for which relative risk was significantly increased, without heterogeneity, showed significant heterogeneity in the analysis that used risk difference, with the exception of low Apgar scores at one minute. Risk difference is sensitive to heterogeneity between trials, and the results of the risk difference analyses highlight the great variability in event rate between trials, which was due, at least in part, to the small sample sizes. The variability in event rates precludes us from extrapolating the results to a specific patient population.
Alternatives to hydralazine
These results are biologically plausible. Rapid or excessive falls in maternal blood pressure may decrease placental perfusion (reflected by abnormal fetal heart rate patterns) and lead to placental abruption, caesarean section, and low Apgar scores at one minute (with recovery by five minutes with resuscitation). The unpredictability of the timing and magnitude of the blood pressure lowering effect of hydralazine may make its use in pregnancy problematic. The results of this meta-analysis do not support recent recommendations favouring initial use of hydralazine over other antihypertensives (including ketanserin).7
Nifedipine seems to be a reasonable alternative to hydralazine. In two case reports, profound muscle weakness and respiratory arrest were associated with concomitant use of nifedipine and magnesium sulphate.18,19 However, no neuromuscular blockade was described in any of the trials comparing hydralazine with nifedipine or isradipine, even though magnesium sulphate was given to all43 or some28 women, and no such blockade was reported in the Magpie trial, in which 29% of women allocated to receive magnesium sulphate also received nifedipine.52 Any risk of neuromuscular blockade is thus likely to be low, and the effect is reversible with calcium gluconate.
Parenteral labetalol also seems to be a reasonable alternative to hydralazine. Although it may be less effective in preventing recurrent severe hypertension, labetalol controlled severe hypertension in 87% of women and was similar to other antihypertensive agents in the need to prescribe further antihypertensives. No new trials were available to update the previously observed association between parenteral labetalol and (usually transient) neonatal bradycardia14; neonatologists should continue to be made aware when intravenous labetalol has been used during labour and delivery.
Ketanserin, an agent investigated most widely in the Netherlands and South Africa, compared favourably with hydralazine.
Of course, there are other limitations to this review that have not been discussed. Meta-analysis is based on a retrospective and observational study design, which relies on published data. However, trials provide the least biased form of information about therapeutic interventions and outcome, and the results of this meta-analysis are biologically plausible.
The most recent Cochrane review found no good evidence that one short acting antihypertensive is better than another, with the exception of ketanserin, which is associated with more persistent hypertension.53 The Cochrane inclusion and exclusion criteria differed somewhat from those in this study, but the most important difference seems to be in the reviews' methods. In the absence of significant between-trial heterogeneity in outcome, we pooled the results from all trials comparing hydralazine and other antihypertensives, whereas the Cochrane review had five subgroups of trials comparing hydralazine and other antihypertensives, with different outcomes reported in each group. In our review, pooling had the advantage of not being based on the assumption that different antihypertensives would cause differences in maternal or perinatal outcome, and where differences between trials existed, pooling informed the reader about how differences in design of the studies and in the intervention may have influenced the results. Pooling resulted in greater statistical power where significant heterogeneity between trials did not exist, and allowed overall conclusions to be drawn from the data.
Conclusions
The results of this review should generate uncertainty about the agent of first choice for treating severe hypertension in pregnancy. Definitive data from adequately powered clinical trials are needed, with the most promising comparison being that of nifedipine with labetalol (or perhaps ketanserin if it is available locally). Such trials should include caesarean section for fetal distress as an outcome. One trial has compared nifedipine with labetalol, but only 50 women were enrolled and caesarean section was not reported.54 The results of this review support the use of antihypertensive agents other than hydralazine for the acute management of severe hypertension in pregnancy.
What is already known on this topic
Hydralazine has been the recommended treatment for severe hypertension in pregnancy, but its side effects mimic symptoms of deteriorating pre-eclampsia
What this study adds
The results of this meta-analysis do not support the use of hydralazine as first line treatment for severe hypertension in pregnancy
Adequately powered clinical trials, starting with a comparison of labetalol and nifedipine, are needed
We thank Ruth Milner for statistical review of the manuscript.
Contributors: LAM, CC, and EJW updated the literature search. LAM, CC, EJW, and PvD abstracted the data; LAM and CC entered the data into Revman. All authors contributed to the analysis of the data and formulation of the results and discussion. All authors reviewed the manuscript. LAM is guarantor.
Funding: PvD and LAM have received establishment grants from the BC Research Institute for Children's and Women's Health, from which PvD also receives salary support. LAM and PvD receive salary support from the BC Women's Hospital and Health Centre Foundation.
Competing interests: LAM has received a speaker's fee and funding for a survey of Canadian practitioners regarding management of hypertension in pregnancy from Shire Pharmaceuticals, the manufacturers of labetalol in Canada.
Ethical approval: Not required.
References
- 1.Rey E, LeLorier J, Burgess E, Lange IR, Leduc L. Report of the Canadian Hypertension Society Consensus Conference: 3. Pharmacologic treatment of hypertensive disorders in pregnancy. CMAJ 1997;157: 1245-54. [PMC free article] [PubMed] [Google Scholar]
- 2.Department of Health. Report on confidential enquiries into maternal deaths in England and Wales 1982-1984. London: HMSO, 1989. [PubMed]
- 3.Department of Health. Report on confidential enquiries into maternal deaths in the United Kingdom 1985-1987. London: HMSO, 1991.
- 4.Department of Health. Report on confidential enquiries into maternal deaths in the United Kingdom 1988-1990. London: HMSO, 1994.
- 5.Department of Health. Report on confidential enquiries into maternal deaths in the United Kingdom 1991-1993. London: HMSO, 1996.
- 6.Lewis G, Drife J, eds. Why mothers die 1997-1999. The confidential enquiries into maternal deaths in the UK. London: RCOG Press, 2001.
- 7.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000;183: S1-22. [PubMed] [Google Scholar]
- 8.Brown MA, Hague WM, Higgins J, Lowe S, McCowan L, Oats J, et al. The detection, investigation and management of hypertension in pregnancy: executive summary. Aust N Z J Obstet Gynaecol 2000;40: 133-8. [DOI] [PubMed] [Google Scholar]
- 9.Impey L. Severe hypotension and fetal distress following sublingual administration of nifedipine to a patient with severe pregnancy induced hypertension at 33 weeks. Br J Obstet Gynaecol 1993;100: 959-61. [DOI] [PubMed] [Google Scholar]
- 10.Olsen KS, Beier-Holgersen R. Fetal death following labetalol administration in pre-eclampsia. Acta Obstet Gynecol Scand 1992;71: 145-7. [DOI] [PubMed] [Google Scholar]
- 11.Vink GJ, Moodley J, Philpott RH. Effect of dihydralazine on the fetus in the treatment of maternal hypertension. Obstet Gynecol 1980;55: 519-22. [PubMed] [Google Scholar]
- 12.Vink GJ, Moodley J. The effect of low-dose dihydrallazine on the fetus in the emergency treatment of hypertension in pregnancy. S Afr Med J 1982;62: 475-7. [PubMed] [Google Scholar]
- 13.Waisman GD, Mayorga LM, Camera MI, Vignolo CA, Martinotti A. Magnesium plus nifedipine: potentiation of hypotensive effect in preeclampsia? Am J Obstet Gynecol 1988;159: 308-9. [DOI] [PubMed] [Google Scholar]
- 14.Magee LA, Ornstein MP, von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ 1999;318: 1332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cutler JA. Calcium-channel blockers for hypertension—uncertainty continues. N Engl J Med 1998;338: 679-81. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998;16: 1823-9. [DOI] [PubMed] [Google Scholar]
- 17.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhager WH, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997;350: 757-64. [DOI] [PubMed] [Google Scholar]
- 18.Ben Ami M, Giladi Y, Shalev E. The combination of magnesium sulphate and nifedipine: a cause of neuromuscular blockade. Br J Obstet Gynaecol 1994;101: 262-3. [DOI] [PubMed] [Google Scholar]
- 19.Snyder SW, Cardwell MS. Neuromuscular blockade with magnesium-sulfate and nifedipine. Am J Obstet Gynecol 1989;161: 35-6. [DOI] [PubMed] [Google Scholar]
- 20.Brown MA, McCowan LM, North RA, Walters BN. Withdrawal of nifedipine capsules: jeopardizing the treatment of acute severe hypertension in pregnancy? Australasian Society for the Study of Hypertension in Pregnancy. Med J Aust 1997;166: 640-3. [PubMed] [Google Scholar]
- 21.Stevens TP, Guillet R. Use of glucagon to treat neonatal low-output congestive heart failure after maternal labetalol therapy. J Pediatr 1995;127: 151-3. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Smith GD. Principles of and procedures for systematic reviews. In: Egger M, Smith GD, Altman DG, eds. Systematic reviews in health care: meta-analysis in context. London: BMJ Books, 2003: 23-42.
- 23.Clarke M, Oxman AD, eds. Cochrane reviewer's handbook 4.2.0. In: Cochrane Library, Issue 2, 2003. www.cochrane.de/cochrane/hbook.htm (accessed 24 Sep 2003).
- 24.Organization of a systematic review for the Cochrane Neonatal Review Group: guidelines for reviewers and editors. Cochrane Collaboration, 2002. hiru.mcmaster.ca/cochrane/centres/canadian/neonatal/checklist.pdf (accessed 16 Sep 2003).
- 25.Bolte AC, van Eyck J, Strack van Schijndel RJ, van Geijn HP, Dekker GA. The haemodynamic effects of ketanserin versus dihydralazine in severe early-onset hypertension in pregnancy. Br J Obstet Gynaecol 1998;105: 723-31. [DOI] [PubMed] [Google Scholar]
- 26.Bolte AC, van Eyck J, Kanhai HH, Bruinse HW, van Geijn HP, Dekker GA. Ketanserin versus dihydralazine in the management of severe early-onset preeclampsia: maternal outcome. Am J Obstet Gynecol 1999;180: 371-7. [DOI] [PubMed] [Google Scholar]
- 27.Harper A, Murnaghan GA. Maternal and fetal haemodynamics in hypertensive pregnancies during maternal treatment with intravenous hydralazine or labetalol. Br J Obstet Gynaecol 1991;98: 453-9. [DOI] [PubMed] [Google Scholar]
- 28.Kwawukume EY, Ghosh TS. Oral nifedipine therapy in the management of severe preeclampsia. Int J Gynaecol Obstet 1995;49: 265-9. [DOI] [PubMed] [Google Scholar]
- 29.Maharaj B, Khedun SM, Moodley J, Madhanpall N, van der Byl K. Intravenous isradipine in the management of severe hypertension in pregnant and nonpregnant patients. A pilot study. Am J Hypertens 1994;7(suppl): 61S-3. [DOI] [PubMed] [Google Scholar]
- 30.Maharaj B, Moodley J, Khedun SM, van der Byl K, Madhanpall N. Intravenous isradipine in the management of severe hypertension of pregnancy in black patients: a pilot study. Blood Press Suppl 1994;1: 54-6. [PubMed] [Google Scholar]
- 31.Maharaj B, Khedun SM, Moodley J, van der Byl K, Rapiti N. A comparative study of intravenous isradipine and dihydralazine in the treatment of severe hypertension of pregnancy in black patients. Hypertens Pregn 1997;16: 1-9. [Google Scholar]
- 32.Moodley J, Gouws E. A comparative study of the use of epoprostenol and dihydralazine in severe hypertension in pregnancy. Br J Obstet Gynaecol 1992;99: 727-30. [DOI] [PubMed] [Google Scholar]
- 33.Steyn DW, Odendaal HJ. Dihydralazine or ketanserin for severe hypertension in pregnancy? Preliminary results. Eur J Obstet Gynecol Reprod Biol 1997;75: 155-9. [DOI] [PubMed] [Google Scholar]
- 34.Wacker J, Lewicka S, Haack D, Bastert G. Hypertension in pregnancy. J Steroid Biochem Mol Biol 1993;45: 65-8. [DOI] [PubMed] [Google Scholar]
- 35.Wacker J, Werner P, Walter-Sack I, Bastert G. Treatment of hypertension in patients with pre-eclampsia: a prospective parallel-group study comparing dihydralazine with urapidil. Nephrol Dial Transplant 1998;13: 318-25. [DOI] [PubMed] [Google Scholar]
- 36.Aali BS, Nejad SS. Nifedipine or hydralazine as a first-line agent to control hypertension in severe preeclampsia. Acta Obstetricia et Gynecologica Scandinavica 2002;81: 25-30. [DOI] [PubMed] [Google Scholar]
- 37.Bolte AC, van Geijn HP, Bekedam DJ, Dekker GA. Ketanserin, a serotonin2 receptor blocker, for hypertension in pregnancy [abstract]. Hypertens Pregn 2002; 9.
- 38.Schulz M, Wacker J, Bastert G. Wirkungen von Urapidil in der antihypertensiven Therapie bei Nraeklampsie auf die Neugeborenen. Zentralbl Gynakol 2001;123: 529-33. [DOI] [PubMed] [Google Scholar]
- 39.Duggan PM, McCowan LM, Stewart AW. Antihypertensive drug effects on placental flow velocity waveforms in pregnant women with severe hypertension. Aust N Z J Obstet Gynaecol 1992;32: 335-8. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez RJW. Manejo de la preeclampsia severa/eclampsia. Comparacion entre nifedipina e hidralazina como medicamentos antihipertensivos. Ginec Obst Mex 1993;61: 76-9. [PubMed] [Google Scholar]
- 41.Ashe RG, Moodley J, Richards AM, Philpott RH. Comparison of labetalol and dihydralazine in hypertensive emergencies of pregnancy. S Afr Med J 1987;71: 354-6. [PubMed] [Google Scholar]
- 42.Bhorat IE, Naidoo DP, Rout CC, Moodley J. Malignant ventricular arrhythmias in eclampsia: a comparison of labetalol with dihydralazine. Am J Obstet Gynecol 1993;168: 1292-6. [DOI] [PubMed] [Google Scholar]
- 43.Fenakel K, Fenakel G, Appelman Z, Lurie S, Katz Z, Shoham Z. Nifedipine in the treatment of severe preeclampsia. Obstet Gynecol 1991;77: 331-7. [PubMed] [Google Scholar]
- 44.Garden A, Davey DA, Dommisse J. Intravenous labetalol and intravenous dihydralazine in severe hypertension in pregnancy. Clin Exp Hypertens B 1982;1: 371-83. [DOI] [PubMed] [Google Scholar]
- 45.Howarth GR, Seris A, Venter C, Pattinson RC. A randomized controlled pilot study comparing urapidil to dihydralazine in the management of severe hypertension in pregnancy. Hypertens Pregn 1997;16: 213-21. [Google Scholar]
- 46.Jegasothy R, Paranthaman S. Sublingual nifedipine compared with intravenous hydrallazine in the acute treatment of severe hypertension in pregnancy: potential for use in rural practice. J Obstet Gynaecol Res 1996;22: 21-4. [DOI] [PubMed] [Google Scholar]
- 47.Mabie WC, Gonzalez AR, Sibai BM, Amon E. A comparative trial of labetalol and hydralazine in the acute management of severe hypertension complicating pregnancy. Obstet Gynecol 1987;70: 328-33. [PubMed] [Google Scholar]
- 48.Martins-Costa S, Ramos JG, Barros E, Bruno RM, Costa CA, Goldin JR. Randomized, controlled trial of hydralazine versus nifedipine in preeclamptic women with acute hypertension. Clin Exper Hypertens Pregn 1992;B11: 25-44. [Google Scholar]
- 49.Rossouw HJ, Howarth G, Odendaal HJ. Ketanserin and hydralazine in hypertension in pregnancy—a randomised double-blind trial. S Afr Med J 1995;85: 525-8. [PubMed] [Google Scholar]
- 50.Seabe SJ, Moodley J, Becker P. Nifedipine in acute hypertensive emergencies in pregnancy. S Afr Med J 1989;76: 248-50. [PubMed] [Google Scholar]
- 51.Sibai BM, Frangieh AY. Management of severe preeclampsia. Curr Opin Obstet Gynecol 1996;8: 110-3. [PubMed] [Google Scholar]
- 52.Duley L, Farrell B, Spark P, Roberts B, Watkins K, Bricker L et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet 2002;359: 1877-90. [DOI] [PubMed] [Google Scholar]
- 53.Duley L, Henderson-Smart DJ. Drugs for rapid treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev 2002;(4); CD001449. [DOI] [PubMed]
- 54.Vermillion ST, Scardo JA, Newman RB, Chauhan SP. A randomized, double-blind trial of oral nifedipine and intravenous labetalol in hypertensive emergencies of pregnancy. Am J Obstet Gynecol 1999;181: 858-61. [DOI] [PubMed] [Google Scholar]