Short abstract
Disseminated intravascular coagulation has long been associated with increased mortality in patients with sepsis. An effective treatment is now available, and the authors of this review describe how improved understanding and earlier diagnosis could lead to targeted treatment and improved prognosis
Although the first clinical observations on disseminated intravascular coagulation (DIC) were reported in the 19th century,1 this condition of widespread and disordered coagulation has probably afflicted mankind for as long as trauma and infection have beset us. Indeed, DIC is generally associated with an adverse outcome by most clinicians, and its acronym has been synonymous with "death is coming." However, a drug targeted at the coagulopathy of severe sepsis (activated protein C) has now emerged as the first successful treatment for the condition.2 We provide an updated overview of DIC and how its onset may indicate the turning point from which an adaptive response becomes maladaptive and potentially injurious to the host. Precise laboratory definition of this process could provide a therapeutic window in critical illness that may finally deliver an improved outcome.
Sources and selection criteria
A systematic search of PubMed with the search term "disseminated intravascular coagulation" and related keywords yielded 10 262 publications, most of which related to pathophysiology and case reports. Owing to a lack of systematic controlled clinical trials, many recommendations are based on expert opinion and consensus driven guidelines rather than a secure evidence base. However, an increasing number of randomised controlled trials studying the diagnosis and management of DIC have been reported in the past five years, and we have incorporated these in the review.
Definition
DIC represents a continuum in clinical-pathological severity, characterised by the increasing loss of localisation or compensated control in intravascular activation of coagulation. It has definable phases that characterise patients at risk for increased mortality. The International Society of Thrombosis and Haemostasis standardisation subcommittee has therefore proposed working definitions to facilitate earlier detection and treatment.3 The new emphasis is on recognising a non-overt stage rather than an overt and late stage of DIC.
Summary points
Disseminated intravascular coagulation (DIC) is caused by the enhanced and abnormally sustained generation of thrombin
DIC is highly relevant to outcome in patients with sepsis; the first successful treatment in sepsis is by targeting generation of thrombin with activated protein C
DIC exemplifies multifaceted interactions between the inflammatory and coagulation pathways
DIC indicates the transition from localised, adaptive, and compensated coagulation processes into maladaptive responses
Identifying circulating biomarkers that propagate generation of thrombin may indicate the timing of dissemination and be a tool for therapeutic targeting
Epidemiology
DIC is an acquired syndrome arising from various different causes (table 1, fig 1). Its classification primarily as a haemostatic complication of diseases has somewhat detracted from a more central pathogenetic consideration. Its importance is supported by two key observations. Firstly, the presence of DIC increases the risk of mortality beyond that associated with the primary disease.4 Secondly, the removal of its underlying cause does not necessarily alleviate the process in all cases. Although removal of products of conception either by surgery or spontaneous delivery usually leads to remission in obstetric causes of DIC, the use of appropriate antibiotics may be insufficient to prevent further deterioration in sepsis.5
Table 1.
Clinical conditions associated with disseminated intravascular coagulation
| Condition | Causes |
|---|---|
| Sepsis or severe infection |
Potentially any micro-organism, including severe acute respiratory syndrome |
| Trauma |
Serious tissue injury |
| Head injury | |
| Fat embolism |
|
| Organ destruction |
Severe pancreatitis |
| Malignancy | Solid tumours |
| Haematological malignancies (for example, acute promyelocytic leukaemia) |
|
| Obstetrical calamities | Placental abruption |
| Amniotic fluid embolism |
|
| Vascular abnormalities | Giant haemangiomas (Kasabach-Merrit syndrome) |
| Large vessel aneurysms (for example, aortic) |
|
| Hepatic failure |
|
| Severe toxic or immunological reactions | Snake bites |
| Recreational drugs | |
| Severe transfusion reactions | |
| Transplant rejection |
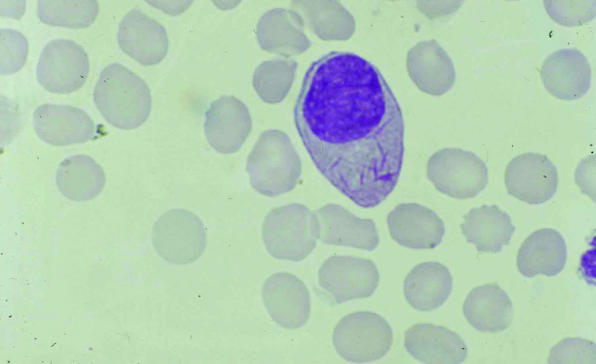
Fig 1.
Blood film from a patient with acute promyelocytic leukaemia, showing a promyeloblast with cytoplasmic auer rods diagnostic of myeloid neoplasia and changes indicative of disseminated intravascular coagulation, including fragmented red blood cells and a marked absence of platelets
Clinical manifestation
The perturbed coagulation of DIC can manifest clinically at any point in the spectrum from bleeding to thrombosis (fig 2). Although bleeding is the archetypal physical manifestation in patients with DIC, as a result of reducing platelets and coagulation factors, organ failure is a much more common finding. Autopsy findings have shown both diffuse bleeding at various sites, with haemorrhagic tissue necrosis, and thrombi in small as well as larger blood vessels.6 Experimental animal models of DIC have shown fibrin disposition in various organs as a cause of organ failure.
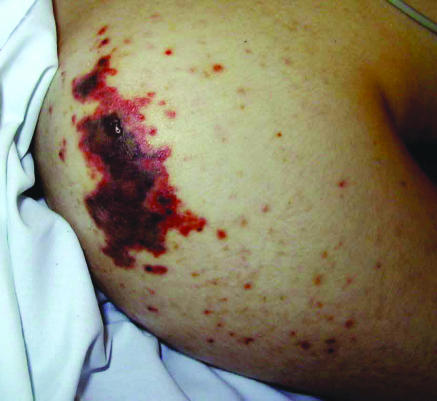
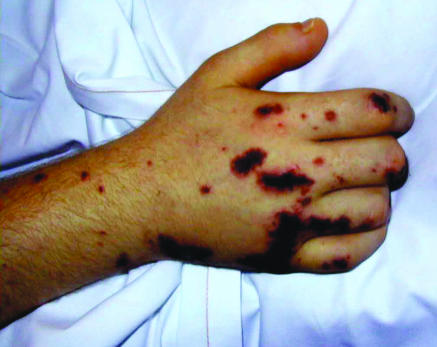
Fig 2.
Typical skin changes in the shoulder (top) and hand (bottom) in purpura fulminans associated with meningococcal septicaemia and disseminated intravascular coagulation
Pathophysiology
Several important themes in the pathophysiology of DIC exist. First is the central part played by the generation of thrombin in vivo.7,8 Second is the fact that mechanisms fuelling and perpetuating the generation of thrombin are pathogenic in its dissemination. Third is the parallel and concomitant activation of the inflammatory cascade. Fourth is the importance of the endothelial microvasculature in this process.
Thrombin generation in vivo
The generation of thrombin in vivo is pivotal to haemostatic control by way of balancing both procoagulant and anticoagulant activities (fig 3). Clot formation through the conversion of fibrinogen to fibrin is simultaneously controlled through activation by thrombin of the protein C anticoagulant regulatory pathway.9 This also serves several potential roles, including the walling off of pathogens, recruitment of cellular processes, and activation of appropriate endothelial response signals.10 This fine homeostatic balance of controlled thrombin generation is lost in DIC.
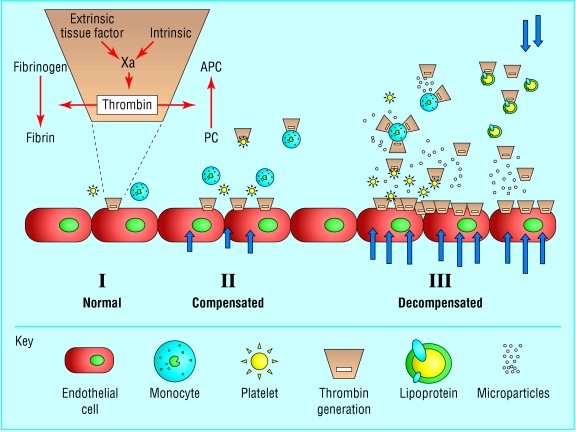
Fig 3.
Generation and dissemination of thrombin. I: normal, localised coagulation activation, via extrinsic and intrinsic pathways, to generate thrombin with a resultant balance in procoagulant conversion of fibrinogen to fibrin and anticoagulant activation of protein C (PC) to its activated form, APC. The orange area denotes the reactions that are highly dependent on phospholipid surfaces. II: compensated increase in coagulation activation with contribution from endothelial, monocyte, and platelet cell surfaces. Arrows denote the intensity of the stress or acute phase response. III: decompensation and dissemination of thrombin generation in vivo, with increased anionic phospholipid surfaces provided by circulating microparticles and lipoproteins released as part of the stress response
Mechanisms disseminating and sustaining thrombin generation
Although the tissue factor or extrinsic pathway of coagulation plays a major part in initiating generation of thrombin, the recruitment of other processes leads to dissemination of intravascular coagulation (box 1). Secondary bursts of thrombin formation are provided by the intrinsic pathway of coagulation, leading to consumption and depletion of the anticoagulant regulatory proteins—protein C, protein S, and antithrombin.11 Additionally, increased exposure of negatively charged phospholipid surfaces further facilitates the assembly and propagation of coagulation kinetics.12 Such surfaces, mainly rich in phosphatidylserine, are provided by externalisation of the inner leaflet of cell membranes on activation and apoptosis.13 Cell damage also leads to the generation of microparticles from platelets, monocytes, and endothelial cells, which increase the circulating surface area available for coagulant reactions to occur.14 Also relevant is the provision of phospholipid surfaces by very low density lipoprotein, which can increase several fold in severe sepsis to further enhance and sustain generation of thrombin.15 Together, these mechanisms form a spatially and temporally expansive response that is the hallmark of DIC.
Box 1: Mechanisms sustaining generation of thrombin
Activation of intrinsic pathway of coagulation
Reduction in levels of endogenous anticoagulant factors (for example, protein C, antithrombin)
-
Increased availability of negatively charged phospholipid surfaces:
externalisation of inner cellular membrane leaflet
cellular microparticle formation
circulating lipoproteins
Links between inflammation and coagulation
Once activated, the inflammatory and coagulation pathways interact with one another to amplify the response further.16 Whereas cytokines and pro-inflammatory mediators can induce coagulation, thrombin and other serine proteases interact with protease activated receptors on cell surfaces to promote further activation and additional inflammation.17 When this process generalises, it escapes the well developed local checks and balances and results in a dysregulated, undirected response that fuels the vicious cycle between inflammation and coagulation.
Endothelial cell activation and dysfunction
The normal and adaptive response of endothelial surfaces is directly relevant to the regulation of coagulation and inflammation.18 Dysfunction and failure of this large, physiological endothelial organ beyond the adaptive nature of the host response can lead to and be indicated by the development of DIC. The degree of coagulopathy and the dominance of thrombotic or bleeding sequelae depend on genetic and other host related factors.19
Diagnosis
In clinical practice, a diagnosis of DIC can be made by a combination of routinely available laboratory tests for coagulation, for which diagnostic algorithms have become available.3 The classically characterised findings are prolonged clotting times (for example, prothrombin time, activated partial thromboplastin time), elevated products of fibrin breakdown (for example, d-dimer), low platelet counts, and low fibrinogen. Results within the normal range do not exclude important consumptive coagulopathy, however, especially as the acute phase response results in shortening of the activated partial thromboplastin time and increased fibrinogen concentrations. For this reason, the International Society of Thrombosis and Haemostasis's guidance on identifying early, non-overt DIC scores not only for abnormal results but also for abnormal trends in these results.3 Emphasis has also been placed on more specific assays that more directly link inflammation and disseminated coagulation processes but that also need to be simple and rapid in performance as well as robust in their evidence base (table 2).15,20
Table 2.
Practical markers of non-overt disseminated intravascular coagulation
| Test | Finding |
|---|---|
| Prothrombin time |
Increasing clotting time |
| Platelets |
Falling count |
| Activated partial thromboplastin time |
Increasing clotting time and abnormal waveform |
| Fibrin related marker (d-dimer, fibrin degradatory products) |
Rising concentrations |
| Fibrinogen |
Falling concentrations |
| Protein C |
Falling concentrations |
| Antithrombin | Falling concentrations |
Treatment
The mainstay of management of DIC is to specifically remove the underlying causative factor. However, as testified by the mortality from this condition, DIC often continues even after appropriate treatment—for example, with the use of antibiotics in sepsis. The efficacy of replacement treatment with plasma or platelets has not been proved in randomised controlled trials. However, replacement treatment seems to be a rational option, particularly when these factors are considerably depleted in a patient who is either actively bleeding or at risk of bleeding (box 2). Furthermore, no clinical or experimental data exist to suggest that such treatment might "add fuel to the fire." A beneficial effect of heparin, which inhibits the excess thrombin generated, on clinically important outcome events in patients with DIC has never been shown in controlled clinical trials. In addition, the safety of heparin treatment is debatable in DIC patients who are prone to bleeding.21
The reported success of a recombinant form of human activated protein C in severe sepsis, whereby mortality at 28 days was significantly reduced from 31% to 25%, is an indication for its prompt consideration in sepsis related DIC (level I evidence).2 In addition to acting as an anticoagulant, activated protein C also has direct anti-inflammatory and antiapoptotic properties.22 This may in part explain why the other endogenous anticoagulants (antithrombin and tissue factor pathway inhibitor) trialled in severe sepsis have not shown such good efficacy.23 Recombinant human activated protein C is currently used as a 96 hour infusion, and caution is needed in patients with severe thrombocytopenia (less than 30x109/l) because of the increased incidence of intracerebral haemorrhage in treated patients. Monitoring of the platelet count and transfusion of platelets as necessary are important considerations.
Although not licensed, unactivated (zymogen) protein C replacement has reported usefulness in meningococcal septicaemia (level IV evidence).24 However, conversion to activated protein C in vivo would depend on the presence of cell surface receptors (thrombomodulin and the endothelial protein C receptor), which can be severely diminished in sepsis.25
Conclusion
New information about the pathophysiology and treatment of DIC promises new hope of an improved prognosis for this disorder, which has long been associated with unacceptably high mortality. Important breakthroughs will need a conceptual shift that emphasises relations between the various mediators and factors that propagate generation of thrombin, especially with regard to the links between inflammation and coagulation. Additional studies promise to provide new insights into DIC, not as an isolated mechanism but rather as a coordinator of events that substantially affects outcome in sepsis and critical illness.
Box 2: Treatment of disseminated intravascular coagulation
Aggressive treatment of the underlying cause
-
Multi-organ support:
circulatory resuscitation or inotropic agents
ventilation
haemodialysis
-
Blood product support if actively bleeding or at high risk (post-surgery, pre-procedure):platelets if less than 50x109/l (4-8 units = 1-2 adult doses)fresh frozen plasma if clotting times prolonged—15 ml/kg (1 litre = 4 units = 1 adult dose)cryoprecipitate to keep fibrinogen > 1.0 g/l—1-1.5 packs/10 kg (10 units = 1 adult dose)
- Recombinant human activated protein C in adults with severe sepsis and multiple organ failure, provided platelet count is not < 30x109/l
-
Additional educational resources
Review articles
Toh CH, Dennis M. DIC 2002: a review of disseminated intravascular coagulation. Hematology 2003;8: 65-7112745654
Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 2003;101: 3765-77
Riewald M, Ruf W. Role of coagulation protease cascades in sepsis. Crit Care 2003;7: 123-9
Websites
www.med.unc.edu/isth—online resource for the Scientific Standardization Committee for the International Society for Thrombosis and Haemostasis
www.DICsepsis.org—international physicians' discussion forum
www.sepsis.com—educational resource from Eli Lilly
We thank Barry Woodcock for the clinical figures and Colin Downey for the illustrations.
Contributors: Both authors contributed to the text, with CHT developing its theme and section headings. MD was responsible for the boxes, tables, and figures.
Competing interests: None declared.
References
- 1.Dupuy M. Injections de matière cérébrale dans les veines. Gaz Med Paris 1834;2: 524. [Google Scholar]
- 2.Bernard GR, Vincent AL, Laterre PF, LaRosa S, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001;344: 699-709. [DOI] [PubMed] [Google Scholar]
- 3.Taylor FB, Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical and laboratory criteria and a scoring system for disseminated intravascular coagulation. Thromb Haemost 2001;86: 1327-30. [PubMed] [Google Scholar]
- 4.Gando S, Kameue T, Nanzaki S, Nakanishi Y. Disseminated intravascular coagulation is a frequent complication of systemic inflammatory response syndrome. Thromb Haemost 1996;75: 224-8. [PubMed] [Google Scholar]
- 5.Hazelzet JA, Risseeuw-Appel IM, Kornelisse RF, Hop WCJ, Dekker I, Joosten KFM, et al. Age-related differences in outcome and severity of DIC in children with septic shock and purpura. Thromb Haemost 1996;76: 932-8. [PubMed] [Google Scholar]
- 6.Shimamura K, Oka K, Nakazawa M, Kojima M. Distribution patterns of microthrombi in disseminated intravascular coagulation. Arch Pathol Lab Med 1983;107: 543-7. [PubMed] [Google Scholar]
- 7.Giles AR, Nesheim ME, Mann KG. Studies of factors V and VIII:C in an animal model of disseminated intravascular coagulation. J Clin Invest 1984;74: 2219-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gando S, Nanzaki S, Sasaki S, Kemmotsu O. Significant correlations between tissue factor and thrombin markers in trauma and septic patients with disseminated intravascular coagulation. Thromb Haemost 1998;79: 1111-5. [PubMed] [Google Scholar]
- 9.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci USA 1996;93: 10212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 2001;167: 2887-94. [DOI] [PubMed] [Google Scholar]
- 11.Von dem Borne PAK, Meijers JCM, Bouma BN. Feedback activation of factor XI by thrombin in plasma results in additional formation of thrombin that protects fibrin clots from fibrinolysis. Blood 1995;86: 3035-42. [PubMed] [Google Scholar]
- 12.Kalafatis M, Swords NA, Rand MD, Mann KG. Membrane-dependent reactions in blood coagulation: role of the vitamin K-dependent enzyme complexes. Biochim Biophys Acta 1994;1227: 113-29. [DOI] [PubMed] [Google Scholar]
- 13.Satta N, Toti F, Feugas O, Bohbot A, Dachary-Prigent J, Eschwege V, et al. Monocyte vesiculation is a possible mechanism for dissemination of membrane associated procoagulant activities and adhesion molecules after stimulation with lipopolysaccharide. J Immunol 1994;153: 3245-55. [PubMed] [Google Scholar]
- 14.Combes V, Simon AC, Grau GE. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest 1999;104: 93-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toh CH, Samis J, Downey C, Walker J, Becker L, Bruffato N, et al. Biphasic transmittance waveform in the APTT coagulation assay is due to the formation of a calcium dependant complex of C-reactive protein with very-low-density-lipoprotein and is a novel marker of impending disseminated intravascular coagulation. Blood 2002;100: 2522-9. [DOI] [PubMed] [Google Scholar]
- 16.Van der Poll T, Buller HR, ten Cate H. Activation of coagulation after administration of tumour necrosis factor to normal subjects. N Engl J Med 1990;322: 1622-7. [DOI] [PubMed] [Google Scholar]
- 17.Coughlin SR. Thrombin signalling and protease activated receptors. Nature 2000;407: 258-64. [DOI] [PubMed] [Google Scholar]
- 18.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 2003;101: 3765-77. [DOI] [PubMed] [Google Scholar]
- 19.Westendorp RG, Langermans JA, Huizinga TW, Verweij CL, Sturk A. Genetic influence on cytokine production in meningococcal disease. Lancet 1997;349: 1912-3. [DOI] [PubMed] [Google Scholar]
- 20.Liaw PCY, Ferrell G, Esmon CT. A monoclonal antibody against activated protein C allows rapid detection of activated protein C in plasma and reveals a calcium ion dependent epitope involved in factor Va inactivation. J Thromb Haemost 2003;1: 662-70. [DOI] [PubMed] [Google Scholar]
- 21.Feinstein DI. Diagnosis and management of disseminated intravascular coagulation: the role of heparin therapy. Blood 1982;60: 284-7. [PubMed] [Google Scholar]
- 22.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein C defines new mechanisms modulating inflammation and apoptosis. J Biol Chem 2001;276: 11199-203. [DOI] [PubMed] [Google Scholar]
- 23.Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, et al. High-dose antithrombin III in severe sepsis: a randomised controlled trial. JAMA 2001; 286: 1869-78. [DOI] [PubMed] [Google Scholar]
- 24.Smith OP, White B, Vaughan D, Rafferty M, Claffey L, Lyons B, et al. Use of protein C concentrate, heparin and haemofiltration in meningococcus-induced purpura fulminans. Lancet 1997;359: 1590-3. [DOI] [PubMed] [Google Scholar]
- 25.Faust SN, Levin M, Harrison OB, Goldin RD, Lockhart MS, Kondaveeti S, et al. Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N Engl J Med 2001;345: 408-16. [DOI] [PubMed] [Google Scholar]