Abstract
The neuroprotective effects of estrogen in young adult rodents are well established. Less well understood is how estrogen neuroprotection is affected by aging and interactions with progesterone. In this study, we investigated the effects of estrogen and continuous progesterone, both alone and in combination, on hippocampal neuron survival following kainate lesion in 14 month-old female rats entering reproductive senescence. Our results show that ovariectomy-induced hormone depletion did not significantly affect the extent of kainate-induced neuron loss. Treatment of ovariectomized rats with estrogen significantly reduced neuron loss, however this effect was blocked by co-administration of continuous progesterone. Treatment of ovariectomized rats with progesterone alone did not significantly affect kainate toxicity. These results provide new insight into factors that regulate estrogen neuroprotection, which has important implications for hormone therapy in postmenopausal women.
Keywords: estrogen, progesterone, neuroprotection, kainite, Alzheimer’s disease
Introduction
The precipitous loss of estrogen and progesterone that occurs after menopause is a significant risk factor for the development of Alzheimer’s disease (AD) in women [19]. However, support for the use of estrogen-based hormone therapy (HT) to reduce the risk of AD in postmenopausal women has been controversial. Although prospective studies have demonstrated that HT can reduce the risk of AD, the Women’s Health Initiative trial indicated that the incidence of dementia was not significantly affected by HT consisting of only conjugated equine estrogen (CEE) initiated several years after the onset of menopause but increased by HT combining CEE plus medroxyprogesterone acetate (MPA). These conflicting results suggested that neural estrogen actions and thus HT efficacy may be attenuated by a variety factors, such as the paradigm of continuous versus cyclic progesterone treatment [19] and advancing age.
Accumulating evidence suggests that there may be a limited window of opportunity for initiation of HT in women after which benefits are not realized [12]. In experimental models, the brain shows age-related alterations in estrogen responsiveness that appear to begin with the onset of reproductive senescence. For example, many neural effects of estrogen are diminished in aged female rats, including induction of brain derived neurotrophic factor [14], compensatory sprouting [27], spatial memory [4] and neuroprotection from stroke [28]. However, middle-aged female rodents retain some estrogen responsiveness on cognition in women [25] and rodents [1]. Taken together, these studies highlight the importance of investigating the neuroprotective effects of female sex steroid hormones in the aging brain.
In addition to aging, neural estrogen actions are also regulated by progesterone, interactions that may affect HT efficacy. For example, we recently reported that estrogen attenuated ovariectomy-induced accumulation of β-amyloid in a mouse model of AD, an effect that was blocked by co-administration of continuous progesterone [5]. Similarly, continuous progesterone can block estrogen-induced increases in the expression of neurotrophins [3] and indices of cholinergic function [10]. When delivered independently, both estrogen [29] and progesterone [24] can exert protective effects in models of neuronal injury such as excitotoxic lesions and ischemia. Co-administration of estrogen and progesterone rescues neuronal loss in some rodent models of injury but not others [24]. For example, continuous exposure to progesterone or the progestin medroxyprogesterone attenuates estrogen neuroprotection against kainate lesion in young adult female rats [23]. How aging affects interactions between estrogen and progesterone in regulation of neuroprotective effects is not well understood. In this study, we begin to investigate in an animal model how both aging and progesterone affect estrogen neuroprotection. We examine the independent and combined effects of estrogen and progesterone on neuron survival in hippocampus following kainate lesion in reproductively senescent female rats.
Materials and Methods
Retired female Sprague-Dawley rat breeders (Harlan Laboratories; Indianapolis, IN) were obtained at 9 mo of age. Vaginal smears were taken 7 consecutive days every 21 days from 12–14 mo of age to identify irregular estrus cycles, a hallmark of reproductive senescence [18]. At 14 months of age, rats underwent ovariectomy (OVX) or sham OVX. Two weeks following surgery, animals were subcutaneously implanted with silastic capsules (1.57 mm I.D., 3.18 mm O.D.; Dow Corning) that were either empty (controls) or contained hormones (Sigma, St. Louis, MO) in a crystalline form. Each group (n=10 per group) received either 1 × 5 mm blank capsule, 1 × 5 mm 17β-estradiol (E2) capsule, 4 × 40 mm progesterone (P4) capsule, or a combination of 1 × 5 mm E2 and 4 × 40 mm P4 capsules designed to maintain plasma E2 and P4 at physiological levels [23; 9; 13]. On the day of sacrifice, uteri were dissected, blotted, and weighed as a bioassay of E2 action to confirm efficacy of estrogen treatment [17]. Further, to confirm efficacy of progesterone treatment, blood was collected and serum P4 levels were measured by ELISA (Demeditec Diagnostics, Germany); our data were consistent with the manufacturer’s reported values for assay sensitivity (0.045ng/ml) and intra-assay (CV= 5.4%) and inter-assay (CV= 9.96%) variability.
Three weeks after initiation of hormone treatment, animals were injected (ip) with either saline or kainate (Sigma; 10 mg/kg in sterile 0.85% NaCl). To assess latency and severity of kainate-induced seizure, animals were monitored continuously for 3 h following injection according to a previously described progressive rating scale ranging from 0 (no seizure) to 5 (continuous seizure) [23]. Animals that displayed a seizure rating of 5 were immediately sacrificed and excluded from further study. Forty-eight hours post-injection, animals were anesthetized with isoflurane and killed by rapid decapitation. Brains were immediately removed, bisected midsagitally, and immersion-fixed for 48 h in cold, freshly prepared 4% paraformaldehyde.
Hemibrains were exhaustively sectioned (40 µm) in the horizontal plane using a vibratome. Every tenth section containing hippocampus (approximately 12 sections per brain) was immunostained with the neuron-specific antibody NeuN (1:250, Chemicon; Temecula, CA) using the ABC Elite immunohistochemistry kit (Vector; Burlingame, CA), as previously described [22]. The number of NeuN immunoreactive cells in the CA2/3 of the hippocampus was counted using a protocol previously described [22]. Briefly, an Olympus BX50 microscope equipped with a motorized stage was computer-controlled by the CAST-Grid software (Olympus, Ballerup, Denmark) so that unbiased, randomly oriented counting frames of 32 µm × 32 µm with X-Y steps of 210 µm × 210 µm were generated throughout the CA2/3 region. The number of positively stained nuclei within each counting frame was recorded by an experimenter blinded to the experimental conditions.
To evaluate treatment effects, raw data were statistically examined using ANOVA and, when appropriate, between group comparisons were made using Fisher LSD tests.
Results
To confirm the efficacy of the hormone manipulations, we measured uterine weight, a bioassay of E2 action, and P4 serum values. We observed a significant overall effect on uterine weight across treatment groups [F(4,36) = 8.61, p <0.001]. In comparison to sham OVX animals, the OVX group had significantly lower uterine weight (530±32 mg versus 230±80 mg; p <0.001). Relative to the OVX group, both of the E2 treated groups, OVX+E2 (376±30 mg) and OVX+E2+P4 (402±23 mg), exhibited significantly higher uterine weights (p = 0.03 and p = 0.02, respectively), indicating effective E2 treatment. Progesterone replacement had no statistically significant effect on uterine weight (284±43 mg; p = 0.42 compared to OVX). We also observed a significant overall effect of treatment on P4 serum values [F(4,23) = 6.28, p = 0.001]. Specifically, compared to sham OVX (36.1±11.2 ng/ml), OVX animals had significantly decreased serum P4 level (9.0±3.5 ng/ml; p = 0.04). Serum P4 levels after P4 replacement (54.7±5.1 ng/ml) and E2+P4 replacement (60.8±12.9 ng/ml) significantly increased levels compared to OVX (p = 0.003 and p = 0.0004, respectively).
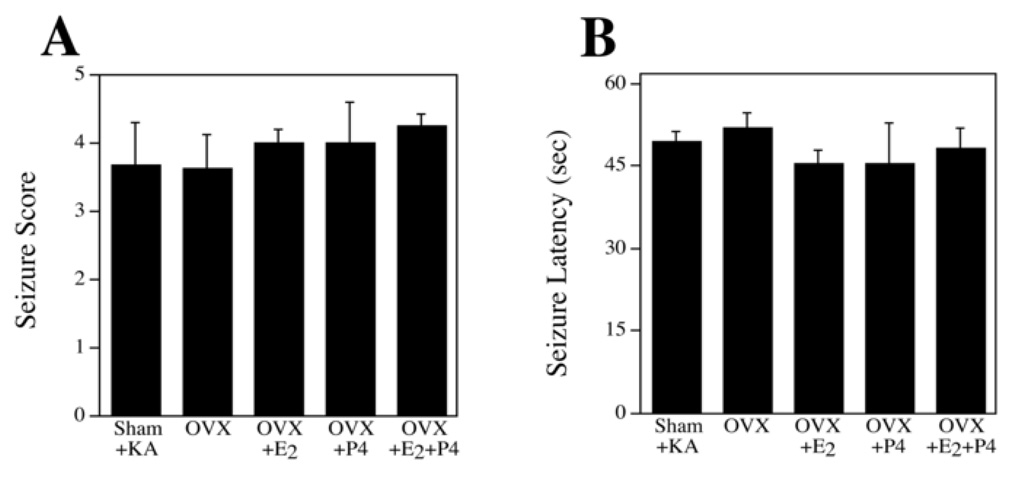
Because kainate-induced seizure severity is related to the extent of neuronal injury, we determined whether hormone manipulations affected seizure parameters. Lesioned animals were monitored for latency to seizure onset and seizure severity. All kainate-treated animals exhibited robust seizures with seizure severity scores of at least 3 out of 5 and an overall mean seizure severity of 3.8. In animals used for neuron survival analysis (<5 seizure score), there was no effect of hormone treatment on either seizure severity [F(4,40) = 0.34, p = 0.85] (Figure 1A) or latency to seizure onset [F(4,40) = 0.42, p = 0.79] (Figure 1B). The proportion of animals achieving maximal seizure rating (score 5; immediate sacrifice and exclusion from study) was higher (50.0%) in the OVX+P4 group compared to all other groups (26.8%).
Figure 1.
Treatment conditions did not significantly affect the severity and latency of kainite-induced seizures. Female rats treated systemically with kainate (KA) were monitored for both seizure severity (A) and latency to seizure onset (B). Seizure scores show mean values (+SEM) of each group (n = 10/group) and represent the severity of seizure-related behavioral indices based on a 0–5 scale with 5 representing the highest level of seizure behavior. Seizure latency shows show mean values (+SEM) for each group of the time period (in minutes) from KA injection to the onset of seizure-related behaviors.
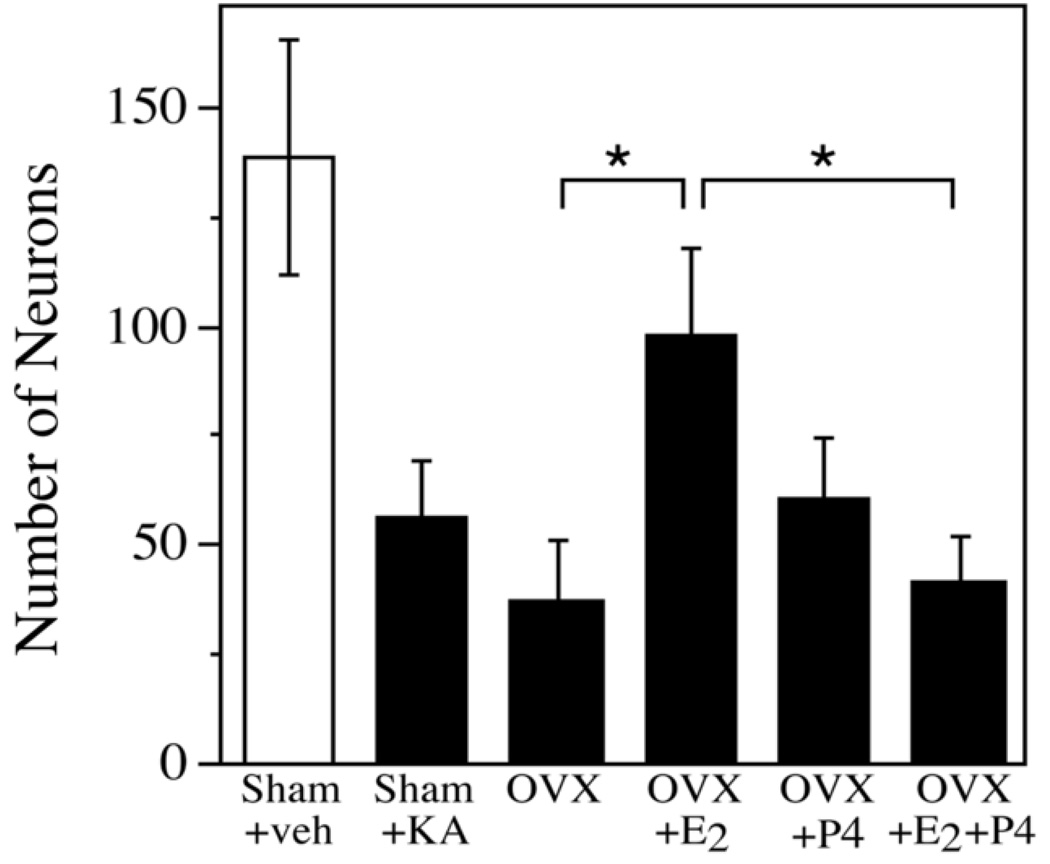
To determine the effect of hormone treatments on the extent of kainate lesion, neuronal survival in the hippocampus CA2/3 was determined. Sham OVX animals suffered ~60% neuron loss. If sex steroids are endogenous regulators of neuron viability, then hormone depletion resulting from OVX should exacerbate kainate-induced cell loss and hormone treatments should protect relative to OVX. Overall, there was a significant effect of treatment on neuron survival [F(5,28) = 8.65, p <0.0001]. However, in contrast to observations in young adult female rats [23], the OVX group did not exhibit a significant decrease in neuron survival relative to the sham OVX group (Figure 2). Treatment of OVX rats with E2 (OVX+E2, p = 0.008) but not P4 (OVX+P4; p = 0.32) significantly increased neuronal survival compared to the OVX group. However, when replaced in combination, the OVX+E2+P4 group suffered significantly more neuron loss compared to the OVX+E2 alone group (p = 0.013), demonstrating that the addition of P4 blocked the neuroprotective effect of E2. Within treatment groups, we did not observe significant correlations between neuron survival and either progesterone levels or uterine weight (data not shown).
Figure 2.
Progesterone blocks the neuroprotective effect of estrogen. The number of NeuN-immunoreactive cells in hippocampal region CA2/3 was quantified in sham OVX rats and in OVX rats treated with either vehicle (veh), E2 and/or P4. Data show mean numbers of counted cells (± SEM) for treatment groups exposed to KA (filled bars) and not exposed to KA (open bars). In all groups exposed to KA except OVX+E2, the number of neurons was significantly decreased relative to Sham-veh. * denotes p<0.05 between indicated groups.
Discussion
The goal of this study was to begin investigating, in an animal model, two variables that may affect efficacy of estrogen-based HT in postmenopausal women: age-related changes in hormone responsiveness and regulatory interactions between estrogen and progesterone. In comparison to our previous study in young adult female rats [23], our current results suggest that the middle-aged female rat shows altered responsiveness to estrogen but can still benefit from estrogen neuroprotection. Further, our data extend to an aging model the growing evidence that progesterone can antagonize at least some neural effects of estrogen.
We applied to reproductively senescent female rats the same paradigm that we previously utilized to demonstrate progesterone antagonism of estrogen neuroprotection in young adult female rats [23]. In the middle-aged rats studied here, we observed a more extensive lesion and more robust seizures than we had in 3 month-old female rats treated with the same dose of kainate [23]. This observed age-related increase in kainate vulnerability is consistent with some [30] but not all [16] prior studies. It is possible that the severity of the lesion created a floor effect, potentially masking subtle hormone effects on neuron survival. Our data are consistent with the hypothesis that middle-aged rodents show diminished estrogen responsiveness. First, we observed that uterine weight decreased by <60% after OVX in 14 mo old rats, a modest effect in comparison to observations in younger female rats. Previous studies also demonstrated that the uterus becomes less responsive to estrogen with increasing age, showing smaller OVX-induced decreases in uterine weight [31] and uterotrophic effects of estrogen only when treated soon after OVX [7]. Second, we did not observe a significant increase in neuron death in OVX rats compared to sham OVX animals, a finding inconsistent with the established role of endogenous estrogen as an important regulator of neuron survival [29]. Similarly, Stone et al. found that OVX impaired compensatory sprouting in young adult but to a lesser degree in middle-aged female rats [27], suggesting that aging may affect the neural response to estrogen depletion.
Although OVX-induced hormone deprivation did not exacerbate kainate lesion, estrogen treatment in middle-aged OVX rats was significantly neuroprotective. This observation adds to a growing literature indicating that although estrogen often exerts diminished effects in the middle-aged female brain, it can retain at least some of its protective effects. In prior studies, estrogen treatment in middle-aged OVX rats was shown to enhance working memory performance on hippocampal-dependent spatial memory tasks [1, 7], regulate cholinergic function [15], and protect against spinal cord [6] and ischemic brain [8, 26, 28] injuries. However, as discussed above, other studies have demonstrated reduced or lost protective actions of estrogen in brain, including regulation of interleukin-1 expression following excitotoxic injury [21], regulation of brain-derived neurotrophic growth factor expression [14], compensatory sprouting [27], neuroprotection from stroke [2], and working memory [7].
The mechanism(s) underlying age-related changes in estrogen responsiveness are not clear, but likely are affected by age changes in ovarian function. We observed that responsiveness to estrogen protection appeared to depend upon the estrus cycle history of middle-aged female rats. Specifically, we noted that estrogen was protective in rats showing persistent vaginal cornification, but not in rats with irregular cycles. The period of persistent vaginal cornification in mice has been characterized by a nearly twofold E2:P4 ratio due to a significant reduction in circulating P4 levels [20]. This increased E2:P4 ratio would expose the brain to a relatively unopposed estrogen-rich environment, perhaps promoting a higher level of estrogen responsiveness.
We also found that the neuroprotective effect of E2 in middle-aged female rats was blocked by co-treatment with P4. This result is consistent with two previous studies from our laboratory. First, using a parallel experimental design, we found that both P4 and medroxyprogesterone acetate antagonize estrogen neuroprotection against kainate in young adult female rats [23]. Second, in young adult female 3×Tg-AD transgenic mice we found that continuous P4 blocked the β-amyloid lowering action of E2 [5]. Our current finding is also consistent with a recent report that progesterone blocks estrogen upregulation of neurotrophins [3]. The mechanism by which progesterone blocks the neuroprotective effect of estrogen remains to be elucidated. Previous studies report that short-term P4 treatment provides neuroprotective effects by attenuating seizure severity however long-term exposure to P4, as used in this study, is not associated with anxiolytic, seizure-reducing effects [23]. Interestingly, there is evidence that CEE+MPA hormone treatment increases seizure frequency in epileptic women, but not in laboratory rodents [11]. One potential strategy with relevance to clinical use of HT is cyclic rather than continuous progesterone exposure, an approach that can increase rather than inhibit estrogen actions in rodent brain [25]. Although abundant experimental and clinical data strongly suggest that estrogen-based HT should yield beneficial neural outcomes in postmenopausal women, the unexpected negative findings from the recent Women’s Health Initiative clinical study have raised serious concerns about the safety and efficacy of HT. Several factors, including advanced age and the use of a continuous progestogen component, have been suggested as potential liabilities that may underlie the recent clinical shortcomings of HT. Our results support this hypothesis. In middle-aged, reproductively senescent female rats our data indicate an altered responsiveness to E2 but also demonstrate a retained ability to exhibit neuroprotection in at least a subset of animals. Further, our findings show that continuous P4 exposure attenuates E2 actions. Additional studies in animal models are needed to elucidate the mechanisms underlying hormone interactions and how they are affected by aging, which should generate the novel insight necessary to develop rational new strategies for effective HT in women.
Acknowledgements
The authors would like to thank Ms. Kelly McMullan for technical assistance. This research was funded by NIH grant AG026572 (RDB) Project 5 (CJP). JCC was supported by NIH grant NS059174. ERR was supported by NIH grant NS52143.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors have no actual or potential conflicts of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately bias our work.
References
- 1.Aenlle KK, Kumar A, Cui L, Jackson TC, Foster TC. Estrogen effects on cognition and hippocampal transcription in middle-aged mice. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31:161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- 3.Bimonte-Nelson HA, Nelson ME, Granholm AC. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004;15:2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- 4.Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- 5.Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaovipoch P, Jelks KA, Gerhold LM, West EJ, Chongthammakun S, Floyd CL. 17beta-estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J Neurotrauma. 2006;23:830–852. doi: 10.1089/neu.2006.23.830. [DOI] [PubMed] [Google Scholar]
- 7.Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- 8.Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J. Cereb. Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Febo M, Jimenez-Rivera CA, Segarra AC. Estrogen and opioids interact to modulate the locomotor response to cocaine in the female rat. Brain Res. 2002;943:151–161. doi: 10.1016/s0006-8993(02)02748-8. [DOI] [PubMed] [Google Scholar]
- 10.Gibbs RB. Oestrogen and the cholinergic hypothesis: implications for oestrogen replacement therapy in postmenopausal women. Novartis Found Symp. 2000;230:94–107. doi: 10.1002/0470870818.ch8. discussion 107-11. [DOI] [PubMed] [Google Scholar]
- 11.Harden CL. Hormone replacement therapy: will it affect seizure control and AED levels? Seizure. 2007;17:176–180. doi: 10.1016/j.seizure.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson VW. Estrogen-containing hormone therapy and Alzheimer's disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138:1031–1039. doi: 10.1016/j.neuroscience.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman GE, Moore N, Fiskum G, Murphy AZ. Ovarian steroid modulation of seizure severity and hippocampal cell death after kainic acid treatment. Exp. Neurol. 2003;182:124–134. doi: 10.1016/s0014-4886(03)00104-3. [DOI] [PubMed] [Google Scholar]
- 14.Jezierski MK, Sohrabji F. Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol. Aging. 2001;22:309–319. doi: 10.1016/s0197-4580(00)00230-x. [DOI] [PubMed] [Google Scholar]
- 15.Kalesnykas G, Puolivali J, Sirvio J, Miettinen R. Cholinergic neurons in the basal forebrain of aged female mice. Brain Res. 2004;1022:148–156. doi: 10.1016/j.brainres.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 16.Kesslak JP, Yuan D, Neeper S, Cotman CW. Vulnerability of the hippocampus to kainate excitotoxicity in the aged, mature and young adult rat. Neurosci. Lett. 1995;188:117–120. doi: 10.1016/0304-3940(95)11415-s. [DOI] [PubMed] [Google Scholar]
- 17.Korach KS, McLachlan JA. Techniques for detection of estrogenicity. Environ Health Perspect. 1995;103:5–8. doi: 10.1289/ehp.95103s75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeFevre J, McClintock MK. Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behavior. Biol. Reprod. 1988;38:780–789. doi: 10.1095/biolreprod38.4.780. [DOI] [PubMed] [Google Scholar]
- 19.Maki P, Hogervorst E. The menopause and HRT. HRT and cognitive decline. Best Pract Res Clin Endocrinol Metab. 2003;17:105–122. doi: 10.1016/s1521-690x(02)00082-9. [DOI] [PubMed] [Google Scholar]
- 20.Nelson JF, Felicio LS, Osterburg HH, Finch CE. Altered profiles of estradiol and progesterone associated with prolonged estrous cycles and persistent vaginal cornification in aging C57BL/6J mice. Biol Reprod. 1981;24:784–794. doi: 10.1095/biolreprod24.4.784. [DOI] [PubMed] [Google Scholar]
- 21.Nordell VL, Scarborough MM, Buchanan AK, Sohrabji F. Differential effects of estrogen in the injured forebrain of young adult and reproductive senescent animals. Neurobiol. Aging. 2003;24:733–743. doi: 10.1016/s0197-4580(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 22.Ramsden M, Shin TM, Pike CJ. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003;122:573–578. doi: 10.1016/j.neuroscience.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 23.Rosario ER, Ramsden M, Pike CJ. Progestins inhibit the neuroprotective effects of estrogen in rat hippocampus. Brain Res. 2006;1099:206–210. doi: 10.1016/j.brainres.2006.03.127. [DOI] [PubMed] [Google Scholar]
- 24.Schumacher M, Guennoun R, Stein DG, De Nicola AF. Progesterone: Therapeutic opportunities for neuroprotection and myelin repair. Pharmacol Ther. 2007;116:77–106. doi: 10.1016/j.pharmthera.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 27.Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Lopez LM, Shick J, Finch CE. Effects of age on gene expression during estrogen-induced synaptic sprouting in the female rat. Exp Neurol. 2000;165:46–57. doi: 10.1006/exnr.2000.7455. [DOI] [PubMed] [Google Scholar]
- 28.Wise PM. Estrogen therapy: does it help or hurt the adult and aging brain? Insights derived from animal models. Neuroscience. 2006;138:831–835. doi: 10.1016/j.neuroscience.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 29.Wise PM. Estrogens and neuroprotection. Trends Endocrinol Metab. 2002;13:229–230. doi: 10.1016/s1043-2760(02)00611-2. [DOI] [PubMed] [Google Scholar]
- 30.Wozniak DF, Stewart GR, Miller JP, Olney JW. Age-related sensitivity to kainate neurotoxicity. Exp. Neurol. 1991;114:250–253. doi: 10.1016/0014-4886(91)90042-b. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Armstrong SJ, Arenas IA, Pehowich DJ, Davidge ST. Cardioprotection by chronic estrogen or superoxide dismutase mimetic treatment in the aged female rat. Am J Physiol Heart Circ Physiol. 2004;287:H165–H171. doi: 10.1152/ajpheart.00037.2004. [DOI] [PubMed] [Google Scholar]