Abstract
Excessive consumption of highly-palatable foods may contribute to the development of weight gain. Therefore medications that selectively suppress eating of such foods would be useful in clinical practice. We compared the effects of the glutamatergic antagonists memantine and MTEP to dexfenfluramine in baboons given periodic access to highly-palatable food and ad libutum access to a standard chow diet. Three days a week baboons received a sugar-coated candy during the first meal and standard diet chow pellets were available in subsequent meals. All baboons derived a greater amount of energy from the single candy meal than from the standard diet across an entire day. Pre-treatment with dexfenfluramine, memantine, and MTEP produced decreases in candy consumption without altering candy-seeking behaviour. At the same time, dexfenfluramine and memantine, but not MTEP, produced a decrease in seeking and consumption of standard chow pellets. Both memantine and MTEP are promising agents for the treatment of obesity.
1 Introduction
The recent dramatic increase in the prevalence of obesity in the United States is often linked to the increased availability of low-cost, highly-palatable (HP) foods which contain greater percentages of carbohydrates and fats and are denser in calories than a standard diet (Drewnowski, 1998, Hill et al., 2003, Ogden et al., 2007). Consumption of HP food has been connected with the increased total energy intake and weight gain in epidemiological (Vartanian et al., 2007), and experimental studies (Raben et al., 2002). Further, it has been argued that increased consumption of HP foods contributes to the development of a non-homeostatic pattern of food intake that can override the metabolic regulatory system responsible for weight maintenance and result in obesity (Berthoud, 2004, Blundell and Finlayson, 2004). HP food is more likely to be consumed between meals and is less responsive to the natural mechanisms regulating food-intake such as hunger and satiety (Erlanson-Albertsson, 2005). Therefore, increased exposure to HP foods may result in the development of abnormal patterns of food consumption such as binge-eating and compulsive over-eating, which may in turn contribute to obesity (Tanofsky-Kraff et al., 2006, Fairburn et al., 2000). Elucidation of the behavioural and neurobiological mechanisms involved in the consumption of HP versus standard diets is an important step in the development of treatments targeting disordered eating and obesity (Yanovski, 2003). Factors controlling consumption of HP foods may differ from those regulating intake of the standard diet. If this is the case, then a pharmacological strategy could be developed to suppress eating of HP foods with minimal effect on intake of less calorie-dense food. Such medications would be particularly useful in clinical practice to normalize disordered eating and to treat obesity.
Several rodent laboratory models have been developed to study the binge-type feeding behaviour observed in some obese individuals (Corwin et al., 1998, Corwin and Buda-Levin, 2004). However, these models use small laboratory animals, which have different feeding patterns and compensatory mechanisms than larger animals, including humans, which may limit the extrapolation of the findings. Modelling binge-eating in a large non-human primate may be more relevant to medication development. One of the paradigms that can be adapted to screen for medications that target binge-eating involves operant responding for food in baboons (Foltin, 2001). In this model, a chain schedule of reinforcement simulates food “seeking” and food “taking” components of feeding, which permits the appetitive and the consummatory phases of feeding behaviour to be studied separately. Providing unrestricted access to standard-diet food with an optional source of HP food, available under a limited access schedule may induce high level of HP food consumption, and be used as a baboon model of binge-eating (Foltin, 2006b, Foltin and Haney, 2007). The first goal of the present study was to assess the utility of this model as a screening tool in binge-eating pharmacotherapy development. To achieve this we have tested the effects of dexfenfluramine, a serotonin and dopamine reuptake inhibitor and a prototypic weight-loss medication that has been found to reduce binge-eating in patients with obesity and binge-eating disorders (Stunkard et al., 1996).
Glutamatergic neurotransmitter systems could be targeted for medication development in eating disorders. Glutamate pathways are an important component of the central reward pathway, involved in regulating reinforced and consummatory behaviours related to the use of drugs of abuse and preferred foods (Parsons et al., 2005). Since the role of glutamatergic pathways in regulating binge-type eating is unknown, the second goal of the present study was to evaluate the effects of glutamatergic compounds, the NMDA receptor antagonists memantine (Parsons et al., 2005) and the mGluR5 antagonist MTEP (Busse et al., 2004) in the laboratory model of binge-eating, and to compare those effects to the effects of dexfenfluramine.
2 Methods
2.1 Animals
Four adult male baboons (Papio cynocephalus anubis), weighing 18.3 to 23.1 kg, and four adult female baboons, weighing 10.7 to 15.8 kg, were individually housed in standard non-human primate cages (0.94 × 1.21 × 1.52 m high) at The New York State Psychiatric Institute. The room was illuminated with fluorescent lighting from 7:00 AM to 7:00 PM daily. In addition to food and candy earned during experimental sessions, two chewable vitamins, two pieces of fresh fruit, and a dog biscuit were also given daily. Water was available ad libitum from a spout located at the back of each cage. Animals had experience responding for candy and chow using this and similar procedures (Foltin, 2006a), and had received acute doses of drugs (Foltin, 2006a, Foltin and Haney, 2007). All aspects of animal maintenance and experimental procedures complied with the U.S. National Institutes of Health Guide for Care and Use of Laboratory Animals, and were approved by the New York State Psychiatric Institute Animal Care and Use Committee.
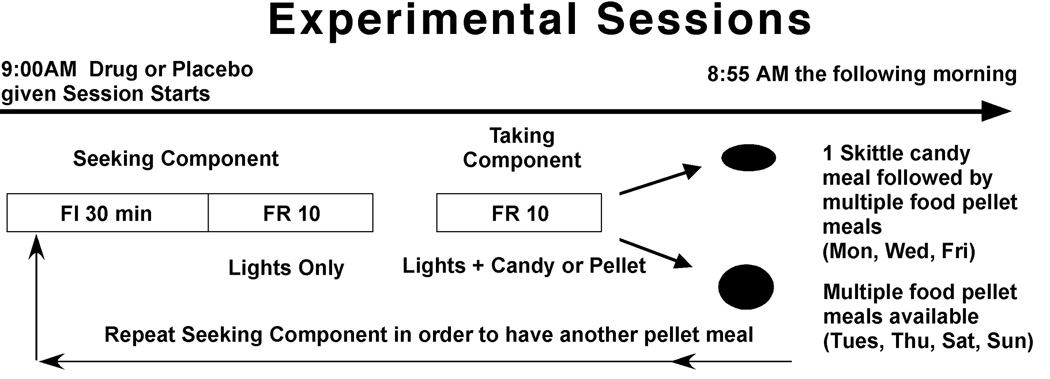
2.2 Schedule of reinforcement (Figure 1)
Figure 1.
Diagram of experimental day. One candy meal followed by multiple food pellet meals were available on Monday, Wednesday and Friday, and only multiple food pellet meals were available on the remaining days of the week. A meal was initiated by the completion of a 30-min seeking interval during which every tenth response was followed by illumination of the lights that accompanied candy or food pellet delivery. Following the 30-min interval, every tenth response was followed by candy or food pellet delivery along with the light presentation. The taking component ended after the animal stopped responding for 10 min. In order to have another food pellet meal the animal had to gain complete the 30-min seeking interval. Thus timing and size of meals were determined individually by each animal.
Responding under each phase of a two-phase chain schedule of reinforcement was on a separate response manipulandum. The session began with the illumination of a single light above the left appetitive manipulanda. Completion of the first 10 responses (Fixed ratio; FR 10) on the appetitive manipulanda began a 30-min timer and illuminated a second light over the appetitive manipulanda, i.e., the 30-min appetitive phase was indicated by the illumination of two lights above the appetitive manipulanda. The appetitive phase was a fixed-interval (FI) 30 min schedule, with a FR 10 second-order phase [FI 30 (FR 10:S)]. Thus, after every 10th response during the FI phase, the stimuli associated with reinforcer delivery during the second phase were presented. There was a 10 min limited hold for the appetitive phase, such that after the expiration of the 30 min FI, the next FR 10 had to be completed within 10 min. Failure to complete a FR 10 within 10 min cancelled that appetitive phase, and extinguished one light over the appetitive manipulanda such that only a single light was illuminated over the appetitive manipulanda. The baboon received no indication that the 30-min interval had elapsed. The first FR 10 completed after 30 min resulted in the two lights above the appetitive lever being extinguished and a single light above the right consumption manipulanda being illuminated, signalling the availability of food under the FR consumption phase of the chain schedule. The consumption phase of the chain schedule was reinforced using a FR 10 schedule of pellet delivery. After a 10-min interval in which no responses on the consumption manipulanda occurred, the consumption phase terminated, i.e., meal size was determined by each baboon. The single light above the consumption manipulanda then extinguished, and the single light above the appetitive manipulanda again illuminated. In order to initiate another meal, the baboon was required to start another 30-min appetitive phase by pulling on the appetitive manipulanda10 times. This schedule was in effect 24 h/day beginning at 9:00 AM, with the exception of a brief period during which the data were backed up and printed, which occurred at 8:55 AM each morning. Baboons had several years of experience responding under these conditions, and showed discriminative control in that they rarely pulled the appetitive manipulanda during a consumption phase, and rarely pulled the consumption manipulanda during an appetitive phase.
During each regular-diet meal, baboons received 1 food pellet (banana-flavored 1-g food pellets containing 3.3 kcal/g: 0.55 g carbohydrate, 0.03 g fat, 0.2 g protein; Bio-Serv, Frenchtown, NJ). Pellet delivery was accompanied by the illumination of all 4 stimulus lights above the 2 levers for 8 s. The illumination of the 4 lights for 8 s also occurred upon completion of each FR 10 during appetitive phases preceding food consumption phases. Responses made while the stimulus lights were flashing did not count toward completion of the FR10 response requirement, i.e., there was an 8 s timeout after each reinforcer delivery. During the candy meal, baboons received 1 Skittle® (1-g; 4.3 kcal: 0.9 g carbohydrate, 0.04 g fat, 0 g protein; Mars Corp., Hackettstown, NJ). Candy delivery was accompanied by the flashing (1 s on:1 s off) of 2 white stimulus lights located above the food hopper for 8 s. The flashing of the 2 white lights for 8 s also occurred upon completion of each FR 10 during the appetitive phase preceding the candy consumption phase.
2.3 Procedure and drugs
Four days a week only food pellets were available and on the other 3 days each week (Monday, Wednesday, and Friday), daily sessions began with a single candy meal. Baboons were free to start responding for pellets or candy beginning at 9:00 AM. Completion of the first 10 responses on the appetitive manipulanda started the appetitive phase, which lasted a minimum of 30 min and a maximum of 40 min. After completion of the appetitive phase, baboons earned 1 pellet or 1 piece of candy after every 10 responses, with the “meal” ending when the baboon stopped pulling the manipulanda for 10 min. On pellet days baboons could have multiple meals during 24 h, but they had to complete a 30 min appetitive phase before each consumption phase. On candy days, after the end of the candy meal, the baboons then could work for pellet meals until 9:00 AM the following morning. There were no stimuli indicating if the first meal of the day would be candy or pellets until the first stimulus presentation during the appetitive phase, i.e, light flashes indicated a candy meal would occur after completion of the appetitive phase, and prolonged illumination of a different set of lights indicated a pellet meal would occur after completion of the appetitive phase.
Memantine HCl (Merz Pharmaceuticals, Frankfurt /M, Germany), Dexfenfluramine HCl (Sigma-Aldrich Chemical Corp., St. Louis. MO, USA) and MTEP (synthetized by Merz Pharmaceuticals) were dissolved in sterile saline. Dexfenfluramine and memantine were administered in doses of 0.25, 0.50, 1.0, 2.0 mg/kg and MTEP was administered in doses of 0.50, 1.0, 2.0, and 4.0 mg/kg. All animals received all doses of all drugs with each dose response assessment determined over a 6-wk period. Drugs were given intramuscularly (i.m.) in a thigh muscle (location varying among sessions) on Monday before a candy session and Thursday before a food pellet session of each week at 0900, with placebo injections given on Tuesday and/or Friday of each week. The 2 smaller doses of each drug were tested before the 2 larger doses of each drug: within each dose pair, dose was counterbalanced such that 2 females and 2 males received the smallest dose of each dose pair first, and 2 females and 2 males received the largest dose of each dose pair first.
2.4 Data analysis
Outcome measures included: 1) total number of reinforcers earned during appetitive and consummatory components, 2) latency to the first consummatory component, 3) number of pellet meals started, and 4) the running rate of responding during the first appetitive and consummatory components. Reinforcers included light presentations during appetitive phases and the candy/pellets deliveries with associated light flashes during consumption phases. Latency to the first candy meal was defined as the number of minutes between 9:00 AM, when the computers were restarted for the day, and the first candy delivery. The latency to the first pellet meal on pellet-only days was defined as the number of minutes between 9:00 AM, when the computers were restarted for the day, and the first pellet delivery. Because the first appetitive component was completed prior to the first consumption component, the minimal latency was 30 min. Running rate was calculated by dividing the total number of responses during each component by the total number of seconds during which the baboon responded; the 10 s timeout after each reinforcer delivery and the interval after each reinforcer delivery that elapsed before the animal started responding again was not included in the total number of seconds. Separate analyses were conducted for: 1) candy (days when candy was available as a first meal of the day), 2) food pellets (days when only food pellets were available), 3) food pellets after candy (days when food meals followed candy meal), and 4) the first food pellet meal of the session on days when only food pellets were available.
Data for each drug were summarized using analyses of variance (ANOVA) with Sex as a between group factor and Drug (placebo vs. active; there was one placebo session for each active dose session, i.e, baseline candy and pellet intake was used a covariate in the analyses), and Dose (4 doses) as 2-within group factors. Data were considered significantly different at P < 0.05, using Huynh–Feldt corrections where appropriate.
3 Results
3.1 Effect of Sex on Candy and Food Pellet Intake and Weight Gain
Although pellet intake varied across the study, males ate on average about 220 food pellets (726 Kcal; 3,035 J) and females ate about 110 pellets (363 Kcal; 1520 J) on food pellet-only days. Although candy intake varied across the study, males ate on average about 170 candies (731 Kcal; 3060 J) in the single candy meal and 120 food pellets (396 Kcal; 1658 J) during the rest of the session, and females ate about 120 candies (516 Kcal; 2160 J) in the single candy meal and about 80 food pellets (264 Kcal; 1105 J) during the rest of the session. Although there were some occasional interactions between a drug dose and sex of the baboon, these interactions were generally accounted for by the baseline differences in food pellet or candy intake. During the six-month period of the study duration, males gained on average 4.1 Kg, which was about 1 Kg greater than previous growth rate while females gained 1.7 Kg, which was about the same as the previous growth rate.
3.2 Appetitive Component (Food Seeking)
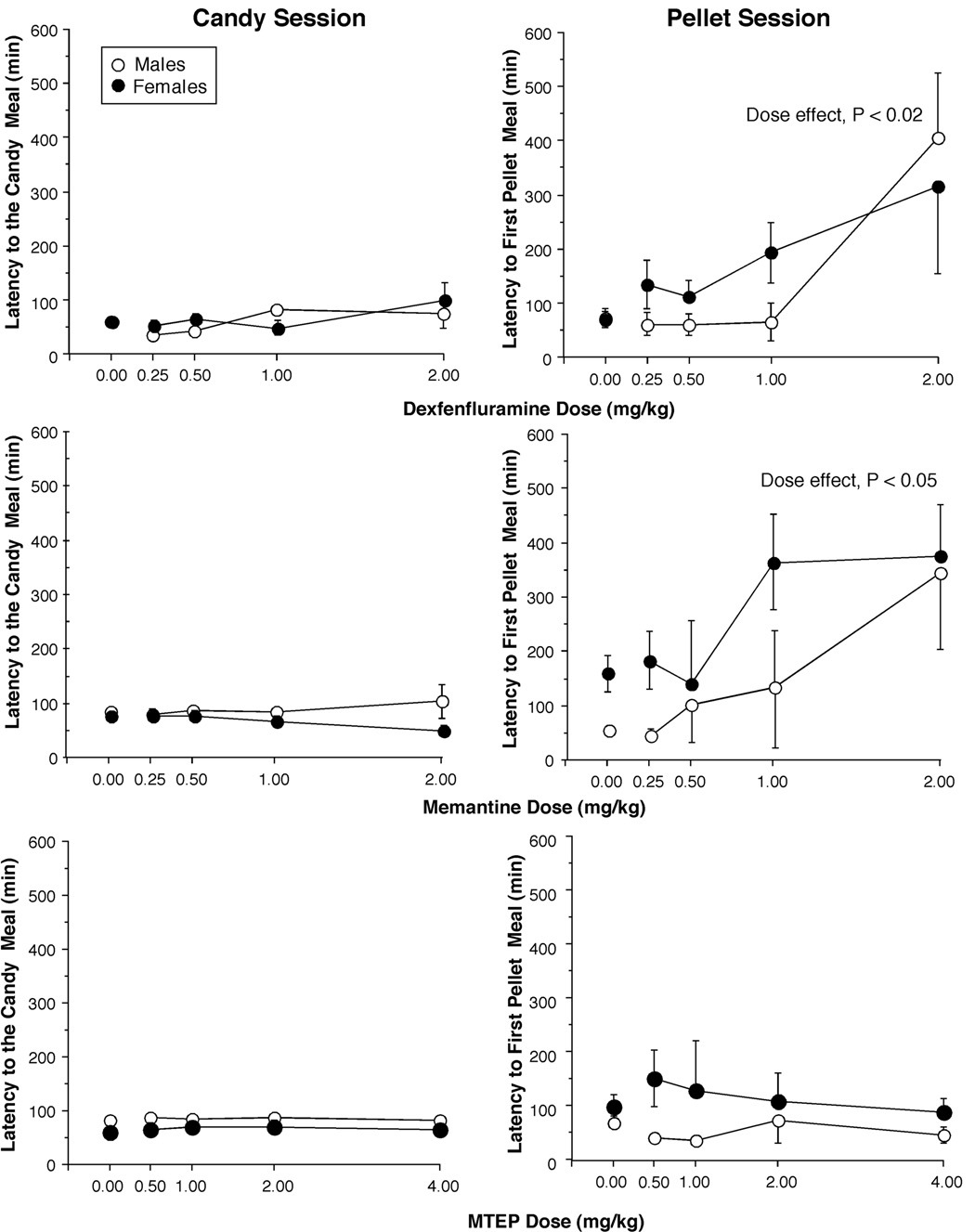
Figure 2 compares the effects of medications on the latency to the candy meal (left panel) and the latency to the first food pellet meal (right panel). The latency to the candy meal was between 50 and 80 min, and there was no effect of any of the tested medications. The latency to the first food pellet meal was between 60 and 100 min. Both dexfenfluramine and memantine produced dose-dependent increases in latency to the first pellet meal. There was a significant main effect of drug and drug × dose interactions for dexfenfluramine (F1,6 = 14.9, P < 0.01; F3,18 = 6.1, P < 0.02), and for memantine (F1,6 = 17.6, P < 0.01; F3,18 = 3.8, P < 0.05). MTEP had no effect on the latency to the pellet meal.
Figure 2.
Latency to the first candy meal (left panel) and the first food pellet meal (right panel) as a function of drug and dose. Error bars represent ±1 SEM. Error bars for the placebo values are based on all 4 placebo days.
None of the studied medications significantly altered the total daily number of light flashes or the rate of responding collected during the appetitive phase, prior to any candy or food pellet meal.
3.3 Consummatory Component (Food Taking)
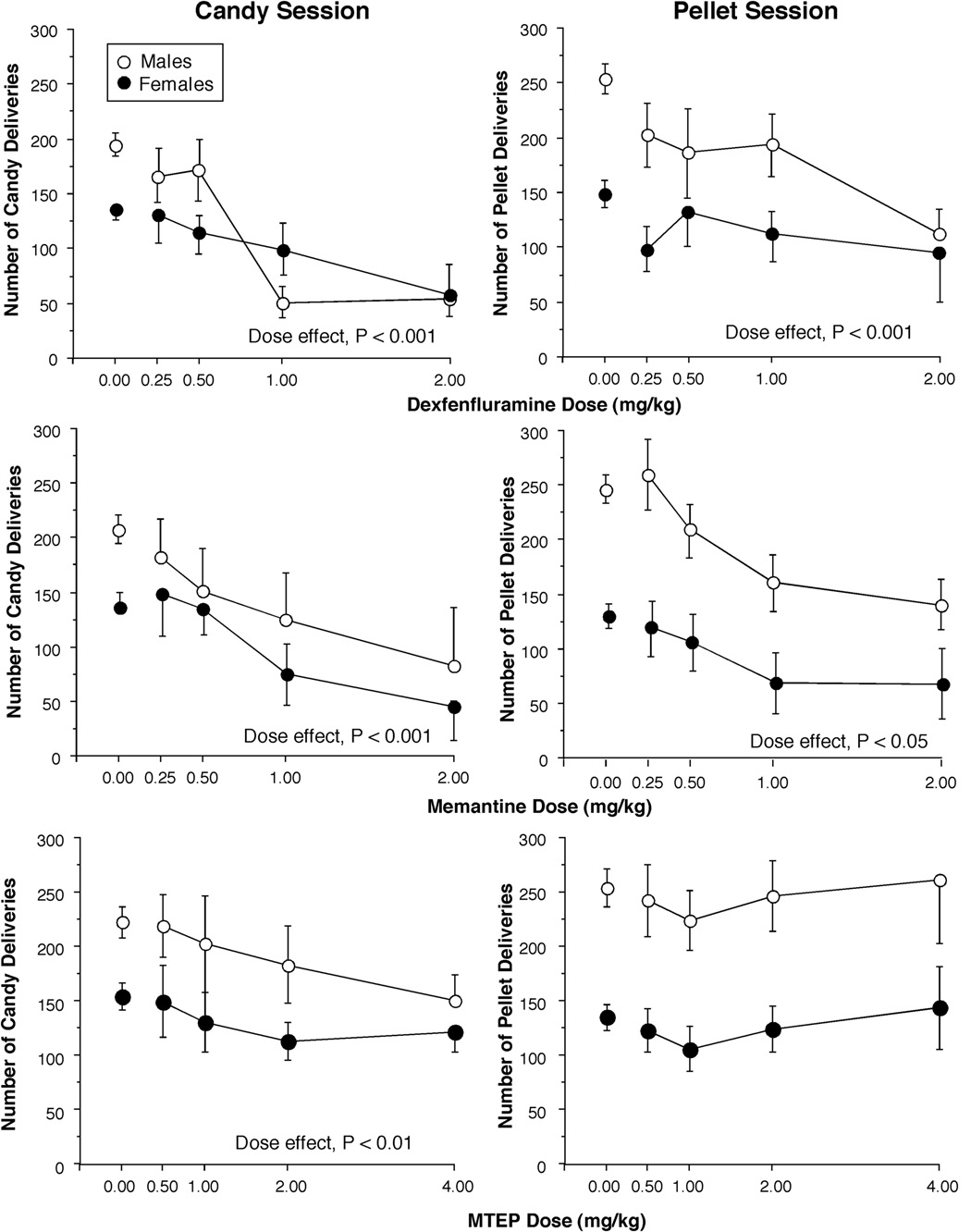
Figure 3 compares the effects of medications on the number of candy deliveries earned during a single candy meal (left panel) and food pellet deliveries earned over the entire day on days when only food-pellets were available (right panel). All medications produced dose-dependent decreases in the number of candies consumed during the single-candy meal. There was a significant main effect of drug and drug × dose interactions for dexfenfluramine (F1,6 = 39.6, P < 0.001; F3,18 = 32.6, P < 0.001), memantine (F1,6 = 34.9, P < 0.001; F3,18 = 21.3, P < 0.001) and MTEP (F1,6 = 13.41, P <0.05; F3,18 = 5.84, P < 0.01). Both dexfenfluramine and memantine produced dose-dependent decreases in total food pellet intake. There was a significant main effect of drug and drug × dose interactions for dexfenfluramine (F1,6 = 44.9, P < 0.001; F3,18 = 4.8, P < 0.05) and for memantine (F1,6 = 17.6, P < 0.01; F3,18 = 3.8, P < 0.05). Similarly, both dexfenfluramine and memantine produced dose-dependent decreases in the number of food pellet meals consumed, i.e., baboons ate about 1 less meal after receiving dexfenfluramine (F3,18 = 4.8, P < 0.05) or memantine (F3,18 = 3.3, P < 0.05). The increase in latency described above would account for baboons missing what would normally have been their first meal of the day. Pretreatment with MTEP had no effect on the food pellet consumption.
Figure 3.
Total number of candies earned during the single candy meal of each candy session (A) and total daily number of food pellets earned on pellet-only days (B) as a function of drug and dose. Error bars represent ±1 SEM. Error bars for the placebo values are based on all 4 placebo days.
Dexfenfluramine, MTEP, and memantine slightly decreased the rate of responding during the candy meal by about 0.5 responses/s from a baseline rate of 5.0 responses/s. This effect was only dose-dependent for dexfenfluramine. Finally, in contrast to the candy meal, none of the medications altered the rate of responding during pellet meals.
4 Discussion
Providing baboons with limited access to a highly palatable food (candy) in addition to a standard diet, yielded a robust, ‘binge-like’ level of candy consumption. Administration of dexfenfluramine, a prototypic weight-loss and anti-binge medication did not alter the motivational or appetitive component of candy consumption (food seeking) but it did produce dose-dependent decreases in the size of the candy meal (food taking). Administration of memantine, an uncompetitive NMDA receptor antagonist and MTEP, an allosteric metabotropic mGlu5 receptor antagonist, produced effects on candy consumption that were comparable to the effect of dexfenfluramine even though their pharmacological effects differ from dexfenfluramine, which is primarily a serotonin releaser (Heal et al., 1998). At the highest doses, memantine, MTEP, and dexfenfluramine reduced the size of the candy-meal to approximately 45%, 65%, and 30% respectively of candy intake under placebo conditions. At the same time, dexfenfluramine and memantine decreased standard food pellet seeking and consumption while MTEP had minimal effect on the standard food pellet seeking and consumption. Most of the differences in pharmacological effects between males and females observed in the present study were accounted for by the baseline differences in food pellet and candy consumption suggesting that the observed pharmacological effect is not sex-specific.
In the present study, irregular and limited access to candy resulted in a level of consumption during a single meal that was comparable to the consumption of food pellets across an entire day. Total amount of energy obtained from a single candy meal was greater than the total daily amount of energy obtained from the standard diet. We suggest that the current behavioural paradigm can be considered a model of binge-eating disorder as it mimics some of clinical features of this disorder, i.e., an overconsumption of HP food and consumption of a large amount of calories in a short period of time (Devlin et al., 2003). The proposed model has good predictive validity as the clinically effective medication dexfenfluramine reduced the size of a candy binge meal, in addition to reducing the consumption of a standard diet. The ability of dexfenfluramine to decrease food consumption in the present model is consistent with the eating-suppressing effect of dexfenfluramine in non-human primates (Foltin, 2001, Foltin, 2006a), healthy human volunteers (Foltin et al., 1996, Goodall et al., 1992), obese patients (Guy-Grand et al., 1989), and individuals with binge-eating disorder (Stunkard et al., 1996). In these clinical studies, fenfluramine appeared to increase the satiating effect of food by decreasing meal size, which is consistent with the effect seen in the present study.
In the proposed model, pretreatment with memantine and MTEP resulted in a smaller size of a candy ‘binge-type’ meal without affecting candy seeking behaviour. Animals continued to work to gain access to a meal of candies, but ate fewer candies. This suggests that memantine and MTEP may reduce the amount and the caloric intake from HP food in humans. As the consumption of large amounts of HP food is presumed to have a disruptive effect on the homeostatic aspects of eating behaviour, decreasing HP food consumption would be desirable in the clinical setting and might lead to the normalization of eating. At the same time, memantine increased the latency to the first food pellet meal, e.g., decreased motivation to eat pellets, and decreased the size of the first pellet meal, in a manner similar to dexfenfluramine. Interestingly, MTEP had no effect on motivation to eat or the size of the standard food pellet meals. This might suggest that in the clinical setting, MTEP may decrease the amount of HP food consumption without altering regular food consumption, an effect particularly relevant to offset weight gain in overweight individuals. Additionally, if some specificity for HP foods is provided by a pharmacological adjunct to weight management, it might be possible to reduce energy intake and normalize disordered eating without triggering compensatory increases of eating which would counteract the effect of medication in the long term. On the other hand, memantine may have stronger effect on the overall food consumption, decreasing caloric intake from all types of food, and may be more effective in producing weight loss.
The finding that systemically administered glutamatergic antagonists decreased food consumption in non-human primates is relatively novel, though not surprising considering that glutamate pathways are involved in regulating food-reinforced and other consummatory behaviours (Parsons et al., 2005). Administering ionotropic glutamatergic agonists (Stanley et al., 1993, Echo et al., 2001, Khan et al., 2004, Stanley et al., 1996), or antagonists (Burns and Ritter, 1997, Ninan and Kulkarni, 1998, Gillespie et al., 2005) increased food intake in rodents. Metabotropic mGluR5 antagonists have been found to decrease food intake (Bradbury et al., 2005, Paterson and Markou, 2005, Semenova and Markou, 2007). The seemingly contradictory effects of glutamatergic manipulations on feeding behaviour may be dependent upon site and type of injection, deprivation state of the animal (e.g., Zeni et al., 2000) and the receptor subtype affected by the drug. It has to be noted however that due to significant differences in feeding behavior between rodents and primates, studies on the mechanisms regulating eating in lower species may not be fully applicable to primates.
We suggest that memantine and MTEP decrease food consumption by altering the reinforcing effects of food, particularly foods with higher palatability that are, therefore, more reinforcing. Glutamatergic antagonists may be more selective in reducing the reinforcing effect of food that is more likely to be consumed for its hedonic rather than homeostatic properties (Saper et al., 2002). Alternatively, glutamatergic agents might have decreased food consumption through the disruption of memory recall (Wozniak et al., 1990), however this is less likely as in such a case we would expect an effect on performance (rate of responding) and we would not expect a differential effect on candy vs. pellet consumption. The effect of pharmacological manipulations tested here on the consumption of HP foods might be analogous to the ability of NMDA or mGluR5 receptor antagonist to decrease self-administration/consumption of other reinforcing agents such as alcohol, cocaine, nicotine and opiates (Holter et al., 2000, Bienkowski et al., 2001, Backstrom et al., 2004, Blokhina et al., 2005, Glick et al., 2001, Newman and Beardsley, 2006, Paterson and Markou, 2005, Paterson et al., 2003, Tessari et al., 2004, Kenny et al., 2005, Semenova et al., 1999, Xi and Stein, 2002). On the other hand, ionotropic and metabotropic glutamate receptor antagonists also decrease drug-seeking behavior (Backstrom and Hyytia, 2006) but in the present study, candy-seeking behavior was not affected.
In the present study, pharmacological manipulations had different effects depending on the type of food. Both dexfenfluramine and memantine reduced motivation to consume standard food pellets (food seeking) by increasing the latency to pellet consumption without an effect on the motivation to consume HP food. MTEP reduced consumption of HP food without affecting seeking and consumption of standard food. It is therefore possible that consumption of standard and HP food may have non-overlapping behavioural control mechanisms (Martel and Fantino, 1996, Cooper and Al-Naser, 2006). Present findings suggests that neurotransmission at the mGluR5 receptor site may be specifically involved in the mediation of satiating effects of HP foods. Although the dopaminergic receptor system, widely believed to be a major system regulating reinforcing effects, might be a primary candidate for regulation of HP food intake, other receptor systems may be also involved. Similarity of behavioural effects observed after administration of memantine and dexfenfluramine suggests that there might be an overlap in glutamatergic and serotonergic control of feeding. Serotonin has been noted to affect reward circuits, and motivated behaviour (Higgins and Fletcher, 2003) and plays an important role in satiety processes (Halford and Blundell, 2000). Alternatively, glutamatergic neurotransmission might be involved in regulating feeding mediated by orexigenic neuropeptides since administering NMDA receptor antagonists blocked increases in food intake produced by NPY (Lee and Stanley, 2005) and orexin (Doane et al., 2007).
Present findings suggest that memantine may have a profile of clinical effectiveness in patients with binge-eating that is comparable to the profile of dexfenfluramine but with a more favourable safety profile (Centers for Disease Control and Prevention, 1997), suggesting that memantine and MTEP are good candidates for clinical testing in patients with eating disorders. There are early uncontrolled clinical reports showing that administration of memantine decreased appetite and suppressed binge-eating (Hermanussen and Tresguerres, 2005), consistent with the effect in baboons observed in the present study. Future controlled clinical studies will need to determine whether the binge-eating suppressant effect will persist with repeated administration, whether it will result in weight-loss in obese individuals who present with disordered eating, and whether clinical improvement will be maintained after medication discontinuation.
However, there is a considerable amount of data showing that patients who receive memantine for the treatment of dementia do not experience weight-loss (McShane et al., 2006). This apparent difference between clinical experience in patients with neurological diseases and the hypothesized efficacy in individuals with disordered eating may be related to the dose, the selectivity of pharmacological effect, and differences between individuals with dementia and healthy individuals in the regulation of eating. Alternatively, memantine may not affect “normal” eating but will be effective in reducing excessive food consumption.
In summary, the present study provides evidence that mechanisms controlling binge-type consumption of HP food are different from those regulating consumption of the standard diet and that modulating glutamatergic neurotransmission using NMDA or mGluR5 receptor antagonists produces decreases in feeding behaviour in an acute treatment paradigm without behavioural toxicity. MTEP may specifically decrease consumption of highly palatable food without altering consumption of the standard diet. This finding suggests that these receptor systems are an attractive target to develop effective pharmacotherapies for eating disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Neural control of appetite: cross-talk between homeostatic and non-homeostatic systems. Appetite. 2004;43:315–317. doi: 10.1016/j.appet.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Krzascik P, Koros E, Kostowski W, Scinska A, Danysz W. Effects of a novel uncompetitive NMDA receptor antagonist, MRZ 2/579 on ethanol self-administration and ethanol withdrawal seizures in the rat. Eur J Pharmacol. 2001;413:81–89. doi: 10.1016/s0014-2999(01)00743-9. [DOI] [PubMed] [Google Scholar]
- Blokhina EA, Kashkin VA, Zvartau EE, Danysz W, Bespalov AY. Effects of nicotinic and NMDA receptor channel blockers on intravenous cocaine and nicotine self-administration in mice. Eur Neuropsychopharmacol. 2005;15:219–225. doi: 10.1016/j.euroneuro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Finlayson G. Is susceptibility to weight gain characterized by homeostatic or hedonic risk factors for overconsumption? Physiol Behav. 2004;82:21–25. doi: 10.1016/j.physbeh.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Bradbury MJ, Campbell U, Giracello D, Chapman D, King C, Tehrani L, Cosford ND, Anderson J, Varney MA, Strack AM. Metabotropic glutamate receptor mGlu5 is a mediator of appetite and energy balance in rats and mice. J Pharmacol Exp Ther. 2005;313:395–402. doi: 10.1124/jpet.104.076406. [DOI] [PubMed] [Google Scholar]
- Burns GA, Ritter RC. The non-competitive NMDA antagonist MK-801 increases food intake in rats. Pharmacol Biochem Behav. 1997;56:145–149. doi: 10.1016/S0091-3057(96)00171-2. [DOI] [PubMed] [Google Scholar]
- Busse CS, Brodkin J, Tattersall D, Anderson JJ, Warren N, Tehrani L, Bristow LJ, Varney MA, Cosford ND. The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology. 2004;29:1971–1979. doi: 10.1038/sj.npp.1300540. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, C. Cardiac valvulopathy associated with exposure to fenfluramine or dexfenfluramine: U.S. Department of Health and Human Services interim public health recommendations. Morb Mortal Wkly Rep. 1997 November;46:1061–1066. 1997. [PubMed] [Google Scholar]
- Cooper SJ, Al-Naser HA. Dopaminergic control of food choice: contrasting effects of SKF 38393 and quinpirole on high-palatability food preference in the rat. Neuropharmacology. 2006;50:953–963. doi: 10.1016/j.neuropharm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- Devlin MJ, Goldfein JA, Dobrow I. What is this thing called BED? Current status of binge eating disorder nosology. Int J Eat Disord. 2003 34 Suppl:S2–S18. doi: 10.1002/eat.10201. [DOI] [PubMed] [Google Scholar]
- Doane DF, Lawson MA, Meade JR, Kotz CM, Beverly JL. Orexin-Induced Feeding Requires Nmda Receptor Activation in the Perifornical Region of the Lateral Hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1022–R1016. doi: 10.1152/ajpregu.00282.2007. [DOI] [PubMed] [Google Scholar]
- Drewnowski A. Energy density, palatability, and satiety: implications for weight control. Nutr Rev. 1998;56:347–353. doi: 10.1111/j.1753-4887.1998.tb01677.x. [DOI] [PubMed] [Google Scholar]
- Echo JA, Lamonte N, Christian G, Znamensky V, Ackerman TF, Bodnar RJ. Excitatory amino acid receptor subtype agonists induce feeding in the nucleus accumbens shell in rats: opioid antagonist actions and interactions with [mu]-opioid agonists. Brain Res. 2001;921:86–97. doi: 10.1016/s0006-8993(01)03094-3. [DOI] [PubMed] [Google Scholar]
- Erlanson-Albertsson C. How palatable food disrupts appetite regulation. Basic Clin Pharmacol Toxicol. 2005;97:61–73. doi: 10.1111/j.1742-7843.2005.pto_179.x. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, Doll HA, Norman P, O'connor M. The natural course of bulimia nervosa and binge eating disorder in young women. Arch Gen Psychiatry. 2000;57:659–665. doi: 10.1001/archpsyc.57.7.659. [DOI] [PubMed] [Google Scholar]
- Foltin RW. Effects of amphetamine, dexfenfluramine, diazepam, and other pharmacological and dietary manipulations on food "seeking" and "taking" behavior in non-human primates. Psychopharmacology (Berl) 2001;158:28–38. doi: 10.1007/s002130100865. [DOI] [PubMed] [Google Scholar]
- Foltin RW. Effects of sibutramine on the appetitive and consummatory aspects of feeding in non-human primates. Physiol Behav. 2006a;87:280–286. doi: 10.1016/j.physbeh.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Foltin RW. "Tasting and wasting" behavior in non-human primates: aberrant behavior or normal behavior in "times of plenty". Physiol Behav. 2006b;89:587–597. doi: 10.1016/j.physbeh.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Effects of the cannabinoid antagonist SR141716 (rimonabant) and d-amphetamine on palatable food and food pellet intake in non-human primates. Pharmacol. Biochem. Behav. 2007;86:766–773. doi: 10.1016/j.pbb.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Haney M, Comer SD, Fischman MW. Effect of fenfluramine on food intake, mood, and performance of humans living in a residential laboratory. Physiol. Behav. 1996;59:295–305. doi: 10.1016/0031-9384(95)02098-5. [DOI] [PubMed] [Google Scholar]
- Gillespie BR, Burns GA, Ritter RC. NMDA channels control meal size via central vagal afferent terminals. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1504–R1511. doi: 10.1152/ajpregu.00169.2005. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA, Kitchen BA. Comparative effects of dextromethorphan and dextrorphan on morphine, methamphetamine, and nicotine self-administration in rats. Eur J Pharmacol. 2001;422:87–90. doi: 10.1016/s0014-2999(01)01066-4. [DOI] [PubMed] [Google Scholar]
- Goodall E, Feeney S, Mcguirk J, Silverstone T. A comparison of the effects of d- and l-fenfluramine and d-amphetamine on energy and macronutrient intake in human subjects. Psychopharmacology (Berl) 1992;106:221–227. doi: 10.1007/BF02801976. [DOI] [PubMed] [Google Scholar]
- Guy-Grand B, Apfelbaum M, Crepaldi G, Gries A, Lefebvre P, Turner P. International trial of long-term dexfenfluramine in obesity. Lancet. 1989;2:1142–1145. doi: 10.1016/s0140-6736(89)91499-2. [DOI] [PubMed] [Google Scholar]
- Halford JC, Blundell JE. Separate systems for serotonin and leptin in appetite control. Ann Med. 2000;32:222–232. doi: 10.3109/07853890008998829. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Cheetham SC, Prow MR, Martin KF, Buckett WR. A comparison of the effects on central 5-HT function of sibutramine hydrochloride and other weight-modifying agents. Br J Pharmacol. 1998;125:301–308. doi: 10.1038/sj.bjp.0702067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanussen M, Tresguerres JA. A new anti-obesity drug treatment: first clinical evidence that, antagonising glutamate-gated Ca2+ ion channels with memantine normalises binge-eating disorders. Econ Hum Biol. 2005;3:329–337. doi: 10.1016/j.ehb.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Fletcher PJ. Serotonin and drug reward: focus on 5-HT2C receptors. Eur J Pharmacol. 2003;480:151–162. doi: 10.1016/j.ejphar.2003.08.102. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Holter SM, Danysz W, Spanagel R. Novel uncompetitive N-methyl-D-aspartate (NMDA)-receptor antagonist MRZ 2/579 suppresses ethanol intake in long-term ethanol-experienced rats and generalizes to ethanol cue in drug discrimination procedure. J. Pharmacol. Exp. Ther. 2000;292:545–552. [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 2005;179:247–254. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- Khan AM, Cheung HH, Gillard ER, Palarca JA, Welsbie DS, Gurd JW, Stanley BG. Lateral hypothalamic signaling mechanisms underlying feeding stimulation: differential contributions of Src family tyrosine kinases to feeding triggered either by NMDA injection or by food deprivation. J Neurosci. 2004;24:10603–10615. doi: 10.1523/JNEUROSCI.3390-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Stanley BG. NMDA receptors mediate feeding elicited by neuropeptide Y in the lateral and perifornical hypothalamus. Brain Res. 2005;1063:1–8. doi: 10.1016/j.brainres.2005.09.045. [DOI] [PubMed] [Google Scholar]
- Martel P, Fantino M. Mesolimbic dopaminergic system activity as a function of food reward: a microdialysis study. Pharmacol Biochem Behav. 1996;53:221–226. doi: 10.1016/0091-3057(95)00187-5. [DOI] [PubMed] [Google Scholar]
- Mcshane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD003154.pub5. CD003154. [DOI] [PubMed] [Google Scholar]
- Newman JL, Beardsley PM. Effects of memantine, haloperidol, and cocaine on primary and conditioned reinforcement associated with cocaine in rhesus monkeys. Psychopharmacology (Berl) 2006;185:142–149. doi: 10.1007/s00213-005-0282-2. [DOI] [PubMed] [Google Scholar]
- Ninan I, Kulkarni SK. Dopamine receptor sensitive effect of dizocilpine on feeding behaviour. Brain Res. 1998;812:157–163. doi: 10.1016/s0006-8993(98)00990-1. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Zieglgansberger W. Excitatory amino acid neurotransmission. Handb Exp Pharmacol. 2005:249–303. doi: 10.1007/3-540-28082-0_10. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology (Berl) 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721–729. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Semenova S, Kuzmin AV, Danysz W, Bespalov AY. Low affinity NMDA receptor channel blockers inhibit initiation of intravenous morphine self-administration in naive mice. Eur. J. Pharmacol. 1999;378:1–8. doi: 10.1016/s0014-2999(99)00431-8. [DOI] [PubMed] [Google Scholar]
- Semenova S, Markou A. The effects of the mGluR5 antagonist MPEP and the mGluR2/3 antagonist LY341495 on rats' performance in the 5-choice serial reaction time task. Neuropharmacology. 2007;52:863–872. doi: 10.1016/j.neuropharm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley BG, Willett VL, 3rd, Donias HW, Dee MG, 2nd, Duva MA. Lateral hypothalamic NMDA receptors and glutamate as physiological mediators of eating and weight control. Am J Physiol. 1996;270:R443–R449. doi: 10.1152/ajpregu.1996.270.2.R443. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Willett VL, 3rd, Donias HW, Ha LH, Spears LC. The lateral hypothalamus: a primary site mediating excitatory amino acid-elicited eating. Brain Res. 1993;630:41–49. doi: 10.1016/0006-8993(93)90640-9. [DOI] [PubMed] [Google Scholar]
- Stunkard A, Berkowitz R, Tanrikut C, Reiss E, Young L. d-fenfluramine treatment of binge eating disorder. Am J Psychiatry. 1996;153:1455–1459. doi: 10.1176/ajp.153.11.1455. [DOI] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Cohen ML, Yanovski SZ, Cox C, Theim KR, Keil M, Reynolds JC, Yanovski JA. A prospective study of psychological predictors of body fat gain among children at high risk for adult obesity. Pediatrics. 2006;117:1203–1209. doi: 10.1542/peds.2005-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health. 2007;97:667–675. doi: 10.2105/AJPH.2005.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DF, Olney JW, Kettinger L, 3rd, Price M, Miller JP. Behavioral effects of MK-801 in the rat. Psychopharmacology (Berl) 1990;101:47–56. doi: 10.1007/BF02253717. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Blockade of ionotropic glutamatergic transmission in the ventral tegmental area reduces heroin reinforcement in rat. Psychopharmacology (Berl) 2002;164:144–150. doi: 10.1007/s00213-002-1190-3. [DOI] [PubMed] [Google Scholar]
- Yanovski SZ. Binge eating disorder and obesity in 2003: could treating an eating disorder have a positive effect on the obesity epidemic? Int J Eat Disord. 2003;34 Suppl:S117–S120. doi: 10.1002/eat.10211. [DOI] [PubMed] [Google Scholar]
- Zeni LA, Seidler HB, De Carvalho NA, Freitas CG, Marino-Neto J, Paschoalini MA. Glutamatergic control of food intake in pigeons: effects of central injections of glutamate, NMDA, and AMPA receptor agonists and antagonists. Pharmacol Biochem Behav. 2000;65:67–74. doi: 10.1016/s0091-3057(99)00153-7. [DOI] [PubMed] [Google Scholar]