CASE SUMMARY
History
A 41 year-old man was referred to the National Institutes of Health (NIH) for evaluation of extensive skin thickening and rippled appearance of his upper extremities, torso, and lower extremities. The patient was day +671 status post a myeloablative 6/6 HLA-matched related (sister) peripheral blood stem cell transplant for acute myelogenous leukemia. The conditioning regimen consisted of busulfan and cyclophosphamide and graft-versus-host disease prophylaxis included tacrolimus and a short course of methotrexate.
The patient’s cutaneous symptoms began approximately eight months post-transplant with the onset of “red dots” on his skin accompanied by decreased range of motion at the wrists. A biopsy a patch on the right forearm demonstrated non-specific histologic findings of a mild superficial perivascular infiltrate with rare neutrophils. Prior to the NIH consultation, the patient had been treated with oral steroids, hydroxychloroquine sulfate, rituximab, thalidomide, and physical therapy without benefit. He had not been treated with phototherapy. Rather, the patient reported that the skin changes and range of motion at several joints continued to worsen, resulting in significant functional limitations. His immunosuppression regimen at the time of referral consisted of methylprednisolone 32 mg daily, tacrolimus 1.5 mg twice daily, hydroxychloroquine 200 mg twice daily, mycophenolate mofetil 1 g twice daily, and thalidomide 200 mg in the evening.
Physical Examination
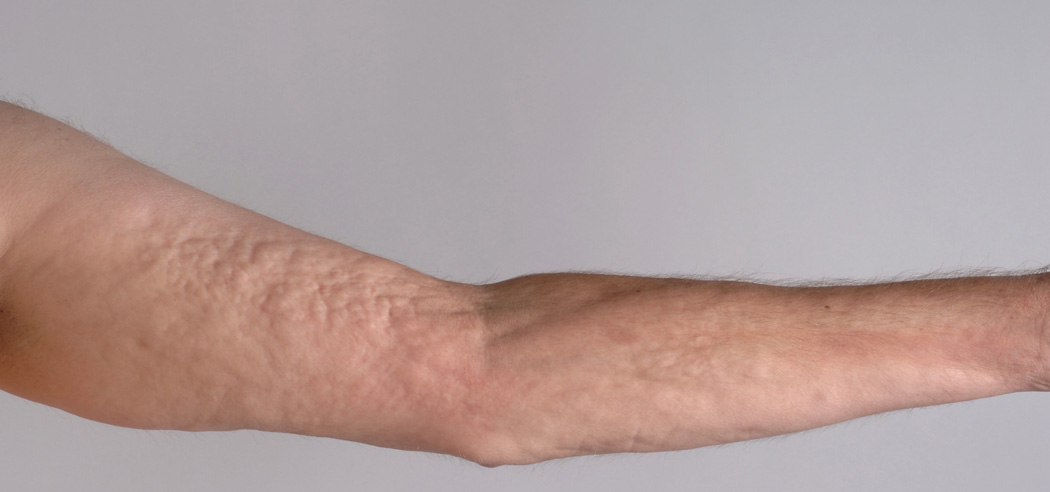
Physical exam was remarkable for a widespread puckered, cellulite-like appearance of the bilateral inner upper arm, majority of the anterior torso, bilateral flanks, medial buttocks, and bilateral inner thighs. The subcutaneous tissue in these areas was firm and nodular by palpation. Deep furrows in the skin extended longitudinally along the forearms (Fig 1A). Alopecia was noted on the anterior legs. The skin of the legs was thickened bilaterally and was fixed to the underlying tibia. The sclerosis extended distally to the mid-dorsum of each foot, inhibiting dorsiflexion and plantarflexion of the ankles. Sclerosis of the popliteal fossae was most prominent in areas of tendinous insertions at the knee. Joint contractures of the shoulders, elbows, wrists, fingers, knees, and ankles were present. Approximately ten 1cm gray atrophic plaques resembling lichen sclerosus were present, however, generalized patchy skin pigmentation (“leopard skin changes”) were not identified. The head and neck region was spared.
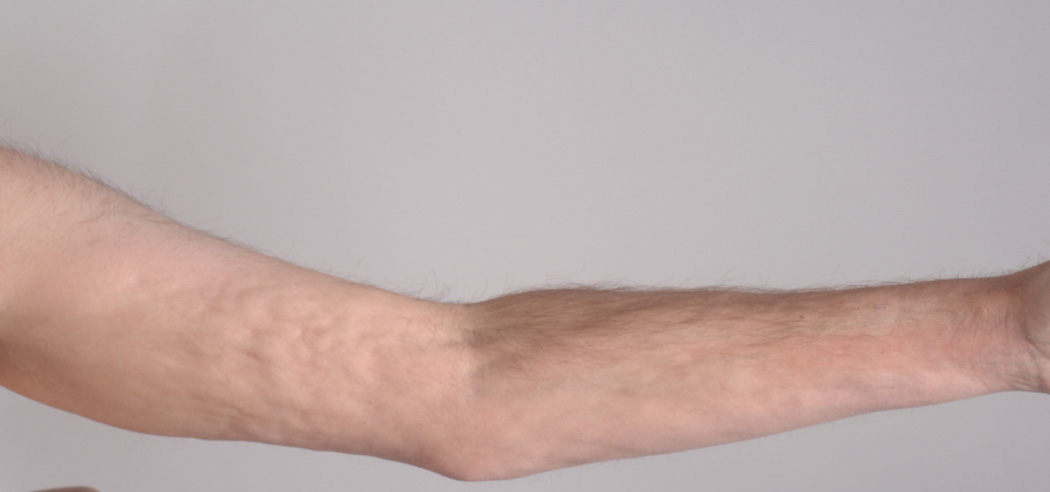
Figure 1.
A. Subcutaneous rippling of left inner arm, grooving of the proximal forearm, and sclerosis of the wrist.
B. Marked improvement in sclerotic manifestations 7 months after initiation of ECP therapy (B).
Histopathologic Examination
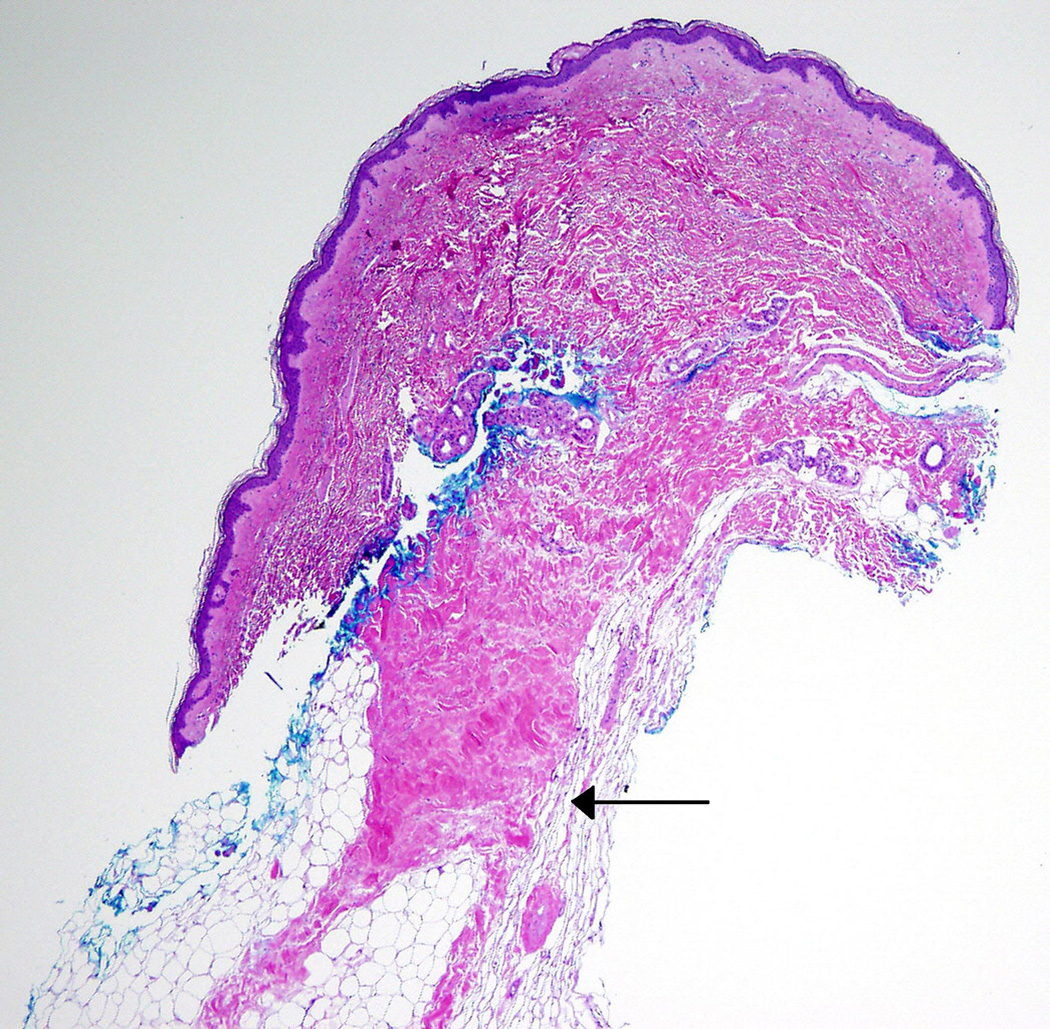
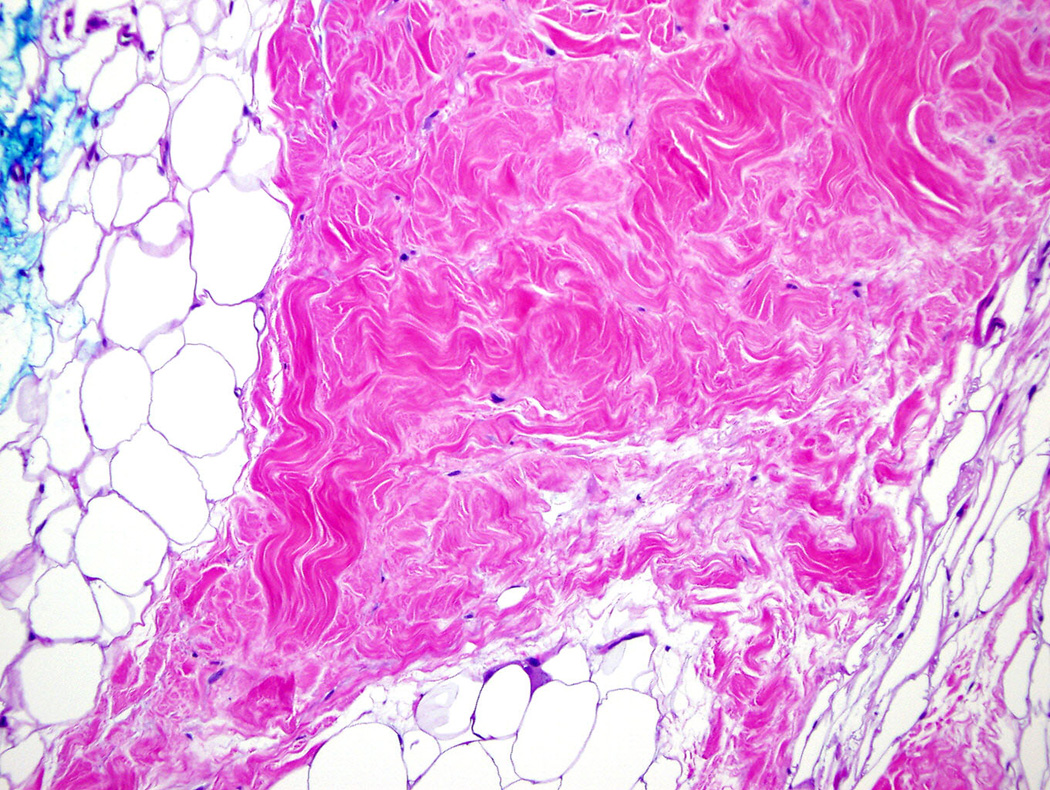
Two 6 mm punch biopsies were performed upon initial evaluation at the NIH. The first was taken from an area of clinically unaffected skin on the right lateral back, and the second from an area of firm, rippled skin on the right medial buttock. Histologic examination of the biopsy from the back was unremarkable. The biopsy from the buttock revealed mild focal thickening of the subcutaneous fat tissue, however, definitive sclerotic changes were not observed. A repeat 5 mm punch biopsy of an area of firm, rippled skin on the left medial upper arm performed several months later revealed focal sclerosis of collagen in the deep dermis extending into the subcutaneous fat and connecting with prominent thickened fat septae (Fig 2A, 2B). Histological features of nephrogenic systemic fibrosis, including spindle-cell proliferation, were not identified. These findings
Figure 2. Left arm.
A. Punch biopsy demonstrates thickening of deep reticular dermis and fat septae. The upper dermis and epidermis are unaffected (Hematoxylin and eosin, original magnification 4x).
B. Higher power view of sclerotic collagen bundles in the subcutaneous fat septum. (Hematoxylin and eosin, original magnification 20x).
Significant Diagnostic Studies
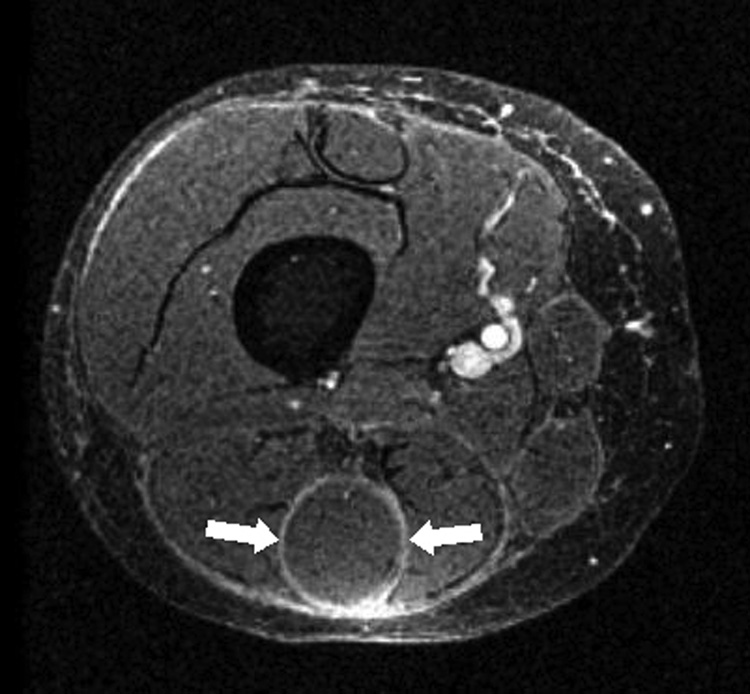
Magnetic resonance imaging (MRI) of the right thigh revealed subcutaneous sclerosis and extensive deep fasciitis with epimysial involvement (Fig 3A).
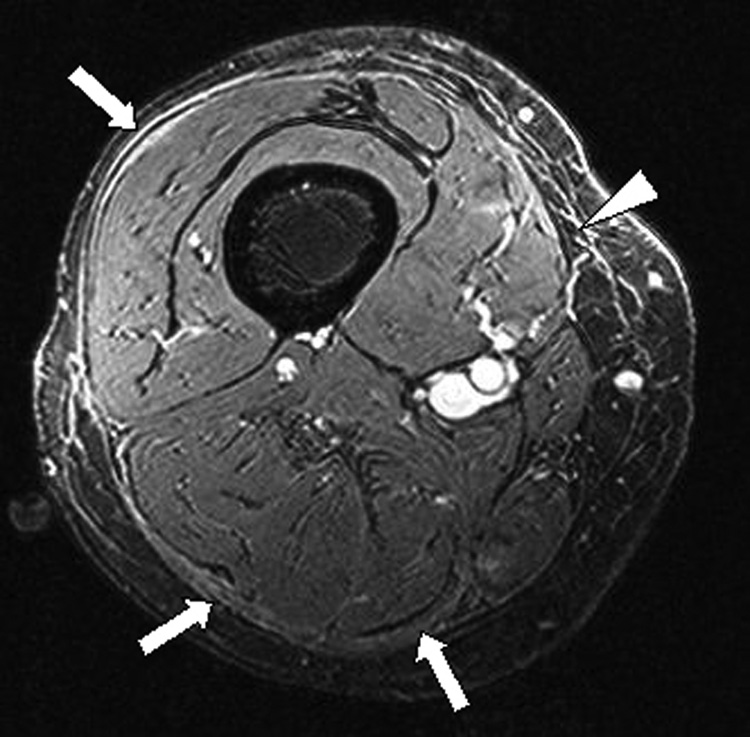
Figure 3. Initial (A) and follow up (B) axial magnetic resonance images of the right thigh.
A. T1 weighted fat suppressed 3D gradient echo (FS-GRE) image, after contrast administration, demonstrates extensive enhancement along the deep fascia, and also along the epimysium of the hamstring musculature, most prominent about the semitendinosus muscle (arrows).
B. T1 weighted FS-GRE image after contrast administration demonstrates mild persistent enhancement along the deep fascia, which appears thickened (arrows). Epimyseal enhancement has regressed. There is residual subcutaneous septal enhancement (arrowhead).
Diagnosis
Cutaneous chronic graft-versus-host disease (cGvHD), sclerotic type, with subcutaneous involvement and fasciitis.
FOLLOW-UP
The patient was enrolled in a phase II NIH protocol studying extracorporeal photopheresis (ECP) for the treatment of cGvHD (Protocol NCT00048789). He underwent ECP three times weekly for one week, followed by twice weekly treatment for thress months, and finally twice weekly on every other week basis. The methylprednisone was converted to prednisone and thalidomide was discontinued due to unexplained neutropenia. A steroid taper was initiated after the patient developed subjective improvement. Five months after initiating therapy, the patient had markedly decreased skin rippling and tightness and increased joint mobility (Fig 1B). After 6 months of therapy, his prednisone dose had been tapered to 20 mg every other day. MRI examination revealed improvement in fasciitis and epimysial inflammation, but the deep fascial thickening and residual enhancement persisted (Fig 3B). The patient continues to receive ECP twice weekly, every other week.
DISCUSSION
cGvHD is the major long-term complication of allogeneic hematopoietic stem cell transplantation (alloHSCT) and the primary cause of non-relapse mortality in alloHSCT recipients.1 cGvHD can involve every organ system, and skin disease is particularly common.
Cutaneous cGvHD involving the epidermis may manifest in a number of clinical patterns, including subtle presentations resembling xerosis, keratosis pilaris, or ichthyosis. Other non-sclerotic presentations include lichen planus-like lesions with erythematous or violaceous flat-topped papules, pityrias rosea-like lesions, eczematous-like plaques, and psoriasiform plaques. Sclerosis may affect the dermis, subcutaneous tissue, or fascia, and may resemble lichen sclerosus, morphea, systemic sclerosis, and eosinophilic fasciitis.2,3 Involvement of the subcutaneous and fascial planes leads to a dimpled or rippled appearance of the skin that resembles cellulite, and also may lead to the “groove sign” on the extremities demarcating muscle/fascial bundles or along the path of superficial vessels. Widespread sclerosis may result in joint contractures and severe limitation of function. Concomitant signs and symptoms of cGvHD-related fasciitis include muscle pain, cramping, weakness, and/or edema.4–6 MRI is under investigation as a potential adjunct to quantify the extent of subcutaneous and fascial disease activity.7,8
Skin sclerosis is a relatively uncommon cGvHD manifestation after alloHSCT. In a retrospective review of 493 alloHSCT patients, only 17 (3.4%) developed sclerotic cGvHD.9 The reported incidence of sclerotic symptoms among patients who manifest cGvHD symptoms is approximately 14%.10,11 However, 58 of 81 (72%) patients with skin involvement evaluated at the NIH via a cross-sectional cGvHD study of patients who have undergone a previous stem cell transplantation (Protocol NCT00331968) presented with evidence of superficial and/or deep sclerotic skin disease (72%)12 based on NIH consensus staging criteria, suggesting that skin sclerosis is a significant problem for a subset of patients with recalcitrant disease.3
Potential risk factors for the development of cGvHD in our patient include a female donor for male recipient and the use of peripheral blood as the stem cell source. Other known risk factors for cGVHD include major HLA allele mismatch between donor and recipient, , older recipient age, , T-cell replete graft, donor lymphocyte infusion, and prior acute GvHD.13 Increased CD3 cell dose at the time of HSCT has been associated with an elevated risk of sclerotic cGvHD in a multivariate analysis.11
Adequate treatment of cGvHD has been hampered by an incomplete understanding of the disease process. Immune dysregulation occurs via subsets of donor-derived T-cells which target mismatched histocompatibility antigens in the recipient. In theory, these donor-derived-T cells should be susceptible to recipient thymic selection. However, the recipient thymus is thought to be compromised due to the effect of previous chemotherapy exposure, including pre-transplant conditioning regimens, radiation therapy, age, and treatment with calcineurin inhibitors and other immunosuppressant agents.14,15 Cytokine dysregulation is also a hallmark of cGvHD, and transforming growth factor-beta (TGF-β) and platelet derived growth factor (PDGF) may have important pathogenic roles in the development of cGvHD-related fibrosis in various organ systems, including the skin.16,17
Management of cutaneous cGvHD must include consideration of other organ system involvement, infectious disease risk, the status of the patient’s underlying malignancy, and the potential for long-term morbidity and mortality from the skin involvement. Sclerotic cGvHD, and deep fibrosis in particular, is poorly responsive to topical therapy Patients typically achieve a partial response, such as softening of fibrosis, with oral corticosteroids or phototherapy.18–20 Other therapeutic agents including calcineurin inhibitors (cyclosporine, FK-506), chimeric or humanized monoclonal antibodies (rituximab, dacluzimab), hydroxychloroquine, intravenous immunoglobulin, mycophenolate mofetil, pentostatin, and thalidomide have been reported to have variable efficacy.18,21–33 Conservative modalities, including physical and occupational therapy, are also important adjuncts to maintain functional range of motion.34
Extracorporeal photopheresis (ECP) was initially developed for the treatment of cutaneous T-cell lymphoma (CTCL)/Sezary syndrome but it has also demonstrated efficacy in the treatment of cGvHD of the skin, oral cavity, and liver. The procedure involves three steps: leukapheresis, photoactivation with UVA light, and reinfusion of treated cells back into the patient. ECP is generally well tolerated, but may be associated with anemia or complications (thrombus, infection) associated with indwelling catheters use. A definitive treatment schedule has not yet been defined for cGvHD and is typically determined by disease severity, the individual’s rate of response, and the physician’s discretion.20 Although the success of ECP for cGVHD of the skin is now well-established, limited availability of centers offering the time-intensive procedure and the cost associated with ECP therapy may impede access for some patients.
KEY TEACHING POINTS
Cutaneous cGvHD may affect the skin at any level from the epidermis to the fascial layer and may manifest with sclerotic or non-sclerotic features.
Sclerotic cGvHD is a potential cause of severe morbidity in alloHSCT recipients. Deep-seated sclerosis and fasciitis present as firm areas of subcutaneous rippling and may result in range of motion limitation and joint contractures.
ECP is emerging as a valuable therapeutic option for the treatment of refractory cutaneous cGvHD; however further study is indicated in order to better understand its biological effect and to standardize treatment regimens.
Acknowledgments
Funding Sources: This research was supported by the Intramural Program of the NIH, Center for Cancer Research, National Cancer Institute. A. R. Patel was supported by the Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer Inc. (via a grant to the Foundation for NIH from Pfizer Inc.), Bethesda, Maryland.
Editor’s Note: Drs. Cowen, Pavletic, and Malech evaluate patients with cGvHD as part of an intramural NIH multi-specialty initiative developed to better study and treat the myriad manifestations of the disease (Protocol NCT00331968). Additional information regarding this program and current cGvHD protocols is available at: ccr.ncifcrf.gov/resources/gvhd/default.asp. Clinicians may refer interested patients to the NIH patient recruitment and referral office at 800-411-1222 or by e-mail at prpl@mail.cc.nih.gov.
Abbreviations/acronyms
- cGVHD
chronic graft-versus-host disease
- ECP
extracorporeal photopheresis
- FS-GRE
fat suppressed 3D gradient echo
- HSCT
hematopoietic stem cell tranplantation
- HLA
human leukocyte antigen
- MRI
magnetic resonance imaging
- TGF-β
transforming growth factor-beta
- PDGF
platelet derived growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper has not been presented previously.
Conflicts of Interest Disclosure: None declared
REFERENCES
- 1.Fraser CJ, Scott Baker K. The management and outcome of chronic graft-versus-host disease. Br J Haematol. 2007 Jul;138(2):131–145. doi: 10.1111/j.1365-2141.2007.06652.x. [DOI] [PubMed] [Google Scholar]
- 2.Hymes SR, Turner ML, Champlin RE, Couriel DR. Cutaneous manifestations of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2006 Nov;12(11):1101–1113. doi: 10.1016/j.bbmt.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 3.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005 Dec;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 4.van den Bergh V, Tricot G, Fonteyn G, Dom R, Bulcke J. Diffuse fasciitis after bone marrow transplantation. Am J Med. 1987 Jul;83(1):139–143. doi: 10.1016/0002-9343(87)90509-2. [DOI] [PubMed] [Google Scholar]
- 5.Janin A, Socie G, Devergie A, Aractingi S, Esperou H, Vérola O, et al. Fasciitis in chronic graft-versus-host disease. A clinicopathologic study of 14 cases. Ann Intern Med. 1994 Jun 15;120(12):993–998. doi: 10.7326/0003-4819-120-12-199406150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Carroll CB, Hilton DA, Hamon M, Zajicek JP. Muscle cramps and weakness secondary to graft versus host disease fasciitis. Eur J Neurol. 2005 Apr;12(4):320–322. doi: 10.1111/j.1468-1331.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- 7.Dumford K, Anderson JC. CT and MRI findings in sclerodermatous chronic graft vs. host disease. Clin Imaging. 2001;25(2):138–140. doi: 10.1016/s0899-7071(01)00240-6. [DOI] [PubMed] [Google Scholar]
- 8.Clark J, Yao L, Turner ML, Cowen EW. Magnetic resonance imaging for the diagnosis of subcutaneous involvement in patients with chronic graft-versus-host disease. J Am Acad Dermatol. 2008 Feb;58(2) Suppl 2:AB85. [Google Scholar]
- 9.Peñas PF, Jones-Caballero M, Aragüés M, Fernández-Herrera J, Fraga J, García-Díez A. Sclerodermatous graft-vs-host disease: clinical and pathological study of 17 patients. Arch Dermatol. 2002 Jul;138(7):924–934. doi: 10.1001/archderm.138.7.924. [DOI] [PubMed] [Google Scholar]
- 10.Chosidow O, Bagot M, Vernant JP, Roujeau JC, Cordonnier C, Kuentz M, et al. Sclerodermatous chronic graft-versus-host disease. Analysis of seven cases. J Am Acad Dermatol. 1992 Jan;26(1):49–55. doi: 10.1016/0190-9622(92)70005-z. [DOI] [PubMed] [Google Scholar]
- 11.Skert C, Patriarca F, Sperotto A, Cerno M, Filì C, Zaja F, Stocchi R, Geromin A, Damiani D, Fanin R. Sclerodermatous chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: incidence, predictors and outcome. Haematologica. 2006 Feb;91(2):258–261. [PubMed] [Google Scholar]
- 12.Patel AR, Pavletic SZ, Turner ML, Cowen EW. The isomorphic response in morphea-like chronic graft-versus-host disease. Arch Dermatol. doi: 10.1001/archderm.144.9.1229. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 14.Johnson ML, Farmer ER. Graft-versus-host reactions in dermatology. J Am Acad Dermatol. 1998 Mar;38(3):369–392. doi: 10.1016/s0190-9622(98)70495-5. [DOI] [PubMed] [Google Scholar]
- 15.Sakoda Y, Hashimoto D, Asakura S, et al. Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease. Blood. 2007;109(4):1756–1764. doi: 10.1182/blood-2006-08-042853. [DOI] [PubMed] [Google Scholar]
- 16.Ochs LA, Blazar BR, Roy J, Rest EB, Weisdorf DJ. Cytokine expression in human cutaneous chronic graft-versus-host disease. Bone Marrow Transplant. 1996 Jun;17(6):1085–1092. [PubMed] [Google Scholar]
- 17.Svegliati S, Olivieri A, Campelli N, Luchetti M, Poloni A, Trappolini S, et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007 Jul 1;110(1):237–241. doi: 10.1182/blood-2007-01-071043. [DOI] [PubMed] [Google Scholar]
- 18.White JM, Creamer D, du Vivier AW, Pagliuca A, Ho AY, Devereux S, et al. Sclerodermatous graft-versus-host disease: clinical spectrum and therapeutic challenges. Br J Dermatol. 2007 May;156(5):1032–1038. doi: 10.1111/j.1365-2133.2007.07827.x. [DOI] [PubMed] [Google Scholar]
- 19.Breuckmann F, Gambichler T, Altmeyer P, Kreuter A. UVA/UVA1 phototherapy and PUVA photochemotherapy in connective tissue diseases and related disorders: a research based review. BMC Dermatol. 2004 Sep 20;4(1):11. doi: 10.1186/1471-5945-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall SR. Technology insight: ECP for the treatment of GvHD--can we offer selective immune control without generalized immunosuppression? Nat Clin Pract Oncol. 2006 Jun;3(6):302–314. doi: 10.1038/ncponc0511. [DOI] [PubMed] [Google Scholar]
- 21.Ratanatharathorn V, Ayash L, Reynolds C, Silver S, Reddy P, Becker M, et al. Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant. 2003;9:505–511. doi: 10.1016/s1083-8791(03)00216-7. [DOI] [PubMed] [Google Scholar]
- 22.Canninga-van Dijk MR, van der Straaten HM, Fijnheer R, Sanders CJ, van den Tweel JG, Verdonck LF. Anti-CD20 monoclonal antibody treatment in 6 patients with therapy refractory chronic graft-versus-host disease. Blood. 2004;104:2603–2606. doi: 10.1182/blood-2004-05-1855. [DOI] [PubMed] [Google Scholar]
- 23.Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamble R, Oholendt M, Carrum G. Rituximab responsive refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2006;12:1201–1202. doi: 10.1016/j.bbmt.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto M, Okano A, Akamatsu S, Ashihara E, Inaba T, Takenaka H, et al. Rituximab is effective for steroid-refractory sclerodermatous chronic graft-versus-host disease. Leukemia. 2006;20:172–173. doi: 10.1038/sj.leu.2403996. [DOI] [PubMed] [Google Scholar]
- 26.Carella AM, Biasco S, Nati S, Congiu A, Lerma E. Rituximab is effective for extensive steroid-refractory chronic graft-vs.host-disease. Leuk Lymphoma. 2007;48:623–624. doi: 10.1080/10428190601094362. [DOI] [PubMed] [Google Scholar]
- 27.Zaja F, Bacigalupo A, Patriarca F, Stanzani M, Van Lint MT, Fili C, et al. Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transplant. 2007;40:273–277. doi: 10.1038/sj.bmt.1705725. [DOI] [PubMed] [Google Scholar]
- 28.Mohty M, Marchetti N, El-Cheikh J, Faucher C, Fürst S, Blaise D. Rituximab as salvage therapy for refractory chronic GVHD. Bone Marrow Transplant. 2008 Feb;18 doi: 10.1038/bmt.2008.12. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 29.Gilman AL, Chan KW, Mogul A, Morris C, Goldman FD, Boyer M, et al. Hydroxychloroquine for the treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6(3A):327–334. doi: 10.1016/s1083-8791(00)70058-9. [DOI] [PubMed] [Google Scholar]
- 30.Sokos DR, Berger M, Lazarus HM. Intravenous immunoglobulin: appropriate indications and uses in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2002;8(3):117–130. doi: 10.1053/bbmt.2002.v8.pm11939601. [DOI] [PubMed] [Google Scholar]
- 31.Vogelsang GB, Arai S. Mycophenolate mofetil for the prevention and treatment of graft-versus-host disease following stem cell transplantation: preliminary findings. Bone Marrow Transplant. 2001 Jun;27(12):1255–1262. doi: 10.1038/sj.bmt.1703076. [DOI] [PubMed] [Google Scholar]
- 32.Jacobsohn DA, Chen AR, Zahurak M, Piantadosi S, Anders V, Bolaños-Meade J, et al. Phase II study of pentostatin in patients with corticosteroid-refractory chronic graft-versus-host disease. J Clin Oncol. 2007 Sep 20;25(27):4255–4261. doi: 10.1200/JCO.2007.10.8456. [DOI] [PubMed] [Google Scholar]
- 33.Browne PV, Weisdorf DJ, DeFor T, Miller WJ, Davies SM, Filipovich A, et al. Response to thalidomide therapy in refractory chronic graft-versus-host disease. Bone Marrow Transplant. 2000 Oct;26(8):865–869. doi: 10.1038/sj.bmt.1702626. [DOI] [PubMed] [Google Scholar]
- 34.Beredjiklian PK, Drummond DS, Dormans JP, Davidson RS, Brock GT, August C. Orthopaedic manifestations of chronic graft-versus-host disease. J Pediatr Orthop. 1998 Sept-Oct;18(5):572–575. doi: 10.1097/00004694-199809000-00002. [DOI] [PubMed] [Google Scholar]