Abstract
Compared to nondrug reinforcers, few studies have examined delay discounting for drug reinforcers. The purpose of the present study was to examine delay discounting in rhesus monkeys using orally delivered phencyclidine (PCP) as the reinforcer and to examine the effects of manipulating reinforcer magnitude and cost on delay discounting for PCP using an adjusting delay task. Monkeys could choose between a single delivery of PCP available immediately or a bundle of PCP deliveries available following a titrated delay. The average of the delays, or the mean adjusted delay (MAD), served as the quantitative measure of delay discounting. In Experiment 1, reinforcer magnitude was manipulated by varying the PCP concentration and the size of the delayed reinforcer (6 or 12 deliveries). The concentration-effect curve for PCP deliveries assumed an inverted U-shaped function, but varying PCP concentration had little effect on MAD values or choice between immediate and delayed reinforcers. Increasing the size of the delayed reinforcer produced an upward and leftward shift in the concentration effect curve. In Experiment 2, the cost of reinforcers was manipulated by increasing the fixed ratio (FR) requirement for each choice. Increasing the FR led to increased MAD values and decreased PCP self-administration.
Keywords: Adjusting delay task, Delay discounting, Magnitude of reinforcement, Phencyclidine, Rhesus monkeys, Self-administration
1. Introduction
The relationship between impulsive behavior and vulnerability to drug abuse has become a major focus of addiction research. A growing body of literature indicates that drug abusers consistently show impulsive behaviors (Coffey et al., 2003; Kirby and Petry, 2004; Madden et al., 1997; Petry, 2001, 2003, 2006). Importantly, results from studies in laboratory animals show that impulsive behavior for food can predict drug self-administration (Perry et al., 2005), reinstatement of drug seeking (Perry and Carroll, 2008), escalation of cocaine self-administration (Anker, Perry, & Carroll, 2008), and sensitization to the behavioral effects of drugs (Mitchell et al., 2006). A number of different procedures for assessing impulsive behavior and drug seeking have been employed (Perry and Carroll, 2008). While impulsivity can be characterized in a number of ways (Evenden, 1999), a commonly used operational concept entails measuring the devaluation, or discounting, of delayed rewards. The delay discounting model assumes that the allocation of choices is directly proportional to the rate and size of the reinforcer, but inversely proportional to the delay to its receipt. Delay discounting is commonly used in human subjects to determine the subjective value of real or hypothetical (Madden et al., 2003), drug (Bickel et al., 1999), and nondrug (Chapman, 1996) rewards. In laboratory animals, delay discounting has been well established with nondrug reinforcers; however, fewer studies have examined the rate of delay discounting of drug reinforcers. Recently, Woolverton et al. (2007), using a delay discounting procedure in monkeys, demonstrated that choice of a dose of cocaine varied as a function of the delay to its receipt, and the relationship between delay and value of the reinforcer was described by a hyperbolic function similar to that shown with nondrug reinforcers (Green et al., 2007; Mazur, 1987).
The adjusting delay procedure measures the indifference between two alternative reinforcers: one that is delivered immediately and another that is larger, but delivered following an adjusting delay. The delay for the larger, delayed reinforcer is adjusted based upon choices made by the subject, such that a choice made for the larger delayed reinforcer increases the delay to the larger, delayed reinforcer by 1 second; whereas, a choice for the smaller immediate reinforcer decreases it by 1 second. The mean adjusted delay (MAD) is calculated following a series of choice trials, and it indicates the point at which the two alternatives are of equal value (i.e., are chosen equally). This equilibrium, known as the indifference point, serves as measure of the subject’s choices between the smaller, immediate reinforcer and the larger, delayed reinforcer. The adjusting delay approach obtains the indifference point efficiently within a few sessions by adjusting the delay to the larger, delayed reinforcer until both alternatives are chosen approximately equally. This procedure allows the subject to titrate the delay. The average delay of each session (MAD) is the dependent variable used as an index of impulsivity. This method is different than that used to construct hyperbolic functions based on experimenter controlled changes in delay and reinforcer magnitude that serve as independent variables in traditional delay discounting tasks. The adjusting delay task has been used in this laboratory to examine the delay discounting for food and cocaine infusions in rats (Perry et al., 2008), and results are consistent with other methods. Our rationale for using the modified adjusting delay method was to study the impact of other variables such as reinforcer magnitude, schedule parameters, and eventually time-limited withdrawal effects (Carroll et al., 2008).
Under choice conditions in which a small dose and a relatively larger doses of a drug are available under identical schedules and temporal conditions, the larger alternative is consistently preferred (Johanson and Schuster, 1975). However, the introduction of various constraints produces shifts in preference. Delay to reinforcer delivery (Anderson and Woolverton, 2003), dose (Iglauer and Woods, 1974; Macenski and Meisch, 1998), and cost or unit price (Bickel et al., 1990; Nader et al., 1993) are well-established determinants of choice among available reinforcers. Using a choice procedure in rhesus monkeys, a higher dose of cocaine was consistently chosen over a smaller one when the delays to each were equal (Anderson and Woolverton, 2003). However, imposing delays prior to the delivery of the larger cocaine dose produced a shift in choice to the smaller, immediate dose. Taken together, these findings suggest that delay and reinforcer magnitude are important factors in choice. The present study extended the analysis of schedule and reinforcement magnitude variables to rhesus monkeys orally self-administering a drug, PCP, under an adjusting delay schedule.
The purpose of the present study was to examine the degree to which magnitude of a reinforcer affects choice for an immediate delivery of a small drug reinforcer vs a delayed delivery of a larger drug reinforcer. PCP was chosen as the drug reinforcer because oral self-administration can be readily obtained, reinforcer magnitudes can be rapidly changed, and the ability to compare a range of variables is not limited by catheter life. Furthermore, several behavioral economic studies have been conducted with this drug in monkeys (Carroll and Campbell, 2000) and the results have been generalized to other drugs, routes of administration, and species (Carroll et al., 2001), and with minimal adverse effects, it is a safe drug to use for oral self-administration. The specific goals of Experiment 1 were to examine the manipulation of the concentration of PCP and the number of PCP deliveries. It was hypothesized that higher PCP concentrations would be discounted less than lower concentrations, and the difference between the magnitude of larger delayed reinforcers (6 vs 12 PCP deliveries) would be reflected in differences in delay discounting, with 12 deliveries discounted less than 6 deliveries. Manipulating reinforcer magnitude in the adjusting delay task has produced different results depending upon the type of reinforcer used. In studies using rats, discounting of delayed cocaine reinforcers was sensitive to an increase in cocaine dose, but discounting for food reinforcers was not sensitive to an increase in number of pellets (Perry et al., 2007). Based on the findings from that study, we expected that PCP self-administration under the adjusting delay procedure would be sensitive to manipulation of reinforcer magnitude.
The goal of Experiment 2 was to examine the effect of altering the schedule of reinforcement (fixed ratio, FR) on choice for an immediate PCP reinforcer (1 delivery) vs a larger, delayed PCP reinforcer (12 deliveries). For Experiment 2, we hypothesized that increasing the amount of effort required to obtain the small, immediate reinforcer and the larger, delayed reinforcer would result in a shift between preference for the small, immediate reinforcer to the larger, delayed reinforcer as reflected in higher indifference points (MADs). Previous research using choice procedures suggests that increasing the FR requirement for a preferred drug reinforcer will lead to a shift in choice to a less preferred drug reinforcer. This has been demonstrated with orally self-administered ethanol (Stewart et al., 2002) and pentobarbital (Meisch and Lemaire, 1989), and intravenous remifentanil (Galuska et al., 2006) in rhesus monkeys. Thus, we expected that increasing the “cost” of the reinforcer would lead the monkeys to maximize their effort by choosing the larger, delayed reinforcer more often.
2. Methods
2.1 Subjects
Eight adult male rhesus monkeys (Macaca mulatta) served as subjects. All monkeys had previous experience self-administering PCP concentrations ranging from 0.0625 to 1.0 mg/ml under various schedules of reinforcement. The monkeys were maintained at 85% of their free-feeding body weights (7.8–13 kg) by adjusting their daily food allotment (Harlan Teklad monkey chow, Bartonville, IL). Monkeys were given fresh fruit, vegetables, or trail mix daily to supplement their diet. Monkeys had free access to water except during the experimental session and for two hours prior to, and 1.5 hours following the experimental session. Following the experimental sessions, movies were played for enrichment. Other enrichment objects including a hanging wooden log and one loose toy were provided to the monkeys at all times. Experiments were conducted in humidity- and temperature-controlled vivarium rooms under a 12-hr light/dark cycle (lights on at 0600 hr) that contained 10 or 12 monkeys each. All procedures and protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee under protocol number 0710A15141. Laboratory facilities were accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC). Laboratory practices were carried out in accordance with the Principles of Laboratory Animal Care (National Research Council, 2003).
2.2 Apparatus
Monkeys were housed in individual, custom-made stainless steel cages (83 cm in width × 76 cm in height × 100 cm in depth; Lab Products, Maywood, NJ) consisting of solid back and side walls, a barred front door, grid floors and a primate perch. One side of each cage was modified to accommodate an operant panel. Each operant panel contained 2 brass spouts (1.2 cm in diameter) that extended 2.7 cm into the cage through circular apertures in the wall about 45 cm above the cage floor, and the spouts were mounted equidistantly from the center and sides of the cage. There were 3 stimulus lamps placed equidistantly on the panel. Two green stimulus lamps were located above the spouts, and one red stimulus lamp was located equidistantly in the center of the panel. Each spout was mounted on clear Plexiglas with 2 green and 2 white stimulus lamps embedded within it. The small green and white stimulus lamps were illuminated upon lip contact for PCP or water, respectively. When the appropriate number of lip contact responses had been made, a solenoid valve opened allowing 0.6 ml of liquid to flow from the 2000-ml reservoirs suspended above and mounted to the outside of the panel attached to the cage. Scheduling and recording of events were accomplished using Med-PC software (Med-PC®. for Windows) and associated interfaces (Med Associates, St Albans, VT) located in an adjacent room.
2.3 Delay discounting procedure
All monkeys were initially trained to self-administer PCP at a concentration of 0.25 mg/ml according to concurrent FR 8 schedules of reinforcement, such that the eighth lip contact response resulted in delivery of the solution. Responses made on one spout counted toward the completion of an FR that terminated in either 1) a single delivery of PCP (0.6 ml/delivery) given immediately (small, immediate reinforcer), or 2) a bundle of multiple (6 or 12) deliveries (0.6 ml each) each given under an FR 1 lip-contact response requirement, after the lapse of a delay (large, delayed reinforcer). Illumination of the red center stimulus lamp signaled the beginning of the experimental session and the availability of a choice on one of two spouts. A choice consisted of the first lip-contact response on either spout in the presence of the red center stimulus light. Once the choice was made, the red center stimulus light extinguished and the green light above the chosen spout was solidly lit to signal the chosen spout. Once the first lip-contact response was made, the choice was “locked in” on that spout until the response requirement was completed. Responding on the other spout had no scheduled consequences. A contingency to prevent switching between spouts (i.e., a change-over delay) was not used, and responses made during the delay did not reset the delay. The FR values were always the same on both spouts. A choice on the large, delayed reinforcer-associated spout consisted of a single response on that spout and completion of the FR requirement was signaled by the flashing of the green stimulus lamp above the spout for the duration of the delay. Once the delay elapsed, the light remained solidly on, and 6 or 12 deliveries were available on an FR 1 schedule of reinforcement. Consumption of the deliveries was not governed by a limited hold. Following the final delivery, the green stimulus lamp above the spout extinguished and 5 sec later, the center red stimulus light was illuminated signaling the availability of the next choice. A choice made on the small, immediate reinforcer-associated spout was signaled by the onset of the green stimulus lamp above it. Upon completion of the FR 8 response requirement, a single delivery of PCP was administered upon the next response. The delay preceding the delivery of the large, delayed reinforcer was determined according to the adjusting delay procedure. Following a choice on the large, delayed reinforcer-associated spout, the delay to the next large reinforcer increased by 1 sec; whereas, a choice made on the immediate, small reinforcer- associated spout resulted in a decrease of the delay to the next large reinforcer by 1 sec. At the beginning of the experiment, the delay was set at 10 sec, but thereafter the beginning delay for each daily experimental session was equal to the ending delay from the previous day’s experimental session, such that it was a continuation from the last session. This approach allowed the monkeys to titrate their delay around their indifference point, and the first several choices of each day were not determined by an effort to return to that indifference point or titration range. Rather, they were determined by the reinforcer magnitude and reinforcement schedule parameters under study. This approach also reduced within-subject variability. The minimum and maximum delays were 0 and 120 sec, respectively. The spout associated with the larger, delayed reinforcer alternated each day with the spout associated with the smaller, immediate reinforcer to discourage the development of spout/side preferences. The monkeys were not restricted to a number of choices during the 3-hr session.
The cumulative delays were calculated and divided by the total number of choices the subject made during the session. The resulting number was the MAD, which served as the indifference point, and a measure of impulsivity. Completion of FR requirements for lip contacts on the two spouts was independent for the 2 spouts (i.e., responding under the FR on one spout was unaffected by responses under the FR on the other spout). Experimental sessions were conducted 7 days per week from 1000 to 1300 hr. Water was available ad libitum (FR 1) on both spouts from 1430 to 0800 hr.
2.4.1. Experiment 1: Effect of PCP concentration and reinforcer magnitude
Following stabilization of delay discounting maintained by 0.25 mg/ml PCP, defined as no increasing or decreasing trends in MAD, other concentrations of PCP (0.0625, 0.125, 0.5, and 1.0 mg/ml) were substituted and tested in a similar manner under FR 8. Subsequent to determining the concentration response relationship for a small, immediate reinforcer and 6 delayed deliveries (larger, delayed reinforcer), the size of the larger, delayed reinforcer was increased from 6 to 12 deliveries and the concentration-response relationship was reexamined.
2.4.2. Experiment 2: Effect of schedule of reinforcement
In this experiment, monkeys self-administered 0.25 mg/ml PCP under the adjusting delay schedule for 1 delivery of PCP available immediately or 12 PCP deliveries available following a delay. Once self-administration became stable, delay discounting for PCP was evaluated at FR 8, 16, 32, 64 and 96. Monkeys self-administered PCP at each FR for 7 days. FR requirements for lip contacts were the same for both the immediate- and delay-associated spouts, and they were tested in an ascending order. Subsequently, delay discounting was reexamined at FR 8, under similar conditions as the first evaluation. After completing testing under all FR requirements, the concentration-response relationship was redetermined at FR 32 to compare the effect of altering the schedule of reinforcement across PCP concentrations.
2.5 Drugs
Phencyclidine HCl was obtained from the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC). PCP solutions were mixed in tap water 24 hr prior to each session, and they were stored at room temperature. The PCP concentrations (0.0625, 0.125, 0.25, 0.5 and 1.0 mg/ml) refer to the weight of the HCl salt.
2.6 Data analysis
For concentration-response determinations in Experiments 1 and 2, data from the first five consecutive days in which there were no upward or downward trends in MAD or total deliveries were used for analysis. Previous oral PCP self-administration studies conducted in this lab used similar 5-day criteria (Newman et al., 2007; Perry et al., 2006). The number of days preceding the observation of stability varied unsystematically across conditions. Typically 5 days were sufficient to reach stability criteria, as the monkeys rapidly adapt to concentration and schedule changes (e.g. Rodefer and Carroll, 1999). For Experiment 2, the last 6 days of a 7-day period were used. While formal trend analyses were not conducted, trends in both MAD and total number of deliveries across the 7-day FR condition were not observed. The 6-day period was used for analyses to control for potential spout preferences by having an equal number of sessions on each spout. The dependent variables of interest were number of PCP deliveries obtained, MAD, choices for immediate and delayed reinforcers, and percent choice for the delayed reinforcers. Mean data obtained for the group of 8 monkeys were subjected to a repeated measures two-way analysis of variance (ANOVA). For Experiment 1 the dependent variables were examined as a function of PCP concentration and size of delayed reinforcer (6 vs 12 deliveries). Similar but separate analyses were conducted to examine the effects of changing the FR requirement from FR 8 to FR 32 on number of deliveries, MAD, and percent choice for the larger, delayed reinforcer in Experiment 2. For Experiment 2, the MAD, numbers of PCP deliveries obtained, and numbers of choices were examined as a function of FR requirement using a one-way repeated measures ANOVA. A repeated measures t-test was used to compare MAD values obtained at FR 8 before (FR 8) and after (FR 8 R) the increase in FR requirements. A one-way repeated measures ANOVA was used to examine numbers of choices at FR 8 before and after (FR 8 R) the increase in FR requirement. For all ANOVA, Newman-Keuls post-hoc tests were performed when a significant overall effect was observed (P < 0.05).
3. Results
3.1. Experiment 1: Effect of PCP concentration and reinforcer magnitude
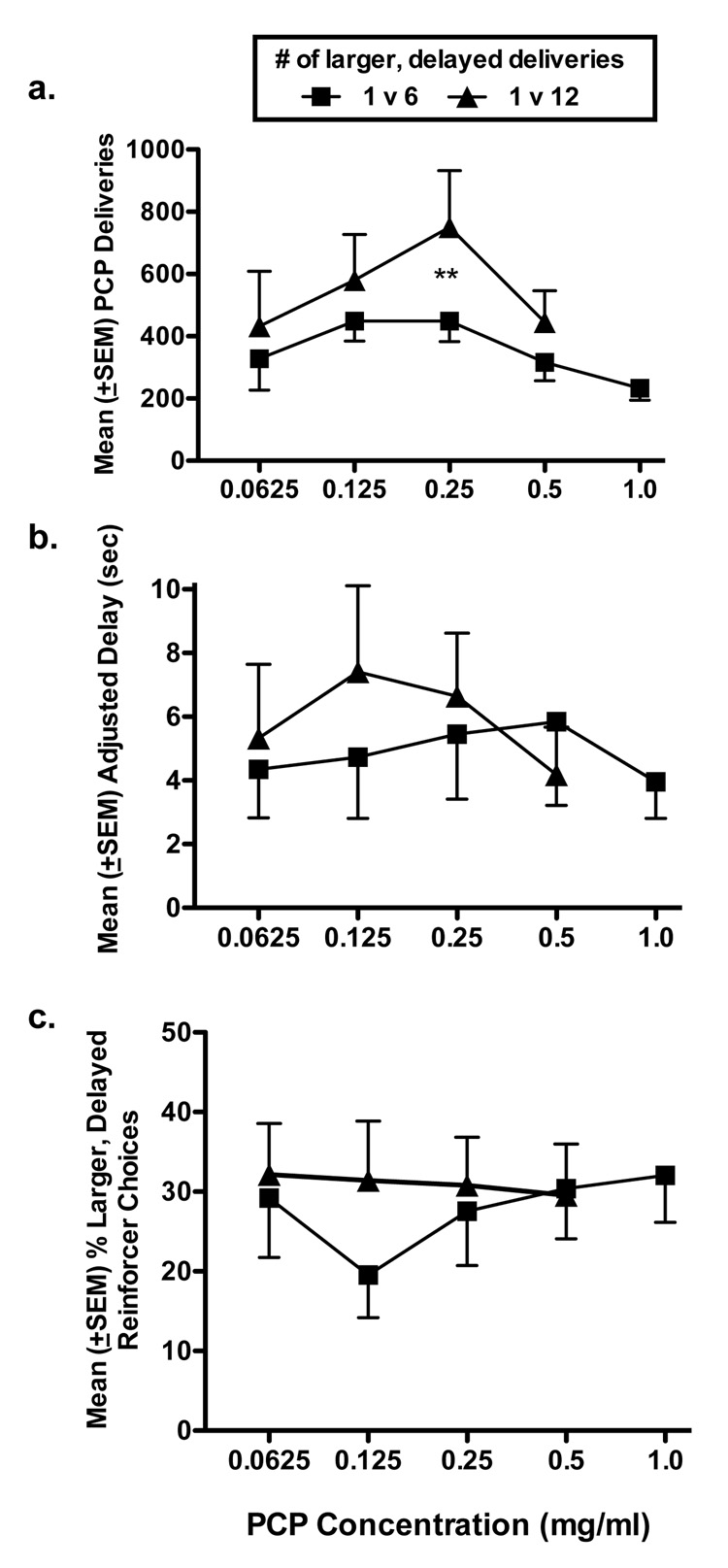
All 8 monkeys reliably self-administered PCP on the concurrent adjusting delay schedule of reinforcement. The 1.0 mg/ml concentration of PCP was tested only under the 1 v 6 delivery condition due to the intake of near-sedative levels of PCP by some monkeys when higher numbers (12) of delayed deliveries were available. Similar levels of intoxication were not observed with other PCP concentrations. Therefore, the data obtained with this 1.0 mg/ml are shown in Figure 1, but they were excluded from statistical analyses because they were not tested in both delivery conditions.
Figure 1.
(a) Mean (±SEM) numbers of PCP deliveries, (b) mean (±SEM) adjusted delay (seconds), and (c) mean (±SEM) percent of choices made for the larger, delayed reinforcer as a function of PCP concentration (0.0625, 0.125, 0.25, 0.5, and 1.0 mg/ml). Data represent the means of 5 sessions obtained in the group of 8 monkeys. Concentration-effect curves were obtained separately for 1 v 6 deliveries (squares) and 1 v 12 deliveries (triangles). **P < 0.01.
The total numbers of PCP deliveries obtained varied in an inverted U-shaped pattern as a function of the PCP concentration available, but deliveries did not differ significantly across PCP concentrations under this adjusting delay schedule [F (3, 63) = 2.29; Figure 1a]. The concentration-response relationship assumed an inverted U-shaped function regardless of the number of deliveries available after the delay. When the delayed reinforcer was 6 deliveries, the concentration-response curve was generally lower than it was for 12 deliveries, and no differences in deliveries were found across concentrations. However, when the size of the delayed reinforcer was increased to 12, the concentration-response curve was relatively steeper, a general upward shift in the concentration response curve was observed, and this overall shift upward was statistically significant [F (1, 63 = 5.77; P < 0.05]. Post hoc analyses indicated that a significant difference between the 6 and 12 delivery conditions was found at the 0.25 mg/ml concentration (P < 0.01).
The MAD is shown in Figure 1b as a function of PCP concentration and reinforcer size. The concentration response curve for 1 v 6 deliveries was only modestly affected by change in the concentration of PCP. The MAD was maintained at around 5 sec (range 4.0 to 5.8 sec) across PCP concentrations. When the number of delayed deliveries was increased from 6 to 12, the concentration curve shifted slightly to the left. Statistically significant differences in MAD were not detected either as a function of concentration [F (3, 63) = 0.50] or size of the reinforcer [F (1, 63) = 0.34].
The percent of larger, delayed reinforcer choices was also generally not affected by concentration of PCP available (Figure 1c). The mean percent of the total choices that were made for the larger, delayed reinforcer was generally between 20 and 30 percent for both the 1 v 6 and 1 v 12 delivery conditions. Statistically significant differences were not observed in percent of larger, delayed reinforcers as a function of PCP concentration [F (3, 63) = 0.41] or as a function of size of the delayed reinforcer [F (1, 63) = 3.73].
3.2. Experiment 2: Effect of schedule of reinforcement
Figure 2a shows the effects of increasing FR requirement on the number of PCP deliveries obtained when the choice was between a single PCP delivery administered immediately after the FR completion or 12 PCP deliveries that were available following a delay. Six of eight monkeys completed testing at FR 96, as ratio strain led to extinction in two monkeys. As FR requirement for each reinforcer (1 v 12 deliveries) was increased, the number of PCP deliveries decreased significantly [F (4, 39) = 22.52, P < 0.0001]. Post-hoc tests indicated that significantly fewer PCP deliveries were obtained at FR 32, FR 64, and FR 96 compared to FR 8 (P < 0.01). Compared to FR 16, fewer deliveries were obtained at FR 32 (P < 0.05), FR 64 (P < 0.05), and FR 96 (P < 0.01), and compared to FR 32, significantly fewer deliveries were obtained at FR 64 (P < 0.05) and FR 96 (P < 0.05).
Figure 2.
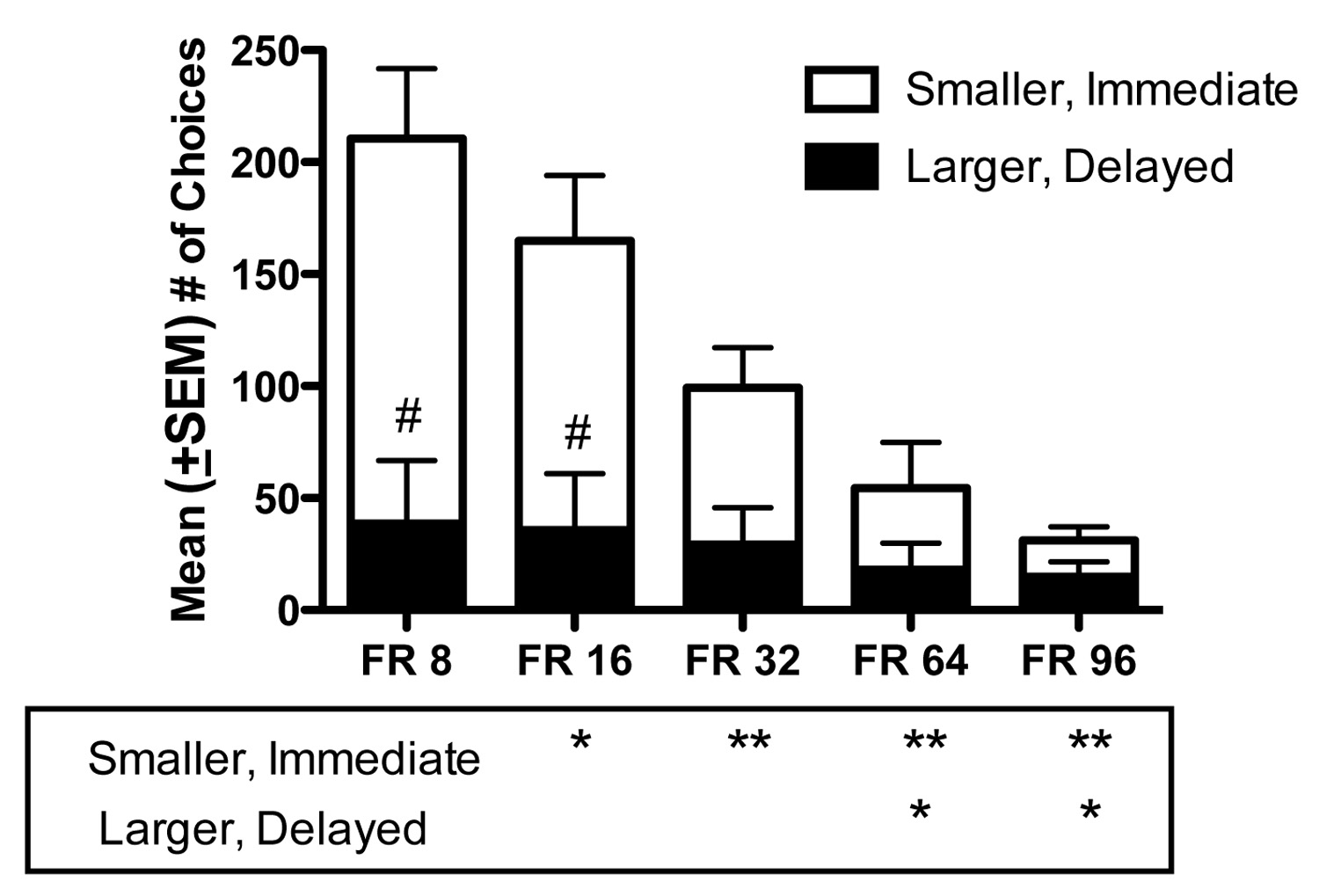
(a) Mean (±SEM) numbers of PCP deliveries, (b) mean (±SEM) adjusted delay (seconds), and (c) mean (±SEM) number of total choices as a function of FR requirement. Divided bars show numbers of smaller, immediate reinforcer choices (white section) and larger, delayed reinforcer choices (black section). Data represent the means of 6 sessions obtained in the group of 8 monkeys, except at FR 96, for which only 6 of 8 monkeys were tested. Solid horizontal lines between bars indicate P < 0.05 and dashed lines indicate P < 0.01. *P < 0.05 and **P < 0.01 compared to FR 8. #P < 0.01 compared to smaller, immediate reinforcer choices.
Figure 2b shows the effects of increasing FR requirement on the MAD values. As FR requirement increased, MAD for the group of monkeys increased significantly [F (4, 39) = 11.27, P < 0.0001]. Compared to FR 8, the MAD was significantly higher at FR 64 (P < 0.05) and FR 96 (P < 0.01). A significant increase in MAD also occurred when the FR value was increased from FR 16 to FR 64 (P < 0.05) and to FR 96 (P < 0.01). Additionally, significant differences were found between FR 32 and FR 96 (P < 0.01) and FR 64 and FR 96 (P < 0.05). Figure 2c shows total numbers of choices made, separated into smaller, immediate and larger, delayed reinforcer choices. Generally, as FR requirement increased, smaller, immediate reinforcer choices decreased to a greater degree than larger, delayed reinforcer choices. A significant main effect was found for choice [F (1, 79) = 8.74, P < 0.001]. Post-hoc tests revealed significant differences between smaller, immediate and larger, delayed reinforcer choices at FR 8 (P < 0.01) and FR 16 (P < 0.01), but not at FR 32, FR 64 or FR 96. An overall significant effect of FR value was also found [F (4, 79) = 24.14, P < 0.001] indicating choices significantly decreased as FR requirement increased. Post-hoc analyses of smaller, immediate reinforcer choices indicated significant differences between FR 8 and FR 16 (P < 0.05), FR 32, FR 64, and FR 96 (P < 0.01). Significant differences were also found between FR 16 and FR 32 (P < 0.05), and between FR 64 and FR 96 (P < 0.01). Post-hoc analyses of larger, delayed reinforcer choices revealed significant differences between FR 8 and FR 64 (P < 0.05) and FR 96 (P < 0.05).
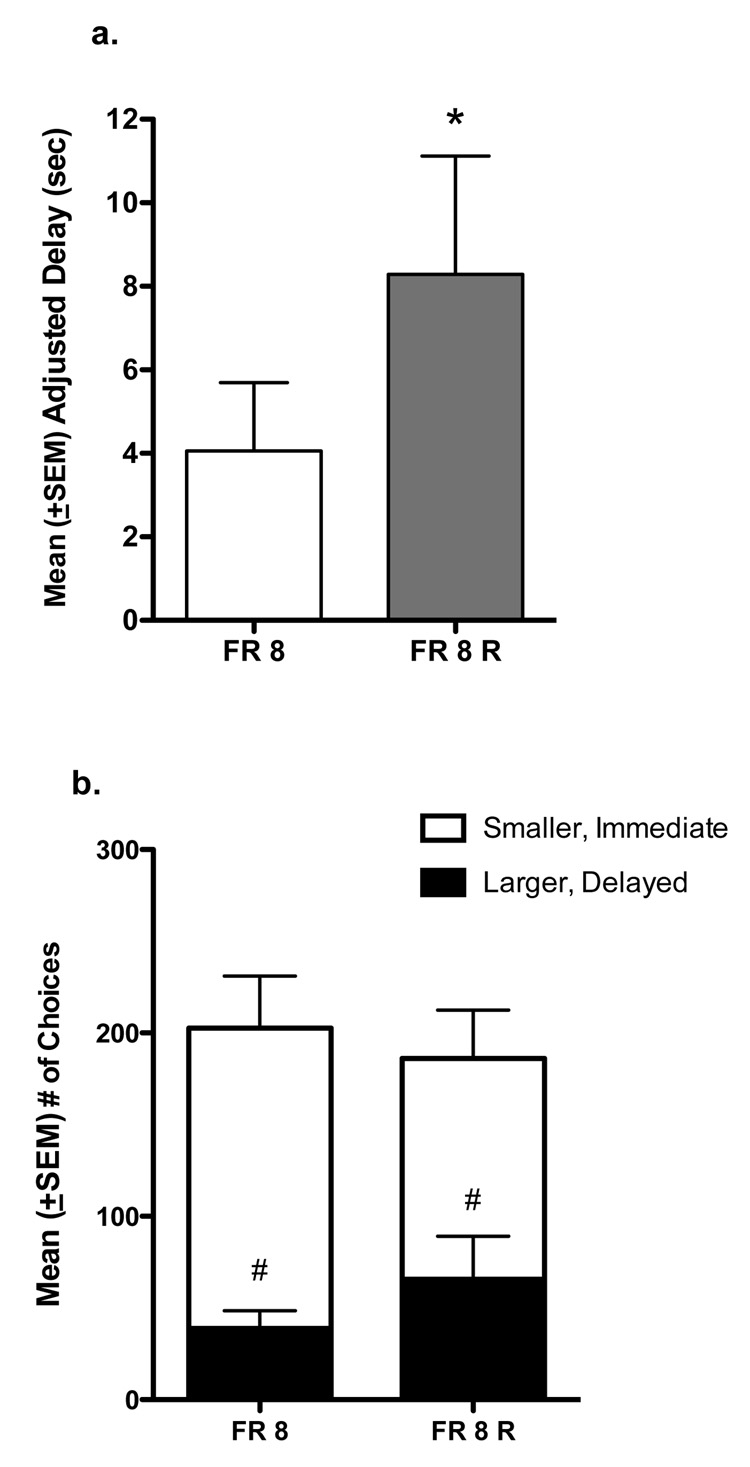
After assessing the change in FR requirement, MAD and choice were re-evaluated under the FR 8 schedule of reinforcement. Figure 3a shows that the MAD obtained at FR 8 following the FR requirement increase was significantly greater compared to the MAD obtained initially [t (7) = 2.71, P < 0.05)]. Figure 3b shows the total number of choices separated by small, immediate reinforcer choices and larger, delayed reinforcer choices. Choice distribution was similar between the initial FR 8 and the FR 8 retest conditions. A repeated measures two-way ANOVA revealed that there were no significant differences between the numbers of larger, delayed reinforcer choices and numbers of smaller, immediate reinforcer choices between the two FR 8 conditions [F (1, 31) = 0.26]. However, the numbers of smaller, immediate reinforcer choices was significantly greater than the numbers of larger, delayed reinforcer choices under both FR 8 conditions [F (1, 31) = 12.52, P < 0.01].
Figure 3.
(a) Mean (+SEM) adjusted delay (seconds) and (b) mean (+SEM) number of total choices under an FR 8 schedule of reinforcement before (FR 8) and after (FR 8 R) exposure to higher FR conditions. Data represents the mean of 6 sessions for the group of 8 monkeys. Divided bars show numbers of smaller, immediate reinforcer choices (white section) and larger, delayed reinforcer choices (black section). *P < 0.05 compared to FR 8; #P < 0.01 compared to smaller, immediate reinforcer choices.
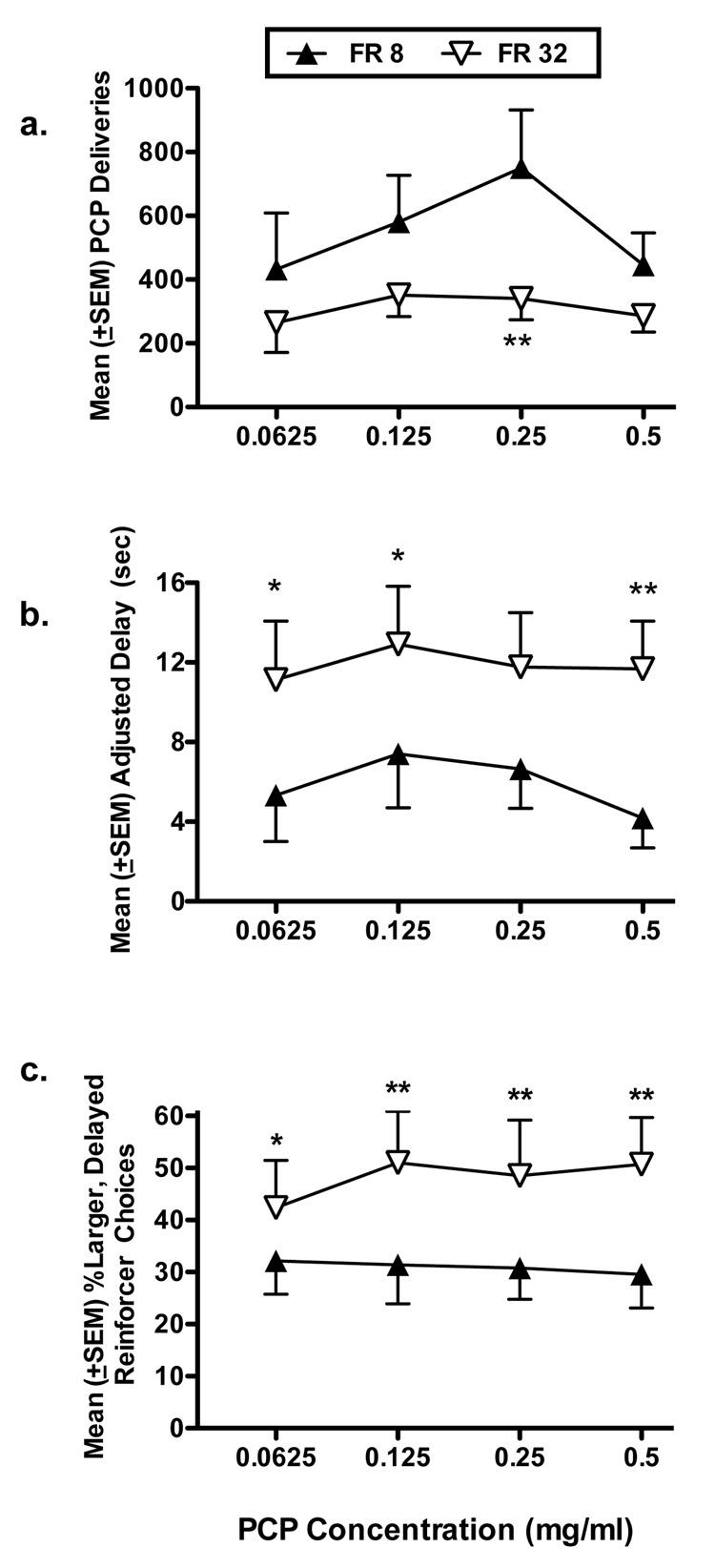
The concentration-response curve was re-examined at FR 32, with the larger delayed reinforcer consisting of 12 individual deliveries. Figure 4a shows the numbers of PCP deliveries obtained across PCP concentrations. Analyses indicate that the numbers of PCP deliveries obtained under FR 32 were significantly less than that obtained at FR 8 [F (1, 63) = 13.94, P < 0.01]. Post-hoc tests revealed that significantly fewer PCP deliveries were obtained under FR 32 than FR 8 when the 0.25 mg/ml concentration was available (P < 0.01). Figure 4b shows MAD across PCP concentrations. A significant effect of FR requirement was found for MAD values indicating that MAD increased under FR 32 [F (1, 63) = 19.33, P < 0.01]. Post-hoc tests indicated that MAD values obtained under FR 32 conditions were significantly greater than those obtained under the FR 8 condition when 0.0625 (P < 0.05), 0.125 (P < 0.05), and 0.5 mg/ml (P < 0.01) PCP concentrations were available. Although the shapes of the concentration-response curves were similar, increasing the FR requirement from 8 to 32 produced a nearly 2-fold increase in MAD at all PCP concentrations. When the percent choice for the delayed reinforcer was examined (Figure 4c), a significant effect of FR condition was observed [F (1, 63) = 15.16, P < 0.01] indicating that the percent of choice for the larger delayed reinforcer was higher at FR 32 than at FR 8. Post-hoc tests revealed significant increases in percent larger, delayed reinforcer choices at FR 32 (vs. FR 8) at 0.0625 (P < 0.05), 0.125 (P < 0.01), 0.25 (P < 0.01), and 0.5 mg/ml PCP (P < 0.01).
Figure 4.
(a) Mean (±SEM) number of PCP deliveries, (b) mean (±SEM) adjusted delay (seconds), and (c) mean (±SEM) percent choices made for the large, delayed reinforcer under FR 8 (filled symbols) and FR 32 (open symbols) as a function of PCP concentration (0.0625, 0.125, 0.25, and 0.5 mg/ml). Data represent the means of 5 sessions obtained in the group of 8 monkeys. *P < 0.05, ** P < 0.01.
4. Discussion
The findings of the present study demonstrate that monkeys discounted delayed self-administered oral deliveries of PCP using an adjusting delay procedure. The concentration-effect relationship for PCP deliveries was characterized by an inverted U-shaped curve, suggesting that self-administration of PCP under adjusting delay conditions was orderly and varied with PCP concentration as it has done under other schedules of reinforcement. In fact, the peak of the delivery concentration-effect functions (Fig. 1 and 4) was similar to that reported previously (Carroll and Meisch, 1980; Carroll and Stotz, 1984). However, the concentration-effect curves were less steep than typically found under conditions in which water is concurrently available under a simple FR schedule. Re-examining the PCP concentration-effect relationship confirmed that a higher FR requirement was more effective than changing reinforcer magnitude in shifting the MAD values.
Under most conditions, monkeys preferred the immediate reinforcer (a single delivery of PCP) to a larger, delayed reinforcer (access to a bundle of 6 or 12 deliveries). None of the monkeys exclusively chose one alternative over the other, as inferred by MAD values being above the minimum (0 s) and below the maximum (120 s) possible values. Temporal distribution of response allocation was not measured in these experiments, and the absence of response distribution data precludes analysis of response patterns. However, because the MAD values did not reach the highest or the lowest possible values, it can be inferred that the monkeys distributed their choices between the two alternatives. Other studies have shown that increasing the delay to larger reinforcers led to a decrease in choices for the larger, delayed reinforcer (and increased choice for the smaller, immediate reinforcer available without delay) (Woolverton et al., 2007). With the current procedure, one possibility is that the subjects consistently chose one alternative for a period and then switched to the other alternative, the end effect being that they repeatedly chose the large alternative until the delay became too high, then ‘drove down’ the delay by repeatedly choosing the small reinforcer. It should be noted that the monkeys did not ‘drive down’ the delay by consistently chose the small, immediate reinforcer (which would effectively eliminate the delay). Assuming that the monkeys were sensitive to the adjusting delay, this suggests the possibility that effort was allocated to maximize reinforcement. Examination of local response allocation would be necessary to support this hypothesis. Interestingly, results of other studies using the adjusting delay task have suggested such oscillating patterns of choice could occur (Cardinal et al., 2002). Such shifting between alternatives within session could explain the lack of a robust effect of reinforcer magnitude. Other data from our laboratory with monkeys self-administering different PCP concentrations under concurrent and independent progressive ratio schedules indicate that the monkeys’ response patterns oscillate from one spout to another over time to optimize the unit price (responses/mg). Thus, monkeys responded under a progressive ratio on the side with the higher concentration and then switched to the side with the lower concentration when the unit price on the higher side exceeded that on the lower side (Rodefer and Carroll, 1999). Although an intricate time analysis was beyond the scope of the current study, it is likely that the monkeys optimized their responding with regard to responses/mg and mg/s.
Examination of magnitude of reinforcer in delay discounting procedures has produced equivocal results. Magnitude of a delayed food reinforcer did not impact the rate of discounting in pigeons or rats (Green et al., 2004). Discounting of a delayed water reinforcer was not significantly related to the volume of the delayed reinforcer (Richards et al., 1997). Both of the aforementioned studies used an adjusting amount procedure. The adjusting amount procedure is similar to the adjusting delay procedure, but instead of controlling the delay to the larger, delayed reinforcer, subjects’ responses increase or decrease the amount of the immediate reinforcer. The adjusting amount procedure and the adjusting delay procedure produce similar estimates of delay discounting (Green et al., 2007). In a study using sucrose solutions as the reinforcer, indifference points varied as a function of sucrose concentration, and the relationship between indifference point and sucrose concentration assumed an inverted U-shaped curve (Farrar et al., 2003). Differences in the effects of reinforcer magnitude across studies may be related to experimental conditions, reinforcers, and species. Using an adjusting delay procedure in rats, the magnitude of cocaine reinforcers (i.e., dose of intravenous infusion), but not size of food reinforcer (i.e., number of pellets), affected rate of discounting (Perry et al., 2007). In studies using rhesus monkeys, increasing the dose of the delayed cocaine reinforcer led to a shift in preference from immediate to delayed reinforcers (Anderson and Woolverton, 2003; Woolverton et al., 2007). Thus, it is possible that procedural differences could account for the absence of a magnitude effect in the present study. A major procedural difference between the present study and others using drugs as reinforcers is that intravenous self-administration allows different magnitudes (dose) of the drug reinforcer to be delivered based upon completion of one response requirement; whereas, the oral self-administration procedure required added effort of lip contact responses to obtain each 0.6 ml delivery of the drug, and the subsequent swallowing response. Thus, additional time and effort differentiate the oral method from the intravenous method. Moreover, manipulation of PCP concentration could be affected by gustatory factors that vary with concentration, as higher concentrations of PCP may have an aversive taste.
In Experiment 2, increasing the work requirement, or FR value, necessary to obtain the reinforcer resulted in a systematic change in the MAD, and a decrease in the numbers of reinforcers. However, the increase in MAD could be attributed to the proportionally greater decrease in number of smaller, immediate reinforcer choices compared to the decrease in the number of larger, delayed reinforcer choices. This indicates that the number of choices for the larger, delayed reinforcer did not increase with increases in FR value, rather the number of choices for the small, immediate reinforcer decreased. This finding suggests that preference for the smaller, immediate reinforcer was more sensitive to increasing cost than the larger, delayed reinforcer. It should be noted that under the present conditions, monkeys were free to make as many choices as they could during the 3-hr session; that is, the choices were not restricted by a set number of trials. The free-operant choice used in the present study may have affected the allocation of choices. For example, if the number of trials were limited, the monkeys’ behavior may have adapted to these constraints such that more economical choices would have been made.
Despite the increase in magnitude of the delayed reinforcer (i.e., 1 v 6 or 1 v 12), the number of choices made for the immediate alternative significantly exceeded the number of choices for the larger alternative until high FR values were reached. The response output required to obtain the immediate reinforcer outweighed the overall response output necessary for obtaining the larger delayed reinforcer and behavior was not dictated by the unit price of the PCP reinforcer, except when the FR requirement for both alternatives was very high (e.g., FR 96). Thus, the relative contributions of cost and benefit to the unit price were not equal, suggesting that these findings cannot be accounted for by traditional economic theory. Further research is needed to explore the behavioral economics underlying delay discounting using adjusting procedures and other FR requirements.
An important consideration when interpreting the findings from Experiment 2 is that the time that it took to complete the FR requirement imposed an additional delay, an effect that would necessarily increase as the FR requirement increased. It has been suggested that the actual number of responses is not as important a determinant as the time it takes to complete it (Killeen, 1969). In procedures using smaller-sooner vs later-larger comparisons, subjects preferred the smaller-sooner alternative when the delay was short, but as the delay to the smaller-sooner reinforcer increased, preference switched to the later-larger alternative even though the difference in timing between the two alternatives was held constant (Ainslie and Herrnstein, 1981; Kirby and Herrnstein, 1995). Accordingly, the increase in time required to complete the FR also increased the time between the trial beginning and delivery of the reinforcer. Thus, relative delay is likely as important as the nominal delay in determining choice.
In the present study, it was demonstrated that experience with increasing FR requirements produced a decrease in discounting (increase in MAD) that persisted when the FR 8 requirement was reinstated. The numbers of choices for the larger, delayed alternative increased, but the effect was not statistically significant. As observed in the other experiments in this study, MAD did not necessarily reflect choice allocation. This discrepancy between MAD and numbers of choices may reflect the differences between the two types of measures, that is, choice was a discrete measure, whereas MAD was a continuous measure. This could lead to a higher MAD as its value was based on the proportion of selections, and could account for the discrepancy between MAD and number of choices. The monkeys used in this study had previous self-administration experience with various PCP concentrations and FR requirements, and generally when a within subjects ‘ABA’ design is employed, a return to baseline levels is observed. However, persistent changes in behavioral effects have been observed following training procedures that require high levels of responding, such as a second-order schedule (Carroll, 1984), and escalation of PCP self-administration during 6-hr sessions (Carroll et al., 2005). Based on these previous findings, the present findings likely reflect an enduring effect of training on higher FR requirements. Findings from other studies indicate that behavioral history can alter impulsivity. Studies using laboratory animals and human subjects have shown that training can lead to increased tolerance to the delays to reinforcement. Mazur and Logue (1978) found that by using a fading procedure to gradually reduce a delay imposed between a response and the delivery of a reinforcer over a history of 11,000 trials, pigeons maintained a preference for the larger, delayed reinforcer instead of switching to the immediately available smaller, immediate reinforcer. Behavioral modifications have also been effective in engendering tolerance to delay of reinforcement in human subjects (Dixon et al., 2003; Schweitzer and Sulzer-Azaroff, 1988).
In addition to schedule of reinforcement and training procedures, delay discounting and impulsive choice are sensitive to pharmacological manipulations. Wolff and Leander (2002) demonstrated that pigeons trained on the adjusting delay task showed increases in adjusted delays (preference for the larger, delayed reinforcer) when chronically treated with selective serotonin reuptake inhibitors, and decreases in adjusted delays when treated with benzodiazepine agonists. Acute treatment with stimulants including methamphetamine (Richards et al., 1999a), amphetamine (Wade et al., 2000; Winstanley et al., 2003), and methylphenidate (van Gaalen et al., 2006) reduced impulsivity; however, others have shown that cocaine and amphetamine increased discounting and impulsive choice (Evenden and Ryan, 1996; Helms et al., 2006; Logue et al., 1992). Other drugs such as ethanol (Evenden and Ryan, 1999; Ortner et al., 2003; Poulos et al., 1998; Richards et al., 1999b) and benzodiazepines (Cardinal et al., 2000; Evenden and Ryan, 1996) have produced varying effects on delay discounting tasks.
Studies to date have generally used nondrug reinforcers to study impulsive behavior defined by delay discounting as well as other measures. Few studies have examined delay discounting of drug reinforcers. Importantly, one should consider the pharmacological effects of the drug reinforcer as they may pertain to measuring impulsivity. As described above, drugs from various pharmacological classes affect delay discounting for nondrug reinforcers, and a behaviorally active dose of the self-administered drug would thus be expected to alter impulsivity of behavior maintained by that drug. PCP, a glutamate antagonist at the NMDA receptor channel, has been shown to disrupt various cognitive tasks. Repeated administration PCP has been shown to impair performance on a five-choice serial reaction time task (Amitai et al., 2007), which provides a measure of cognitive impulsivity. Previous research has shown that cognitive deficits reflective of impaired behavioral inhibition can be produced by PCP administered on a subchronic basis in rats (Jentsch and Taylor, 2001) and in nonhuman primates (Jentsch et al., 2000), and these deficits could affect impusive behavior. Additional research is necessary to determine whether monkeys with histories of PCP self-administration discount drug and nondrug reinforcers differently than monkeys without histories of PCP self-administration.
In summary, the present series of experiments demonstrate that under a delay discounting task, PCP self-administration can be described as a function of concentration available. However, the rate of discounting was not robustly affected as a function of either PCP concentration or the size of bundled reinforcer delivery. The rate of discounting, and allocation of choices appeared to be affected by the amount of effort required to produce the reinforcer.
Acknowledgements
This research was supported by National Institute on Drug Abuse grant R01 DA02486, K05 DA15267 (MEC), and T32 DA0709 (JLN). The authors wish to thank Alayna Fogal, Jami Mach, Rachel LaNasa, and Jenny Falor for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainslie G, Herrnstein RJ. Preference reversal and delayed reinforcement. Animal Learning and Behavior. 1981;9:476–482. [Google Scholar]
- Amitai N, Semenova S, Markou A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology. 2007;193:521–537. doi: 10.1007/s00213-007-0808-x. [DOI] [PubMed] [Google Scholar]
- Anderson KG, Woolverton WL. Effects of dose and infusion delay on cocaine self-administration choice in rhesus monkeys. Psychopharmacology. 2003;167:424–430. doi: 10.1007/s00213-003-1435-9. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, Hughes JR. Behavioral economics of drug self-administration. I. Functional equivalence of response requirement and drug dose. Life sciences. 1990;47:1501–1510. doi: 10.1016/0024-3205(90)90178-t. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Daw N, Robbins TW, Everitt BJ. Local analysis of behaviour in the adjusting-delay task for assessing choice of delayed reinforcement. Neural Netw. 2002;15:617–634. doi: 10.1016/s0893-6080(02)00053-9. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology. 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Carroll ME. The effect of second-order schedule history on fixed-ratio performance maintained by orally-delivered phencyclidine in rhesus monkeys. Pharmacology, biochemistry, and behavior. 1984;20:779–787. doi: 10.1016/0091-3057(84)90199-0. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Mach JL, Newman JL, Perry JL. Delay discounting as a predictor of drug abuse. In: Madden GJ, CT, Bickel WK, editors. Impulsivity: Theory, Science, and Neuroscience of Discounting. Washington, DC.: American Psychological Association; 2008. [Google Scholar]
- Carroll ME, Batulis DK, Landry KL, Morgan AD. Sex differences in the escalation of oral phencyclidine (PCP) self-administration under FR and PR schedules in rhesus monkeys. Psychopharmacology. 2005;180:414–426. doi: 10.1007/s00213-005-2182-x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Bickel WK, Higgins ST. Nondrug Incentives to Treat Drug Abuse: Laboratory and Clinical Developments. In: Overmier JB, editor. Animal Research and Human Health. Washington D.C.: American Psychological Association; 2001. pp. 139–154. [Google Scholar]
- Carroll ME, Campbell UC. A behavioral economic analysis of the reinforcing effects of drugs: transition states of addiction. In: BWaV R, editor. Reframing Health Behavior Change with Behavioral Economics. New Jersey: Lawrence Erlbaum Associates, Inc; 2000. pp. 63–87. [Google Scholar]
- Carroll ME, Meisch RA. Oral phencyclidine (PCP) self-administration in rhesus monkeys: effects of feeding conditions. The Journal of pharmacology and experimental therapeutics. 1980;214:339–346. [PubMed] [Google Scholar]
- Carroll ME, Stotz DC. Increased phencyclidine self-administration due to food deprivation: interaction with concentration and training conditions. Psychopharmacology. 1984;84:299–303. doi: 10.1007/BF00555202. [DOI] [PubMed] [Google Scholar]
- Chapman GB. Temporal discounting and utility for health and money. Journal of experimental psychology. 1996;22:771–791. doi: 10.1037//0278-7393.22.3.771. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Experimental and clinical psychopharmacology. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Dixon MR, Rehfeldt RA, Randich L. Enhancing tolerance to delayed reinforcers: the role of intervening activities. Journal of applied behavior analysis. 2003;36:263–266. doi: 10.1901/jaba.2003.36-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1999;146:413–421. doi: 10.1007/pl00005486. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Kieres AK, Hausknecht KA, de Wit H, Richards JB. Effects of reinforcer magnitude on an animal model of impulsive behavior. Behav Processes. 2003;64:261–271. doi: 10.1016/s0376-6357(03)00139-6. [DOI] [PubMed] [Google Scholar]
- Galuska CM, Winger G, Hursh SR, Woods JH. Assessing unit-price related remifentanil choice in rhesus monkeys. Journal of the experimental analysis of behavior. 2006;86:181–195. doi: 10.1901/jeab.2006.108.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Holt DD, Slevin JR, Estle SJ. Discounting of delayed food rewards in pigeons and rats: is there a magnitude effect? Journal of the experimental analysis of behavior. 2004;81:39–50. doi: 10.1901/jeab.2004.81-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Shah AK, Estle SJ, Holt DD. Do adjusting-amount and adjusting-delay procedures produce equivalent estimates of subjective value in pigeons? Journal of the experimental analysis of behavior. 2007;87:337–347. doi: 10.1901/jeab.2007.37-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Reeves JM, Mitchell SH. Impact of strain and D-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology. 2006;188:144–151. doi: 10.1007/s00213-006-0478-0. [DOI] [PubMed] [Google Scholar]
- Iglauer C, Woods JH. Concurrent performances: reinforcement by different doses of intravenous cocaine in rhesus monkeys. Journal of the experimental analysis of behavior. 1974;22:179–196. doi: 10.1901/jeab.1974.22-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH, Taylor JR. Object retrieval/detour deficits in monkeys produced by prior subchronic phencyclidine administration: evidence for cognitive impulsivity. Biological psychiatry. 2000;48:415–424. doi: 10.1016/s0006-3223(00)00926-4. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impaired inhibition of conditioned responses produced by subchronic administration of phencyclidine to rats. Neuropsychopharmacology. 2001;24:66–74. doi: 10.1016/S0893-133X(00)00174-3. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. A choice procedure for drug reinforcers: cocaine and methylphenidate in the rhesus monkey. The Journal of pharmacology and experimental therapeutics. 1975;193:676–688. [PubMed] [Google Scholar]
- Killeen P. Reinforcement frequency and contingency as factors in fixed-ratio behavior. Journal of the experimental analysis of behavior. 1969;12:391–395. doi: 10.1901/jeab.1969.12-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Herrnstein RJ. Preference reversals due to myopic discounting of delayed reward. Psychological Science. 1995;6:83–89. [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction (Abingdon, England) 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Logue AW, Tobin H, Chelonis JJ, Wang RY, Geary N, Schachter S. Cocaine decreases self-control in rats: a preliminary report. Psychopharmacology. 1992;109:245–247. doi: 10.1007/BF02245509. [DOI] [PubMed] [Google Scholar]
- Macenski MJ, Meisch RA. Ratio size and cocaine concentration effects on oral cocaine-reinforced behavior. Journal of the experimental analysis of behavior. 1998;70:185–201. doi: 10.1901/jeab.1998.70-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Begotka AM, Raiff BR, Kastern LL. Delay discounting of real and hypothetical rewards. Experimental and clinical psychopharmacology. 2003;11:139–145. doi: 10.1037/1064-1297.11.2.139. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Experimental and clinical psychopharmacology. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An Adjusting Procedure for Studying Delayed Reinforcement. In: Commons M, Mazur J, Nevin J, Rachlin H, editors. Qualitative Analyses of Behavior: The Effect of Delay of Intervening Events on Reinforcement Value. Hillsdale, NJ: Erlbaum; 1987. pp. 55–73. [Google Scholar]
- Mazur JE, Logue AW. Choice in a "self-control"paradigm: effects of a fading procedure. Journal of the experimental analysis of behavior. 1978;30:11–17. doi: 10.1901/jeab.1978.30-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisch RA, Lemaire GA. Oral self-administration of pentobarbital by rhesus monkeys: maintenance of behavior by different concurrently available volumes of drug solution. Journal of the experimental analysis of behavior. 1989;52:111–126. doi: 10.1901/jeab.1989.52-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH, Reeves JM, Li N, Phillips TJ. Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcoholism, clinical and experimental research. 2006;30:429–437. doi: 10.1111/j.1530-0277.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- Nader MA, Hedeker D, Woolverton WL. Behavioral economics and drug choice: effects of unit price on cocaine self-administration by monkeys. Drug and alcohol dependence. 1993;33:193–199. doi: 10.1016/0376-8716(93)90060-4. [DOI] [PubMed] [Google Scholar]
- Newman JL, Perry JL, Carroll ME. Social stimuli enhance phencyclidine (PCP) self-administration in rhesus monkeys. Pharmacology, biochemistry, and behavior. 2007;87:280–288. doi: 10.1016/j.pbb.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortner CN, MacDonald TK, Olmstead MC. Alcohol intoxication reduces impulsivity in the delay-discounting paradigm. Alcohol and alcoholism (Oxford, Oxfordshire) 2003;38:151–156. doi: 10.1093/alcalc/agg041. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008 doi: 10.1007/s00213-008-1173-0. In press. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacology, biochemistry, and behavior. 2007;86:822–837. doi: 10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Normile LM, Morgan AD, Carroll ME. Sex differences in physical dependence on orally self-administered phencyclidine (PCP) in rhesus monkeys (Macaca mulatta) Experimental and clinical psychopharmacology. 2006;14:68–78. doi: 10.1037/1064-1297.14.1.68. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM. Discounting of money, health, and freedom in substance abusers and controls. Drug and alcohol dependence. 2003;71:133–141. doi: 10.1016/s0376-8716(03)00090-5. [DOI] [PubMed] [Google Scholar]
- Petry NM. Early-onset alcoholism: a separate or unique predictor of delay discounting? Comment on Dom et al. (2006) Addiction (Abingdon, England) 2006;101:292. doi: 10.1111/j.1360-0443.2005.01307.x. author reply 293–294. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Parker JL, Le DA. Increased impulsivity after injected alcohol predicts later alcohol consumption in rats: evidence for "loss-of-control drinking"and marked individual differences. Behavioral neuroscience. 1998;112:1247–1257. doi: 10.1037//0735-7044.112.5.1247. [DOI] [PubMed] [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, Seiden LS. Determination of discount functions in rats with an adjusting-amount procedure. Journal of the experimental analysis of behavior. 1997;67:353–366. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology. 1999a;146:432–439. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. Journal of the experimental analysis of behavior. 1999b;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodefer JS, Carroll ME. Concurrent progressive-ratio schedules to compare reinforcing effectiveness of different phencyclidine (PCP) concentrations in rhesus monkeys. Psychopharmacology. 1999;144:163–174. doi: 10.1007/s002130050990. [DOI] [PubMed] [Google Scholar]
- Schweitzer JB, Sulzer-Azaroff B. Self-control: teaching tolerance for delay in impulsive children. Journal of the experimental analysis of behavior. 1988;50:173–186. doi: 10.1901/jeab.1988.50-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RB, Wang NS, Bass AA, Meisch RA. Relative reinforcing effects of different oral ethanol doses in rhesus monkeys. Journal of the experimental analysis of behavior. 2002;77:49–64. doi: 10.1901/jeab.2002.77-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biological psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology. 2003;170:320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Wolff MC, Leander JD. Selective serotonin reuptake inhibitors decrease impulsive behavior as measured by an adjusting delay procedure in the pigeon. Neuropsychopharmacology. 2002;27:421–429. doi: 10.1016/S0893-133X(02)00307-X. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Myerson J, Green L. Delay discounting of cocaine by rhesus monkeys. Experimental and clinical psychopharmacology. 2007;15:238–244. doi: 10.1037/1064-1297.15.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]