Abstract
Background
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a newly identified neurodegenerative disorder due to intermediate expansion of trinucleotide CGG repeats (55 – 200 repeats) in the 5′ untranslated region (UTR) of the Fragile X mental retardation 1 (FMR1) gene. FXTAS is now considered to be one of the most common inherited neurodegenerative disorders in males.
Objective
To examine the future of potential therapies for this late-onset disease.
Methods
Examination of relevent literature.
Results/conclusions
Accumulating evidence indicates that overproduced riboCGG repeats in the 5′ UTR of FMR1 mRNA are toxic. Recently, proteins that bind specifically to rCGG repeats were identified. Progress in understanding the molecular pathogenesis of FXTAS, plus the availability of different animal models are discussed.
Keywords: animal models, CUGBP1, FXTAS, hnRNP A2/B1, non-coding RNA, Pur α, rCGG repeats, RNA toxicity, trinucleotide repeats
1. Introduction
Expansion of trinucleotide repeats is the cause of many heritable human diseases such as Fragile X syndrome and Huntington’s disease [1–3]. Expansion of trinucleotide repeats could occur in the exon region of a gene (for example Huntington’s disease). This kind of expansion results in the generation of polypeptides with a poly-glutamine or poly-alanine stretch, which has been shown to be toxic [2–5]. In Fragile X syndrome, the trinucleotide expansion occurs in the 5′ untranslated region (UTR), resulting in hypermethylation of the promoter region, and eventually, shutting down the transcription of the Fragile X mental retardation 1 (FMR1) gene [6]. In the normal population, the CGG repeat number in the 5′ UTR of FMR1 gene is between 5 and 54, and an expansion to 200 or higher (full mutation) leads to Fragile X syndrome. Repeat numbers between 55 and 200 are called ‘premutation’. Premutation was previously considered not to directly cause any human disorder, and only to pose an increased risk of expanding into a full mutation when passed to the next generation. Recently neurologists have started recognizing unique neurological symptoms that are associated with Fragile X premutation carriers, which are distinguished from Fragile X syndrome [7–11]. This newly identified disorder is named Fragile X-associated tremor/ataxia syndrome (FXTAS). Major symptoms of this disorder include intention or postural (action) tremor, cerebellar gait and limb ataxia and Parkinsonism [7–11]. Neurodegenerative features, such as intracellular inclusion bodies, have been identified from neuroimaging and postmortem brain tissue [12–14].
It has been known that FMR1 mRNA level is elevated 5 – 10 times in premutation carriers, while the FMR protein (FMRP) level is about normal [15]. This phenomenon leads us and others to argue that the excess of FMR1 mRNA with expanded CGG repeats in its 5′ UTR is toxic. Indeed riboCGG (rCGG) repeats have been shown to be toxic in human neural cell culture as well as in transgenic flies [16,17]. In fly model of FXTAS, non-coding rCGG repeats have been further shown to cause the formation of inclusion bodies and lead to neurodegeneration, cellular hallmarks of FXTAS in patients [17]. Emerging evidence suggests that the RNA-mediated gain-of-function mechanism could underlie several additional inherited diseases, including myotonic dystrophy type 1 and 2 (DM1 and DM2), spinocerebellar ataxia type 8 (SCA8), and Huntington’s disease-like syndrome 2 (HDL2) [18].
As a new and possibly currently under-diagnosed disease, FXTAS is considered to be one of the most common heritable neurodegenerative disorders in males: 1 in 813 males in the general population are carriers of an FMR1 premutation with about 40% penetrance for males aged > 50 years [19]. Therefore, there is a great need to develop therapies to alleviate and cure this disease.
2. Perspective
Currently, there is no specific treatment for FXTAS to target the underlying pathological mechanism. Treatments to relieve specific symptoms are the main medical practice at present [19,20].
Comprehensive research into the molecular mechanism of FXTAS is needed to aid the development of FXTAS therapies. This review concentrates on current understanding of molecular pathology and construction of animal models of FXTAS. How advances in these areas may aid the development of future therapy will then be discussed.
Recently, two studies published by this laboratory and others have advanced our understanding of the pathogenesis of FXTAS one step further by identifying proteins interacting specifically with rCGG repeats [21,22]. Using biochemical and genetic approaches, three proteins, purine-rich element containing-protein α(Purα), heterogeneous nuclear ribonucleoprotein (hnRNP) A2/B1 (two protein isoforms from one gene), and CUG vbinding protein 1(CUGBP1) were found to bind rCGG repeats either directly (Pur α and hnRNP A2/B1) or indirectly (CUGBP1, through interaction with hnRNA A2/B1) (Figure 1)[21,22]. Thus, there are protein complexes physically interacting with rCGG repeats. All these proteins are RNA-binding proteins, and have been shown to function in transcription, mRNA trafficking, splicing and translation. Interestingly, Pur α knockout mice appeared normal at birth, but developed severe tremors and spontaneous seizures at 2 weeks of age due to markedly reduced numbers of neurons in regions of the hippocampus and cerebellum [23]. The hypothesis is that over-produced rCGG repeats in FXTAS sequester these proteins from their normal cellular functions, contributing significantly to the pathology of this disorder. This idea is strongly supported by the fact that overexpressing either Pur α or hnRNA A2/B1 alleviated neurodegeneration in a fly model of FXTAS [21,22]. These results provide direct insights into the development of future therapies. Presumably, drugs that specifically suppress the interaction between the rCGG repeats and the protein complexes would release these proteins from this requisition. Compounds boosting the expression or function of these proteins may also be found to be useful. Identifying further downstream RNA targets of these proteins is also crucial, and specific therapies based on their affected RNA targets in FXTAS may then be explored.
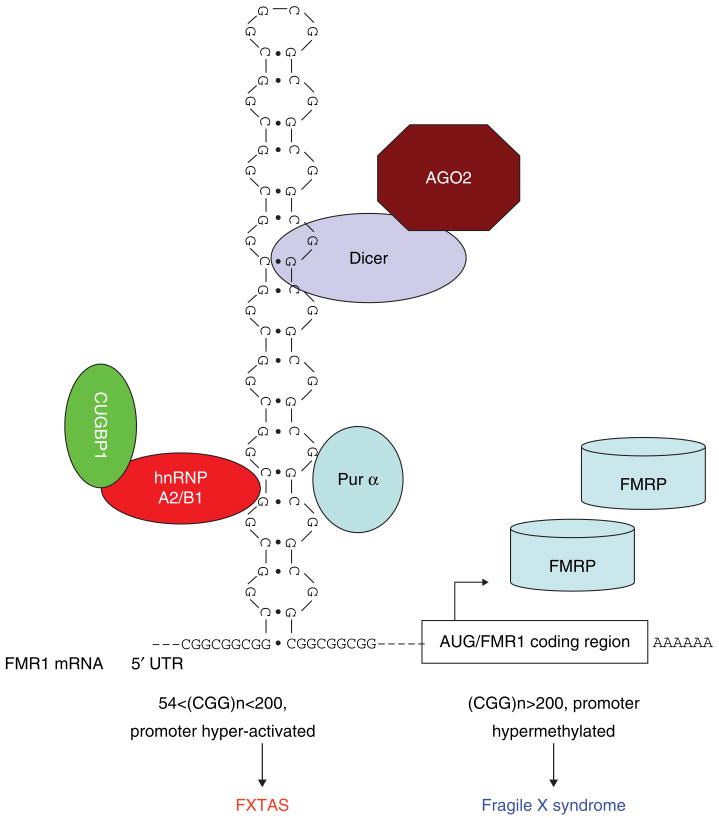
Figure 1. The normal human FMR1 gene has a CGG repeat size of between 5 and 54.
A CGG repeat size > 200 triggers hypermethylation of the FMR1 promoter and leads to the shutdown of transcription, which results in FRAXA in males. When CGG repeat size is between 55 and 200, the promoter is hyper-activated, leading to overproduction of FMR1 mRNA with an expanded 5′UTR with toxic riboCGG repeats, while the FMRP level remains about normal. The rCGG repeats can form a hairpin structure, and can be processed by Dicer and the RNAi machinery, including AGO2. Recently, Purα, CUGBP1, and hnRNP A2/B1 were shown to bind specifically to rCGG repeats. Sequestrations of these proteins by rCGG repeats has been shown to underlie the molecular pathology of FXTAS.
AGO2, Argonaute 2; CUGBP1, CUG-binding protein 1; FMR1, Fragile X mental retardation 1; FRAXA, Fragile X syndrome; FRMP, FMR protein;
FXTAS, Fragile X-associated tremor/ataxia syndrome; hnRNP, Heterogeneous nuclear ribonucleoprotein; Purα, Purine-rich element containing-protein alpha; RNAi, RNA interference; UTR, Untranslated region.
rCGG repeats form, at least in vitro, double-stranded RNA hairpins (Figure 1), which is a structural feature shared by most trinucleotide repeats [24]. This structure resembles the hairpin of microRNA precursors, and indeed has been shown to be processed by Dicer, core component of the RNA interference (RNAi) machinery [24]. Interestingly, crossing rCGG and rGCC, two complementary repeats each pathogenic by itself, into transgenic flies rescued phenotypes generated by each individual repeat [25]. This rescue relies on Argonaute 2 (AGO2), another core component of the RNAi machinery [25]. RNAi is now under enthusiastic pursuit as a great hope for therapies for many varieties of diseases [26]. Approaches to promote the degradation of rCGG repeats by the RNAi machinery would provide a new direction in exploring therapies of FXTAS. Small-interfering (si)GCC RNA oligonucleotides, constructs or viruses that could generate siGCC in cells may be applied to treat FXTAS. Compounds enhancing the processing of rCGG hairpins by RNAi machinery may be identified and found to be beneficial. It is also crucial to investigate whether the binding of Purα, hnRNP A2/B1 and CUGBP1 to rCGG repeats depends on the hairpin structure. If the answer is positive, compounds that disrupt the rCGG hairpins would release the sequestering of these proteins, another possibility for attacking this disease. A caution that must be kept in mind is the possibility of side effects from these kinds of compounds. Careful research need to be performed to make sure each drug is rCGG repeats-specific.
3. Expert opinion
The concept that non-coding repeats in certain mRNAs can be pathogenic is relatively new. The etiology of FXTAS has now been traced to a toxic mRNA sequence. An ideal therapy for this disease would be one to neutralize or reduce significantly the presence of the toxic rCGG repeats, while keeping the FMRP level above the minimal requirement. Presently, this is not close to realization.
Animal models of FXTAS should give strong support to the development of specific therapies. By using the FXTAS fly model, we have already revealed and will continue to uncover aspects of the pathology of the rCGG repeats. This model can also be used directly in drug discovery. Currently, our laboratory is screening the library of small molecules using the FXTAS fly model (P Jin, unpublished data). Chemical compounds identified from these screens can be further investigated, and, there will still be a long wait before one, if any, of them eventually becomes a therapeutic drug. By identifying compounds known to target specific a biochemical pathway, this chemical biology approach could also give us indications regarding the pathology of rCGG repeats. To aid these efforts, we are constructing another invertebrate model of FXTAS, which expresses rCGG repeats in the nematode worm, Caenorhabditis elegans (G Shan and P Jin, unpublished). The C. elegans genome does encode an obvious FMR1 homologue. In this model, the pathology of rCGG repeats can be investigated independently without considering the role of FMRP. A mouse model of FXTAS is also available, in which both elevated Fmr1 mRNA levels and intranuclear inclusions were detected in neurons; both the number and the size of the inclusions were increased during the aging process, which correlates with the progressive character of FXTAS in humans [27]. Although all these models may not be ideal in terms of modeling the human phenotypes, they would complement each other and provide interfaces to work with this disease at the current stage.
Many features of FXTAS are still obscure. Like a lot of other neurodegenerative diseases, FXTAS is late onset. The pathology in the context of aging requires more investigation. Better understanding of this feature will provide new angle for therapy development and help to delay and maybe ease the symptoms. We are still unclear about how the toxic rCGG repeats lead to neural cell death. How rCGG repeats trigger the formation of the inclusion bodies in neurons and astrocytes, and neural cell loss in FXTAS patients requires more investigation. Understanding of these mechanisms would shed new light on future therapy development. Preventing the formation of the inclusion bodies and cell death will certainly offer another tactic for disease treatment.
More effort is in need to decode FXTAS, and hopefully excellent treatments and even a cure for this disease will emerge in the near future.
Acknowledgments
P Jin is supported by NIH grants R01 NS051630 and R01 MH076090 and is the recipient of a Beckman Young Investigator Award and a Basil O’Connor Scholar Research Award and is an Alfred P Sloan Research Fellow in Neuroscience.
Footnotes
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as of interest(•) to readers.
- 1.Mirkin SM. Expandable DNA repeats and human disease. Nature. 2007;447:932–40. doi: 10.1038/nature05977. [DOI] [PubMed] [Google Scholar]
- 2.•.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Ann Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. Most recent review on trinucleotide repeat disorders. [DOI] [PubMed] [Google Scholar]
- 3.Nithianantharajah J, Hannan AJ. Dynamic mutations as digital genetic modulators of brain development, function and dysfunction. Bioessays. 2007;29:525–35. doi: 10.1002/bies.20589. [DOI] [PubMed] [Google Scholar]
- 4.Riley BE, Orr HT. Polyglutamine neurodegenerative diseases and regulation of transcription: assembling the puzzle. Genes Dev. 2006;20:2183–92. doi: 10.1101/gad.1436506. [DOI] [PubMed] [Google Scholar]
- 5.Albrecht A, Mundlos S. The other trinucleotide repeat: polyalanine expansion disorders. Curr Opin Genet Dev. 2005;15:285–93. doi: 10.1016/j.gde.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Warren ST. The expanding world of trinucleotide repeats. Science. 1996;271:1374–5. doi: 10.1126/science.271.5254.1374. [DOI] [PubMed] [Google Scholar]
- 7.•.Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–30. doi: 10.1212/wnl.57.1.127. First paper to recognize FXTAS. [DOI] [PubMed] [Google Scholar]
- 8.Jacquemont S, Hagerman RJ, Hagerman PJ, et al. Fragile-X syndrome and fragile X-associated tremor/ataxia syndrome: two faces of FMR1. Lancet Neurol. 2007;6:45–55. doi: 10.1016/S1474-4422(06)70676-7. [DOI] [PubMed] [Google Scholar]
- 9.Hagerman RJ, Ono MY, Hagerman PJ. Recent advances in fragile X: a model for autism and neurodegeneration. Curr Opin Psychiatry. 2005;18:490–6. doi: 10.1097/01.yco.0000179485.39520.b0. [DOI] [PubMed] [Google Scholar]
- 10.Willemsen R, Mientjes E, Oostra BA. FXTAS: a progressive neurologic syndrome associated with Fragile X premutation. Curr Neurol Neurosci Rep. 2005;5:405–10. doi: 10.1007/s11910-005-0065-5. [DOI] [PubMed] [Google Scholar]
- 11.Baba Y, Uitti RJ. Fragile X-associated tremor/ataxia syndrome and movements disorders. Curr Opin Neurol. 2005;18:393–8. doi: 10.1097/01.wco.0000168332.99305.50. [DOI] [PubMed] [Google Scholar]
- 12.•.Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–78. doi: 10.1086/374321. First paper to summarize the phenotype and frequency associated FXTAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen S, Masyn K, Adams J, et al. Molecular and imaging correlates of the fragile X-associated tremor/ataxia syndrome. Neurology. 2006;67:1426–31. doi: 10.1212/01.wnl.0000239837.57475.3a. [DOI] [PubMed] [Google Scholar]
- 14.•.Greco CM, Hagerman RJ, Tassone F, et al. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–71. doi: 10.1093/brain/awf184. First paper to report the presence of inclusions in FXTAS brain tissues. [DOI] [PubMed] [Google Scholar]
- 15.Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001;10:1449–54. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- 16.Arocena DG, Iwahashi CK, Won N, et al. Induction of inclusion formation and disruption of lamin A/C structure by premutation CGG-repeat RNA in human cultured neural cells. Hum Mol Genet. 2005;14:3661–71. doi: 10.1093/hmg/ddi394. [DOI] [PubMed] [Google Scholar]
- 17.•.Jin P, Zarnescu DC, Zhang F, et al. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in Drosophila. Neuron. 2003;39:739–47. doi: 10.1016/s0896-6273(03)00533-6. First paper to report the fly model of FXTAS and demonstrate that rCGG alone is sufficient to cause neuronal toxicity. [DOI] [PubMed] [Google Scholar]
- 18.Ranum LP, Cooper TA. RNA-mediated neuromuscular disorders. Ann Rev Neurosci. 2006;29:259–77. doi: 10.1146/annurev.neuro.29.051605.113014. [DOI] [PubMed] [Google Scholar]
- 19.Berry-Kravis E, Abrams L, Coffey SM, et al. Fragile X-associated tremor/ataxia syndrome: clinical features, genetics, and testing guidelines. Mov Disord. 2007;22:2018–30. doi: 10.1002/mds.21493. [DOI] [PubMed] [Google Scholar]
- 20.Hagerman PJ, Hagerman RJ. Fragile X-associated tremor/ataxia syndrome – an older face of the fragile X gene. Nat Clin Pract Neurol. 2007;3:107–12. doi: 10.1038/ncpneuro0373. [DOI] [PubMed] [Google Scholar]
- 21.•.Sofola OA, Jin P, Qin Y, et al. RNA-binding proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat-induced neurodegeneration in a Drosophila model of FXTAS. Neuron. 2007;55:565–71. doi: 10.1016/j.neuron.2007.07.021. This and ref. [22] are the first two papers to report the identification of specific rCGG-binding proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.•.Jin P, Duan R, Qurashi A, et al. Pur α binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile X tremor/ataxia syndrome. Neuron. 2007;55:556–64. doi: 10.1016/j.neuron.2007.07.020. This and ref. [21] are the first two papers to report the identification of specific rCGG-binding proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalili K, Del Valle L, Muralidharan V, et al. Purα is essential for postnatal brain development and developmentally coupled cellular proliferation as revealed by genetic inactivation in the mouse. Mol Cell Biol. 2003;23:6857–75. doi: 10.1128/MCB.23.19.6857-6875.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Handa V, Saha T, Usdin K. The fragile X syndrome repeats form RNA hairpins that do not activate the interferon-inducible protein kinase, PKR, but are cut by Dicer. Nucleic Acids Res. 2003;31:6243–8. doi: 10.1093/nar/gkg818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sofola OA, Jin P, Botas J, Nelson DL. Argonaute-2 dependent rescue of a Drosophila model of FXTAS by FRAXE premutation repeat. Hum Mol Genet. 2007;16:2326–32. doi: 10.1093/hmg/ddm186. [DOI] [PubMed] [Google Scholar]
- 26.De Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443–53. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.•.Willemsen R, Hoogeveen-Westerveld M, Reis S, et al. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet. 2003;12:949–59. doi: 10.1093/hmg/ddg114. This paper reports the first mouse model for FXTAS. [DOI] [PubMed] [Google Scholar]