Abstract
This paper summarizes and quantifies the current evidence relating dietary intake of animal products and endometrial cancer. Literature searches were conducted to identify peer-reviewed manuscripts published up to December 2006. Twenty-two manuscripts from three cohort studies and 16 case-control studies were identified. One of these cohort studies evaluated only fried meat and another only milk consumption; they were not included in our meta-analyses. The third cohort study identified did not present exposure levels and could not be included in dose-response meta-analysis. This cohort study did not show an association with meat or red meat consumption. Random-effects dose-response summary estimates for case-control studies evaluating these foods were 1.26 (95% CI: 1.03–1.54) per 100 g/day of total meat, 1.51 (95% CI: 1.19–1.93) per 100 g/day of red meat, 1.03 (95% CI: 0.32–3.28) per 100 g/day of poultry, 1.04 (95% CI: 0.55–1.98) per 100 g/day of fish, and 0.97 (95% CI: 0.93–1.01) per serving of dairy. Our meta-analysis, based on case-control data, suggests that meat consumption, particularly red meat, increases endometrial cancer risk. The current literature does not support an association with dairy products, while the evidence is inconsistent for poultry, fish, and eggs. More studies, particularly prospective studies, are needed.
Keywords: endometrial carcinoma, diet, meat, eggs, fish, poultry, dairy products, milk, animal foods, meta-analysis, systematic literature review
INTRODUCTION
Endometrial cancer is the most common female gynecological cancer in the United States, ranking fourth among all cancers in women in age-adjusted incidence [1]. Endometrial cancer is a model of hormonal carcinogenesis, with convincing evidence that most endometrial cancers are caused by excessive exposure to unopposed estrogens [2]. Although obesity is an established and strong risk factor for endometrial cancer [3], the role of individual dietary factors is not well understood. There is evidence pointing to a possible role of meat consumption on cancer etiology. The WCRF/AICR 1997 Report [4] concluded that meat consumption was a “probable” cause of colorectal cancer and that it “possibly” increased the risk for cancers of the pancreas, breast, prostate, and kidney. Additional support for a relationship between meat intake and colorectal cancer was provided by a recent meta-analysis of 15 prospective studies [5]. However, the role of meat consumption on endometrial cancer risk has received little attention.
Because animal foods are known to contain estrogens at various concentrations [6], conceivably they may affect endometrial cancer risk. Although the association between animal products and endometrial cancer was reviewed in the WCRF/AICR 1997 Report [4], no judgment was possible for meat, fish, or eggs because the evidence was limited and inconsistent. No papers were identified for meat or dairy products. For fish, three case-control studies [7–9] were mentioned with inconsistent results. For eggs, three case-control studies were identified; two of them [7, 9] found elevated risk associated with higher egg consumption, whereas the third [8] reported similar mean egg consumption in cases and controls.
Commissioned by the WCRF, we conducted a systematic and comprehensive literature review of the nutritional epidemiology of endometrial cancer [10]. The purpose of this review was to enhance and update the previous 1997 review conducted for the First WCRF/AICR Report on Food, Nutrition, and the Prevention of Cancer [4]. The objective of this manuscript is to summarize the evidence from the epidemiologic literature examining the role of consumption of animal products (i.e., meat, poultry, fish, eggs, and dairy products) on endometrial cancer risk.
METHODS
In general, we followed the methodology in the WCRF Specification Manual (available online at www.wcrf.org) to conduct the overall systematic literature review (SLR) and meta-analyses. More details in our methods can be found in our protocol posted at the WCRF website. The methods used in this manuscript diverge from the WCRF instructions in the following aspects:
This systematic review and meta-analysis is limited to case-control and cohort studies. Randomized trials of animal foods intake and risk of endometrial cancer would have been included, but none exist. Ecological and cross-sectional studies were excluded.
Analyses were repeated excluding studies that did not meet certain a priori criteria (i.e., population-based studies with more than 200 cases, known hysterectomy status among controls, and adjustment for total energy intake and body mass).
Interpretation of the evidence may not represent the views of WCRF and may differ from those in the upcoming WCRF report summarizing evidence related to food, nutrition, physical activity, and cancer risk (expected November, 2007).
Search strategy
Searches were conducted in July 2003, October 2004, and December 2005. Databases included Medline, ISI Web, Embase, Biosis, Ingenta, CINAHL, Science Direct, LILACS, Pascal, ExtraMed, and Allied CompMed. Results from the 2003 searches indicated that most citations were found in Medline and, therefore, some of the databases that did not produce any new results were not used in subsequent searches. These searches were complemented with manual searches of bibliographies in published papers. We made explicit efforts to include manuscripts in foreign languages. Translations were provided by WCRF when necessary. For this manuscript, we also monitored the literature using PubMed Alerts for all new papers on endometrial cancer from January through December 2006.
Exposure terms for PubMed were provided by WCRF and can be found in Appendix 1. General terms included diet[tiab] OR diets[tiab] OR dietetic[tiab] OR dietary[tiab] OR eating[tiab] OR intake[tiab] OR nutrient*[tiab] OR nutrition[tiab] OR vegetarian*[tiab] OR vegan*[tiab] OR “seventh day adventist”[tiab] OR macrobiotic[tiab] OR food and beverages[MeSH Terms]. Specifically for animal foods, we used meat[tiab] OR beef[tiab] OR pork[tiab] OR lamb[tiab] OR poultry[tiab] OR chicken[tiab] OR turkey[tiab] OR duck[tiab] OR fish[tiab] OR egg[tiab] OR eggs[tiab] OR shellfish[tiab] OR seafood[tiab] OR dairy[tiab] OR milk[tiab].
Following WCRF instructions, our searches included endometrial hyperplasia, as this includes precancerous lesions. However, we found few papers evaluating the role of diet and nutrition on endometrial hyperplasia, and none evaluated animal products. Outcomes search terms included: (1): endometrial neoplasm [MeSH]; (2): malign* [tiab] OR cancer*[tiab] OR carcinoma*[tiab] OR tumor*[tiab] OR tumour*[tiab]; (3): endometr* [tiab] OR corpus uteri [tiab] OR uterine [tiab]; (4): #2 AND #3; (5): #3 AND hyperplasia [tiab]
Manuscript selection and data extraction
Overall search results and manuscript selection have been described elsewhere [11]. In brief, citations identified from these searches were reviewed independently by two of us (LHK, EVB) for relevance. For citations that appeared relevant, the full paper was retrieved, reviewed, and classified as “included” or “excluded”. Of the 285 papers identified evaluating some aspect of nutrition, diet, physical activity and endometrial cancer, 28 mentioned animal foods (i.e., meat, poultry, fish, eggs, or dairy products), all written in English. Through monitoring the endometrial cancer literature in 2006 using PubMed Alerts nine additional papers were identified that evaluated nutrition, diet, or physical activity and endometrial risk; one of these evaluated animal products and was added to this review.
For this manuscript, we decided a priori to exclude ecological [12, 13] and cross-sectional studies (none found). We also excluded publications that reported mean intakes for animal foods but did not present risk estimates [8, 14–16] or other studies that did not collect information at the individual level [17]. Two additional publications [18, 19] from the same case-control study were also excluded because insufficient information was provided on the methodology used (e.g., ascertainment of cases and controls, dietary assessment method used). Furthermore, this study [18, 19] evaluated only fried meat and cooked meat and showed only crude risk estimates. The remaining 22 papers, from 3 cohort studies and 16 case-control studies, were included in this systematic literature review. Two manuscripts from Terry et al. were from the same case-control study in Sweden, but both were included because one presented results on meat and dairy products [20] and the other on fish intake [21]. Three other manuscripts were from the same on-going hospital-based case-control study in Italy [22–24], but again the three were included because they evaluated different exposures. Studies included in the review are listed in Table 1.
Table 1.
Characteristics of observational studies evaluating animal products and endometrial cancer risk
| Reference | Country | Cases/controls or cohort size | Age | Dietary assessment | Time frame† | Hysterectomies Excluded | Animal foods evaluated |
|---|---|---|---|---|---|---|---|
| COHORT STUDIES | |||||||
| Ursin et al., 1990[41] | Norway | 11/15,914 | <75 | Questionnaire | Current intake | ? | Milk |
| Knekt et al., 1997[32] | Finland | 21/9,990 | 15–99 | Dietary history | 1 year | ? | Fried meat |
| Zheng et al., 1995[29] | United States | 216/23,070 | 55–69 | FFQ (127 items) | Current intake | Yes | Animal foods, total meat, red meat, poultry, seafood, processed meat/fish, dairy products, eggs. |
| CASE-CONTROL STUDIES: Population-based | |||||||
| Shu et al., 1993 [9] | China | 268/268 | 18–74 | FFQ (63 items) | 10 years | Yes | Meat, read meat, poultry, fresh fish, other seafood, eggs. |
| Potischman et al., 1993 [33] | United States | 399/296 | 20–74 | FFQ (Block, 60 items) | “past few years” | Yes | All meats, red meats, dairy foods. |
| Goodman et al., 1997 [34] | United States | 332/511 | 18–84 | Dietary history(250 items) | 1 year | Yes | All meat, beef, pork, poultry, fish, processed meat, red meat, dairy products, butter, milk, cheese, eggs. |
| Jain et al., 2000[39] | Canada | 552/562 | 30–79 | Dietary history (n Items ?) | 1 year | Yes | Read meat, fish, chicken, milk, cheese. |
| McCann et al., 2000 [35] | United States | 232/639 | 40–85 | FFQ (172 items) | 2 years | Yes | Red meat, poultry, fish/seafood, processed meats, total meat, dairy |
| Littman et al., 2001[36] | United States | 679/944 | 45–74 | FFQ (modified Block, 98 items) | 5 years | Yes | Dairy products, meat. |
| Terry et al., 2002[20] ‡ | Sweden | 709/2887 | 50–74 | FFQ (32 Items plus fish questions) | 1 year | Yes | Dairy products, meat. |
| Terry et al., 2002[21] ‡ | Sweden | 698/2720 | 50–74 | FFQ (32 Items plus fish questions) | 1 year | Yes | Fatty fish, lean fish, total fish. |
| Xu et al., 2006 [37] | China | 1204/1212 | 30–69 | FFQ (76 foods) | 5 years | Yes | Total meat, red meat, organ meat, poultry, total fish, marine fish, fresh water fish, shrimp and crab, eel, shellfish, eggs, milk. |
| CASE-CONTROL STUDIES: Hospital-based | |||||||
| Tzonou et al, 1996[30] | Greece | 145/298 | FFQ (115 items) | 1 year | ? | “Meats, fish or eggs”, “milk or milk products”. | |
| La Vecchia et al., 1988 [22] § | Italy | 434/1385 | <75 | Questionnaire(frequency of consumption per week of 14 items) | “before symptoms” | Yes | Total meat. |
| Mettlin et al., 1990[42] | United States | 231/1300 | 18.97 | FFQ (n items?) | ? | No | Whole milk, 2% milk, skim milk. |
| Levi et al., 1993 [7] | Italy, Switzerland | 274/572 | 30–75 | Questionnaire(Weekly frequency of intake of 50 items) | “before symptoms” | Yes | Beef, pork, poultry, fish, liver, raw ham, boiled ham, salami and sausages, canned meat, other meats, milk, cheese, eggs. |
| Barbone et al., 1993 [43] | United States | 103/236 | FFQ (Willett, 116 items) | 1 year | Yes | Skim milk, sour cream, yogurt, cheese other than cream. | |
| Hirose et al., 1996[40] | Japan | 145/26751 | >20 | Questionnaire (n items ?) | ? | ? | Milk, boiled or broiled fish, sashimi, egg, chicken, beef, pork. |
| Fernandez et al., 1999[24] § | Italy | 750/7,990 | <75 | Questionnaire(weekly frequency of intake of 14–37 items) | 1 year | ? | Fish. |
| Tavani et al., 2000[23] § | Italy | 750/7,990 | <75 | Questionnaire(weekly frequency of approximately 40 items) | 2 years | ? | Red meat. |
| Petridou et al., 2002 [31] | Greece | 84/84 | FFQ (110 items) | 1 year | Yes | “Meats, fish, and eggs”, “milk and milk products” | |
| Salazar-Martinez et al., 2005 [38] | Mexico | 85/629 | 18–81 | FFQ (116 items) | 1 year | Yes | Dairy products, meat. |
Abbreviations: FFQ, food frequency questionnaire; ?, unspecified
Time frame for dietary assessment.
Same case-control study in Sweden.
Same case-control study in Italy.
Data were extracted on study characteristics and results using an Access® program developed by Leeds University under WCRF sponsorship. Data extraction was conducted by trained research staff and reviewed by at least one of us.
Statistical analysis
We followed WCRF criteria to decide when to conduct meta-analysis. For a given exposure, meta-analyses were conducted if there were at least two randomized clinical trials, at least two cohort studies, and/or at least five case-control studies evaluating that exposure. Meta-analyses were conducted separately by study design. Because there were only a few studies evaluating any particular animal foods exposure, we had limited ability to assess publication bias through funnel plots, or to conduct sensitivity analyses and meta-regression.
For total meat, red meat, poultry, fish, dairy products, and eggs there was a sufficient number of studies to conduct meta-analyses. Most of the studies categorized relevant variables into four or five groups, and reported odds ratios for each category relative to the lowest exposure category. A few studies reported parameter estimates from a continuous logistic regression analysis. To compare these studies, it was necessary to convert the grouped results into what would have resulted from continuous logistic regression analysis, had those been reported.
In order to conduct dose-response meta-analyses, we transformed exposure levels into a common scale. Studies reported consumption in qualitative scales (such as low, medium, high), or in servings, g, or times per month, week, or day. We transformed the quantitative exposure units into g/day by assuming, when exposure was reported as frequency of consumption, that a “serving” or “time” corresponded to 100 g consumption. For studies not reporting confidence intervals, these were estimated based on the number of cases and controls in each category of exposure [25].
For studies reporting only categorical analyses, an estimate of mean intake for each category was computed following the methodology developed by Chêne and Thompson [26]. The iterative method described in Greenland and Longnecker [27] was used to estimate a single logistic regression parameter per study. This method imputes expected numbers of cases and controls (or cases for a prospective study) based on the observed distributions and categorical relative risk, and computes the logistic regression slope parameter (which may be interpreted as the log relative risk) and standard error. Finally, we estimated fixed effects and random effects pooled logistic regression coefficients across studies. We used the random effects models in forest plots and for interpretation of the evidence, since it uses a combination of the “within study” variance and the “between study” variance for computing weights. The Chêne and Thompson [26] and Greenland and Longnecker [27] algorithms described above were implemented in the statistical language R (R Development Core Team, 2003). Fixed- and random-effects pooled estimates and forest plots were produced using the “metan” package (version 1.86) for STATA. Heterogeneity was assessed by conducting Q tests (i.e., testing for presence/absence of heterogeneity) and quantifying the degree of heterogeneity by estimating the I2 index [28].
Assessment of study quality and sensitivity analyses
For the purpose of this manuscript we decided not to grade individual papers according to a quality score for two reasons: 1) there is not a current widely-accepted tool for quality assessment of epidemiologic studies, and 2) we had few papers evaluating separately each animal food variable. Instead, we decided to present all the evidence from the several case-control studies, and then repeated certain analyses excluding studies that did not meet certain a priori quality criteria. In the forest plots, excluded studies are marked and the specific reasons for exclusion are indicated in the footnotes. The a priori quality criteria were: 1) population-based studies, as the appropriateness of hospital controls in diet and cancer studies is controversial; 2) sample size of at least 200 cases for more optimal statistical power; 3) exclusion of hysterectomies from the control group; and 4) adjustment for important confounders such as total energy intake and body size.
RESULTS
This systematic literature review included 22 manuscripts from three cohort studies and 16 case-control studies evaluating a variety of animal food exposures. The characteristics of these studies and the foods evaluated are listed in Table 1. As shown in the table, these studies were conducted in several countries and varied considerably in the quality of dietary assessment, as well as in the evaluation of animal foods.
Animal foods combined
One cohort study [29] and two hospital-based case-control studies [30, 31] evaluated the risk associated with animal foods combined with little evidence of an association (data not shown).
Meat (type unspecified)
Studies evaluating meat consumption are listed in Table 2. The Iowa Women’s Health Study found no association [29], whereas the other cohort study conducted in Finland [32] suggested increased risk associated with fried meat intake, but the confidence interval included the null value. In addition, we found nine case-control studies evaluating this association [9, 20, 22, 33–38]. Of these, six suggested elevated risk [9, 20, 22, 34, 37, 38], whereas in the other three studies, odds ratios for meat intake were one [33, 36] or below one [35]. It should be noted that the definition of meat varied substantially by study, as shown in the Appendix Table. Furthermore, three of the studies [20, 22, 35] did not adjust for total energy intake.
Table 2.
Studies evaluating meat intake and endometrial cancer risk
| Reference | Country | Age | Cases/Controls or | Exposure evaluated | Contrast | OR (95% CI) | P for trend | Covariates Considered* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | E | S | H | R | ||||||||
| COHORT STUDIES | |||||||||||||
| Knekt et al., 1994[32] | Finland | 15–99 | 21/9,990 | Fried meat | Q3 vs. Q1 | 2.11 (0.61–7.27) | 1 | 1 | 1 | 1 | 1 | ||
| Zheng et al., 1995[29] | United States | 55–69 | 216/23,070 | Total meat | Q3 vs. Q1 | 1.1 | >0.05 | 1 | 1 | 1 | 2 | ||
| CASE-CONTROL STUDIES: Population-based | |||||||||||||
| Shu et al., 1993 [9] | China | 18–74 | 268/268 | Meat | > 576 vs. < 243 g/week | 2.5 | <0.01 | 1 | 1 | 1 | 1 | ||
| Potischman et al., 1993 [33] | United States | 20–74 | 399/296 | All meats | > 10.8 vs. < 5.1 times/week | 1 (0.6–1.7) | 1 | 1 | 1 | 1 | 1 | 1 | |
| Goodman et al., 1997 [34] | United States | 18–84 | 332/511 | All meat | > 171 vs. < 72 g/day | 1.5 | 0.33 | (A) | 1 | 1 | 1 | ||
| McCann et al., 2000[35] | United States | 40–85 | 232/639 | Total meat | >64 vs. <38 times/month | 0.8 (0.5–1.3) | 0.27 | 1 | 1 | 1 | 1 | 3 | |
| Littman et al., 2001[36] | United States | 45–74 | 679/944 | Meat | >1.3 vs. <0.9 servings/day | 1 (0.75–1.4) | 0.85 | 1 | 1 | 1 | 1 | 1 | |
| Terry et al., 2002[20] | Sweden | 50–74 | 709/2,877 | Meat (all types) | Q4 (median 24 servings/week) vs. Q1(median 4 servings/week) | 1.3 (1–1.8) | 0.11 | 1 | 1 | 1 | |||
| Xu et al., 2006 [37] | China | 30–69 | 1,204/1,212 | Total meat | >81.7 vs. <31.2 g/day | 1.6 (1.3–2.0) | <0.01 | 1 | 1 | 1 | 1 | ||
| CASE-CONTROL STUDIES: Hospital-based | |||||||||||||
| La Vecchia et al., 1988 [22] | Italy | <75 | 434/1,385 | Total meat | > 7 vs. < 4 portions/week | 1.6 (1.22–2.12) | 1 | 1 | 1 | 4 | |||
| Salazar-Martinez et al., 2005 [38] | Mexico | 18–81 | 85/629 | Meat | T3 vs. T1 | 1.96 (0.97–3.95) | 0.36 | 1 | 1 | 1 | 1 | ||
Adjustment columns: A = Age; B = BMI/weight; E = Total Energy; S = Smoking; H = HRT/ERT use; R = Reproductive factors; (A): matched on age. Numbers in columns refer to the number of covariates adjusted for under that grouping. Abbreviations: Q: quantile; T: tertile.
Appendix Table.
Foods included in food groups in papers contributing to meta-analyses.
| Reference | Meat | Red meat | Poultry | Fish | Dairy products |
|---|---|---|---|---|---|
| Fernandez et al., 1999[24] | -- | -- | “Fish” | -- | |
| Goodman et al., 1997[34] | All meat, including beef, pork, poultry, fish, processed meat, red meat | “Red meat” | “Poultry” | “Fish” | Butter, milk and cheese |
| Hirose et al., 1996 [40] | -- | -- | “Chicken” | -- | |
| Jain et al., 2000 [39] | -- | All beef, pork, veal, lamb, game, meat stews, meat soups | “Chicken” | “Fish” | -- |
| La Vecchia et al., 1988 [22] | “Total meat” | -- | -- | -- | -- |
| Levi et al., 1993 [7] | -- | -- | “Poultry” | -- | -- |
| Littman et al., 2001 [36] | Hamburger, beef burrito, meatloaf, beef, beef stew, pot pie, liver, pork, fried chicken, other chicken, fried fish, tuna, shellfish, fish broiled or baked, hot dogs, ham, lunch meats, bacon, and sausage | -- | -- | -- | Cottage cheese, cheese, cheese spread, flavored yogurt, frozen yogurt, milk, low-fat yogurt, low-fat cottage cheese |
| McCann et al., 2000 [35] | “Total meats” | “Red meat” | “Poultry” | “Fish and seafood” | “Dairy” |
| Petridou et al., 2002 [31] | -- | -- | -- | -- | Feta cheese, kaseri cheese, other cheese, whole milk, skim milk, full-fat yogurt, reduced-fat yogurt, milk pudding, rice milk pudding, ice cream, cheese pie (1/2), pizza (1/2) |
| Potischman et al., 1993 [33] | Hamburgers, cheeseburgers, or meat loaf; beef such as steaks or roast; beef stew or pot pie; liver; port such as port chops or roasts; fried chicken; baked, stewed, or broiled chicken or turkey; fried fish or fish sandwiches; spaghetti, lasagna, or pasta with tomato sauce; hot dogs;ham or lunch meats; bacon; sausage | Hamburgers, cheeseburgers or meat loaf; beef such as steaks or roasts; beef stew or pot pie; liver; pork such as pork chops or roasts;spaghetti, lasagna, or pasta with tomato sauce; hot dogs; ham or lunch meats; bacon; sausage | -- | -- | Cheese or cheese spreads; butter; ice cream; whole milk; 2%milk; skim milk, 1% milk or buttermilk; milk in coffee or tea; cream or half-and-half in coffee or tea |
| Salazar-Martinez et al., 2005 [38] | “Meat” | -- | -- | -- | “Dairy products” |
| Shu et al., 1993 [9] | Pork chops, pork spareribs, pig’s feet, pork (fat only), pork (lean only), salted pork, pork liver, chicken liver, other pork organ meats, beef, lamb, chicken, duck | Pork chops, pork spareribs pig’s feet, pork (fat only), pork (lean only), salted pork, beef, lamb, | “Chicken and duck” | “Fresh fish” | -- |
| Tavani et al., 2000 [23] | -- | Beef, veal, and pork (excluding canned and preserved meat) | -- | -- | -- |
| Terry et al., 2002 [21] | -- | -- | -- | Fatty fish (e.g., salmon, herring, mackerel) and lean fish (e.g., cod, flounder, shellfish) | -- |
| Terry et al., 2002 [20] | “Meat” | -- | -- | -- | “Dairy products” |
| Tzonou et al., 1996 [30] | -- | -- | -- | -- | Feta cheese, kaseri cheese, other cheese, whole milk, skim milk, full-fat yogurt, reduced-fat yogurt, milk pudding, milk pudding, rice pudding, ice cream, cheese pie (1/2), pizza (1/2) |
| Xu et al., 2006 [37] | Total meat, including red meat, organ meat, and poultry | Pork, beef, and mutton meat | “Poultry” | Total fish, including marine fish, fresh fish, shrimp, crab, eel and shellfish | -- |
| Zheng et al., 1995 [29] | Total meat, including red meat and poultry | “Red meat” | “Poultry” | -- | -- |
Meta-analysis
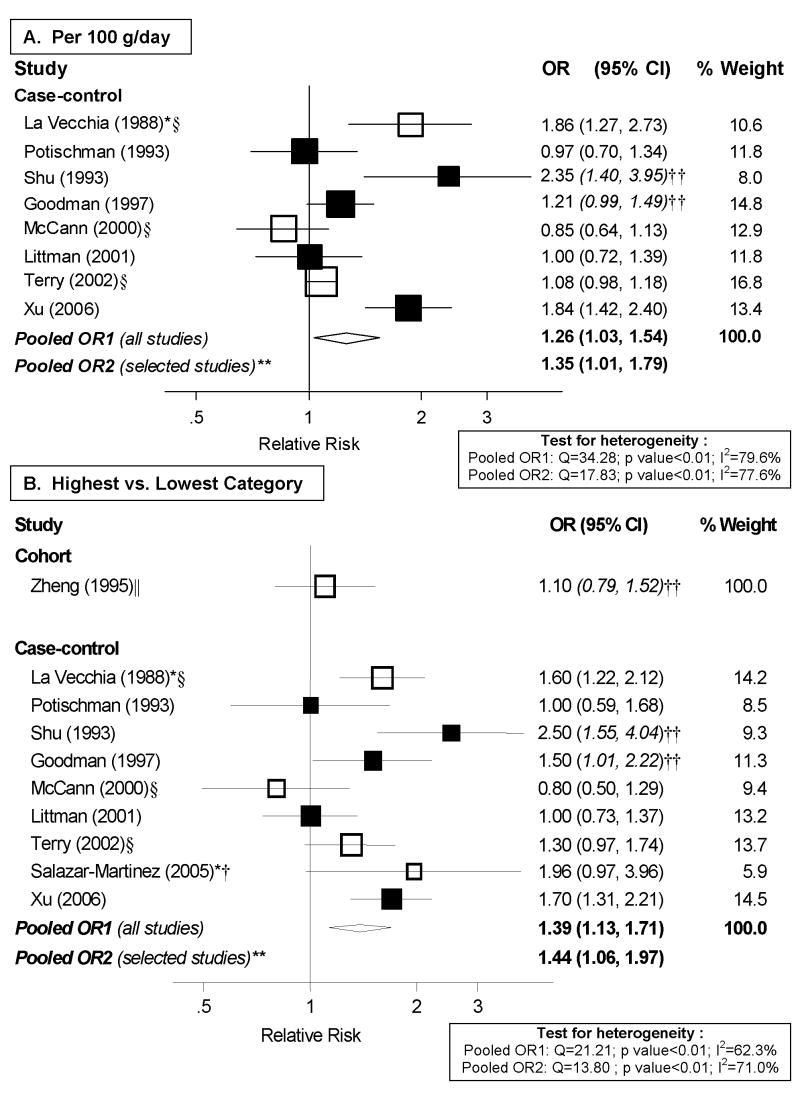
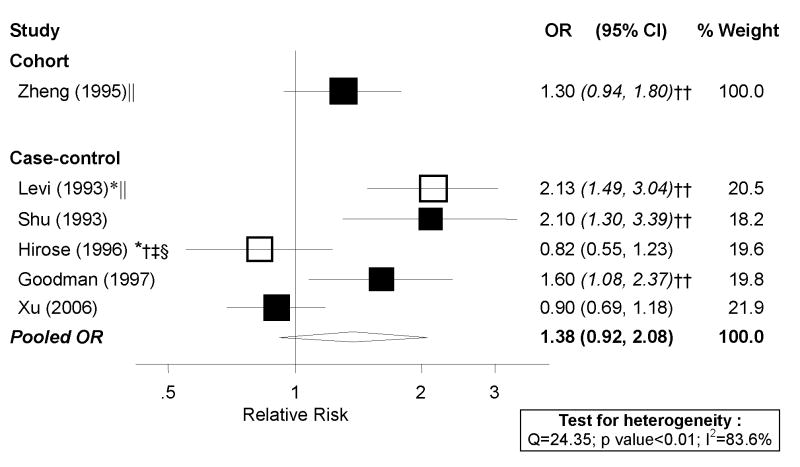
Out of the two cohort studies identified, only Zheng et al. [29] reported on total meat. However, it did not present cutpoints and, therefore, could not be included in dose-response analyses. Of the nine case-control studies identified, eight were included in dose-response meta-analyses, shown in Figure 1.A. The study by Salazar-Martinez et al. [38] could not be included because it did not present cutpoints. There was high heterogeneity among studies (I2: 79.6%, p<0.01) and some indication of an association with meat intake (random effects pooled OR: 1.26; 95% CI: 1.03–1.54 per 100 g/day). High vs. low analyses, shown in Figure 1.B., included the nine case-control studies identified and also suggested elevated risk associated with meat consumption. These findings should be viewed with caution due to the high heterogeneity of both risk estimates and exposure definitions among studies. The one cohort study evaluating this association found little evidence of an association.
Figure 1. Random-effects meta-analysis of meat consumption (unspecified type) and endometrial cancer risk.
**Excluding studies for the following reasons: * Hospital-based; † Less than 200 cases; ‡ exclusion of hysterectomies not clearly specified; § not adjusted for total energy intake; || not adjusted for BMI/weight †† estimated confidence interval.
Dose-response and high vs. low meta-analyses were repeated excluding the studies that did not meet certain a priori quality criteria, but pooled risk estimates essentially did not change and heterogeneity remained high and significant (Figure 1).
Red meat
Case-control studies have generally supported an increased risk of endometrial cancer associated with red meat consumption (Table 3). Of the seven case-control studies evaluating the association [9, 23, 33–35, 37, 39], all but one [35] reported an OR greater than 1 comparing the highest to lowest category of intake. In contrast, the only cohort study evaluating this relationship provided little support for an association [29]. The cohort study [29] and one case-control study [23] did not adjust for BMI, and two studies [23, 35] did not control for total energy intake.
Table 3.
Studies evaluating red meat and endometrial cancer risk.
| Reference | Country | Age | Cases/Controls or total cohort | Type of study | Exposure | Contrast | OR (95% CI) | P for trend | Covariates considered* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | E | S | H | R | |||||||||
| Zheng et al., 1995[29] | United States | 55–69 | 216/23,070 | Cohort | Red meat | Q3 vs. Q1 | 1.1 | >0.05 | 1 | 1 | 1 | 2 | ||
| Potischman et al., 1993 [33] | United States | 20–74 | 399/296 | Population-based cc | Red meat | >8 vs. <2.9 times/week | 1.3 (0.8–2.4) | 1 | 1 | 1 | 1 | 1 | 1 | |
| Shu et al., 1993 [9] | China | 18–74 | 268/268 | Population-based cc | Red meat | >576 vs. <200 g/week | 2.5 | <0.01 | 1 | 1 | 1 | 1 | ||
| Goodman et al., 1997 [34] | United States | 18–84 | 332/511 | Population-based cc | Red meat | >98.6 vs. <28.2 g/day | 2 | 0.03 | (A) | 1 | 1 | 1 | ||
| Jain et al., 2000[39] | Canada | 552/562 | 30–79 | Population-based cc | Red meat | >53 vs. <15 g/day | 1.21 (0.83–1.77) | 0.55 | 1 | 1 | 1 | 1 | 1 | 2 |
| McCann et al., 2000 [35] | United States | 40–85 | 232/639 | Population-based cc | Red meat | >17 vs. <8 times/month | 0.9 (0.5–1.4) | 0.79 | 1 | 1 | 1 | 1 | 3 | |
| Xu et al., 2006 [37] | China | 30–69X | 1,204/1,212 | Population-based cc | Red meat | >61.9 vs. <22.4 g/day | 1.4 (1.1–1.9) | <0.01 | 1 | 1 | 1 | 1 | ||
| Tavani et al., 2000 [23] | Italy | <75 | 750/7,990 | Hospital-based cc | Red meat (categorical) | High vs. low | 1.5 (1.2–1.8) | <0.01 | 1 | 1 | ||||
| Red meat(continuous) | 1 serving/day | 1.5 (1.2–1.9) | 1 | 1 | ||||||||||
Adjustment columns: A = Age; B = BMI/weight; E = Total Energy; S = Smoking; H = HRT/ERT use; R = Reproductive factors; (A): matched on age. Numbers in columns refer to the number of covariates adjusted for under that grouping. Abbreviations: Q: quantile; T: tertile; cc: case-control.
Only three case-control studies have evaluated the association with specific types of red meat (i.e., beef and pork; data not shown). Both Goodman et al. [34] and Levi et al. [7] found elevated risk for pork and beef, whereas Hirose et al. [40], in a hospital-based case-control study in Japan, did not find an association with either. Overall, the data are insufficient at the present time to warrant any conclusions regarding risk by type of red meat.
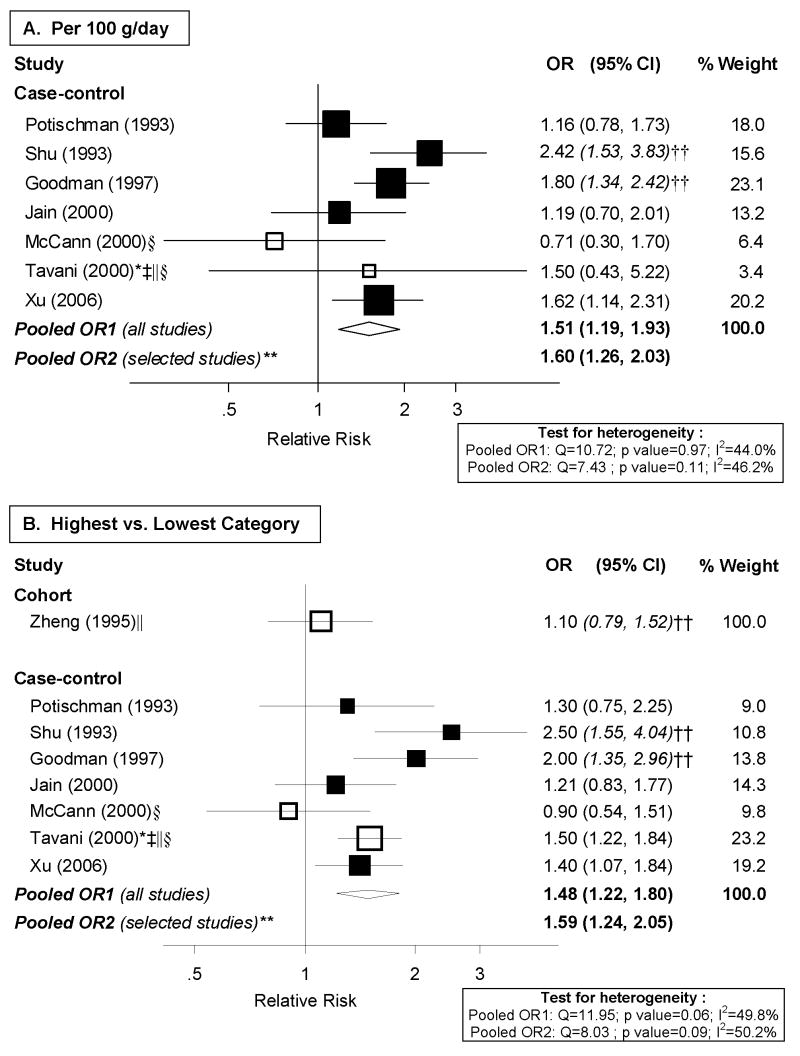
Meta-analysis
The seven case-control studies reporting risk estimates for total red meat were included in meta-analyses, shown in Figure 2. We were unable to derive a continuous RR for the cohort study because cutpoints were not provided. Continuous analyses indicated a 51% increase in endometrial cancer risk per 100 g/day of red meat consumption (random effects pooled OR: 1.51; 95% CI: 1.19–1.93; I2: 44%; p for heterogeneity: 0.097). The magnitude of the association in high vs. low meta-analysis was similar (Figure 2.B.), with moderate heterogeneity among studies (p for heterogeneity: 0.97). When we excluded studies that did not meet our quality criteria, the pooled risk dose-response and high vs. low risk estimates became stronger (Figure 2.A. and 2.B.).
Figure 2. Random-effects meta-analysis of red meat intake and endometrial cancer risk.
**Excluding studies for the following reasons: * Hospital-based; † Less than 200 cases; ‡ exclusion of hysterectomies not clearly specified; § not adjusted for total energy intake; || not adjusted for BMI/weight †† estimated confidence interval.
Processed meat
The association with processed meat has been evaluated in only a few studies (data not shown). In the Iowa Women’s Health Study [29] there was some indication of an increased risk with processed meat and fish intake (RR: 1.5, p for trend≤0.05 for the highest tertile compared to the lowest). Three case-control studies evaluating this association found inconsistent results. Goodman et al. [34] suggested elevated risk, whereas there was little evidence of an association in the Western New York Diet Study [35]. An additional case-control study in Italy [7] examined risk associated with various individual processed meat products, and suggested an increased risk for raw ham, boiled ham, salami and sausages, and canned meat.
Liver and other meats
The risk associated with liver and organ meats have been evaluated in two case-control studies with conflicting results (data not shown). One study conducted in Italy and Switzerland [7] suggested elevated risk with liver consumption and with other meats (i.e., other than beef, pork, poultry, fish, liver, boiled ham, raw ham, canned meat, and salami and sausages) (OR: 1.83; confidence interval not presented). In contrast, a more recent study in China did not find an association with intake of organ meats [37].
Poultry
Studies evaluating poultry consumption and endometrial cancer risk are shown in Table 4. Zheng et al. [29] examined the association with poultry intake in the Iowa Women’s Health Study and reported a modest increased risk comparing high to low intake (RR=1.3, confidence interval not provided). As shown in Table 4, the seven case-control studies [7, 9, 34, 35, 37, 39, 40] that reported on poultry or chicken have offered inconsistent results.
Table 4.
Studies evaluating poultry intake and endometrial cancer risk.
| Reference | Country | Age | Cases/Controls or total cohort | Type of study | Exposure | Contrast | OR (95% CI) | P for trend | Covariates considered* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | E | S | H | R | |||||||||
| Zheng et al., 1995[29] | United States | 55–69 | 216/23,070 | Cohort | Poultry | Q3 vs. Q1 | 1.3 | >0.05 | 1 | 1 | 1 | 2 | ||
| Shu et al., 1993 [9] | China | 18–74 | 268/268 | Population-based cc | Poultry | Q4 vs. Q1 | 0.9 | 0.42 | 1 | 1 | 1 | 1 | ||
| Goodman et al., 1997 [34] | United States | 18–84 | 332/511 | Population-based cc | Poultry | >38 vs. <10.5 g/day | 0.6 | 0.006 | (A) | 1 | 1 | 1 | ||
| McCann et al., 2000 [35] | United States | 40–85 | 232/639 | Population-based cc | Poultry | >10 vs. <5 times/month | 0.7 (0.4–1.1) | 0.09 | 1 | 1 | 1 | 1 | 3 | |
| Jain et al., 2000[39] | Canada | 552/562 | 30–79 | Population-based cc | Chicken | >33.4 vs. <9.2 g/day | 1.23 (0.85–1.78) | 0.65 | 1 | 1 | 1 | 1 | 1 | 2 |
| Xu et al., 2006 [37] | China | 30–69 | 1,204/1,212 | Population-based cc | Poultry | >18.5 vs. <4 g/day | 1.6 (1.3–2.1) | <0.01 | 1 | 1 | 1 | 1 | ||
| Levi et al., 1993 [7] | Italy and Switzerland | 30–75 | 274/572 | Hospital-based cc | Poultry | Q3 vs. Q1 | 0.87 | 1 | ||||||
| Hirose et al., 1996[40] | Japan | >20 | 145/26,751 | Hospital-based cc | Chicken | >3 vs. <3 servings/week | 1.02 (0.64–1.62) | 1 | ||||||
Adjustment columns: A = Age; B = BMI/weight; E = Total Energy; S = Smoking; H = HRT/ERT use; R = Reproductive factors; (A): matched on age. Numbers in columns refer to the number of covariates adjusted for under that grouping. Abbreviations: Q: quantile; T: tertile; cc: case-control.
Meta-analysis
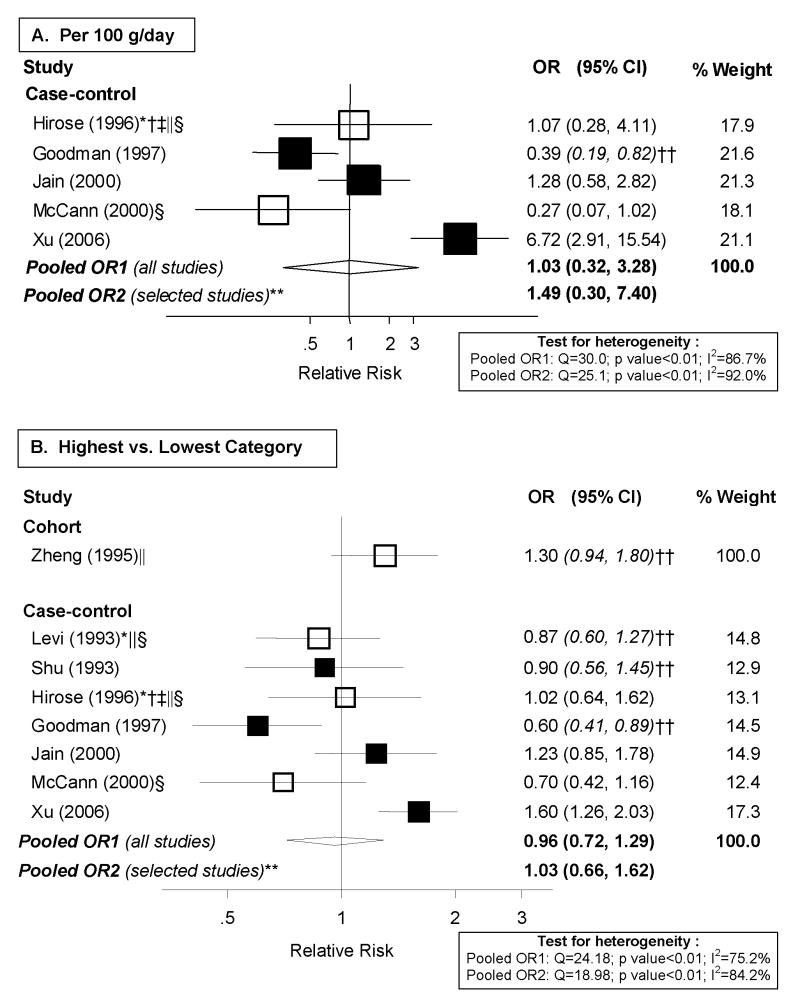
The seven case-control studies [7, 9, 34, 35, 37, 39, 40] and cohort study [29] identified were considered for meta-analyses. However, two of the case-control studies [7, 9] and the cohort study [29] could not be included in the dose-response analyses, shown in Figure 3.A, because exposure levels were not presented. There was high heterogeneity among case-control studies (p<0.01) with an I2 of 86.7% and a random-effects pooled OR of 1.03 (95% CI: 0.32–3.28) per 100 g/day of poultry intake. To be able to include the three additional studies without cutpoints, we conducted high vs. low analyses, shown in Figure 3.B. The summary OR was of similar magnitude (OR: 0.96; 95% CI: 0.72–1.29; I2: 75.2%). The cohort study did not present confidence limits, but we estimated them based on the distribution of cases and non-cases by exposure category. The RR for those in the highest tertile of poultry consumption compared to the lowest was 1.3 (estimated 95% CI: 0.94–1.80). We repeated analyses excluding selected studies that did not meet our quality criteria. Overall, there was substantial inconsistency and little evidence of an association with poultry consumption.
Figure 3. Random-effects meta-analysis of poultry intake and endometrial cancer risk.
**Excluding studies for the following reasons: * Hospital-based; † Less than 200 cases; ‡ exclusion of hysterectomies not clearly specified; § not adjusted for total energy intake; || not adjusted for BMI/weight.
†† estimated confidence interval.
It should be noted that pooling the seven case-control studies may not be appropriate, as two of them examined only chicken and the other four “poultry” (Table 4). There were not enough studies to separately evaluate overall poultry consumption and chicken consumption.
Fish (total)
One cohort study reported on the association of total seafood intake and endometrial cancer risk (Table 5). There was suggestion of an increased risk with high vs. low intake (RR=1.4) with a p for trend of 0.05 (confidence limits not provided). We identified nine case-control studies reporting on fish intake, with conflicting results (Table 5). Three studies, conducted in Sweden [21], the U.S. [35], and Italy [24], provided some indication of an inverse association, while four studies conducted in China [9, 37], Japan [40], and Hawaii [34] reported increased risk associated with fish consumption. Odd ratios were close to the null in the other studies [7, 39]. The three studies that suggested an inverse association [21, 24, 35] did not adjust for total energy intake.
Table 5.
Studies evaluating fish intake.
| Reference | Country | Age | Cases/Controls or total cohort | Type of study | Exposure | Contrast | OR (95% CI) | P for trend | Covariates considered* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | E | S | H | R | |||||||||
| Zheng et al., 1995[29] | United States | 55–69 | 216/23,070 | Cohort | Seafood | Q3 vs. Q1 | 1.4 | >0.05 | 1 | 1 | 1 | 2 | ||
| Shu et al., 1993 [9] | China | 18–74 | 268/268 | Population-based cc | Fresh fish | >300 vs. <100 g/week | 1.7 | 0.12 | 1 | 1 | 1 | 1 | ||
| Goodman et al., 1997 [34] | United States | 18–84 | 332/511 | Population based cc | Fish | >41.7 vs. <10.1 g/day | 1.5 | 0.21 | (A) | 1 | 1 | 1 | ||
| McCann et al., 2000 [35] | United States | 40–85 | 232/639 | Population based cc | Fish/seafood | >9.5 vs. <4.5 times/month | 0.7 (0.4–1.1) | 0.23 | 1 | 1 | 1 | 1 | 3 | |
| Jain et al., 2000[39] | Canada | 30–79 | 552/562 | Population based cc | Fish | >35.6 vs. <7.6 g/day | 0.97 (0.67–1.4) | 0.79 | 1 | 1 | 1 | 1 | 1 | 2 |
| Terry et al., 2002[21] | Sweden | 50–74 | 698/2720 | Population based cc | Total fish | Q4 vs. Q1(median: 3.5 vs. 0.9 servings/week) | 0.8 (0.6–1) | 0.05 | 1 | 1 | 1 | |||
| Xu et al., 2006 [37] | China | 30–69 | 1204/1212 | Population-based cc | Total fish | >65.7 vs. <17.6 g/day | 2.4 (1.8–3.1) | <0.01 | 1 | 1 | 1 | 1 | ||
| Levi et al., 1993 [7] | Italy and Switzerland | 30–75 | 274/572 | Hospital-based cc | Fish | Q3 vs. Q1 | 0.93 | 1 | ||||||
| Hirose et al., 1996[40] | Japan | >20 | 145/26,751 | Hospital-based cc | Boiled or broiled fish, sashimi | >1–2/week vs. <3/month | 1.46 (0.78–2.71) | 1 | 1 | 1 | 2 | |||
| Fernandez et al., 1999 [24] | Italy | <75 | 750/7,990 | Hospital-based cc | Fish (categorical) | >2 vs. <1 servings/week | 0.8 (0.6–0.9) | <0.05 | 1 | 1 | 1 | |||
| Fish (continuous) | Per serving/week | 0.9 (0.8–1) | 1 | 1 | 1 | |||||||||
Adjustment columns: A = Age; B = BMI/weight; E = Total Energy; S = Smoking; H = HRT/ERT use; R = Reproductive factors; (A): matched on age. Numbers in columns refer to the number of covariates adjusted for under that grouping. Abbreviations: Q: quantile; T: tertile; cc: case-control.
Some studies have evaluated fish subtypes (data not shown). Shu et al. [9] evaluated “other seafood” (i.e., other than “fresh fish”) and reported no association. Terry et al. [21] found that the inverse association found for total fish was much stronger for fatty fish (e.g., salmon, herring, mackerel), whereas lean fish (e.g., cod, flounder, shellfish) was unrelated to risk. The adjusted OR (although not adjusted for total energy intake) for those in the highest quartile of consumption (median: 2 servings/week) of fatty fish compared to the lowest (median: 0.2 servings/week) was 0.6 (95% CI: 0.5–0.8) with a significant test for trend (p=0.0002). Xu et al. [37] reported elevated risk in the Shanghai case-control study for total fish, marine fish, fresh water fish, shrimp and crab, eel, and shellfish. ORs for the highest intake of these fish subtypes compared to the lowest ranged from 1.3 to 2.4 (none of the confidence intervals included one).
The suggestion of an increased risk in case-control studies conducted in China, Japan, and Hawaii, and decreased risk in other populations may indicate that different fish preparation methods or other population characteristics may play a role. This kind of information was not presented and therefore cannot be evaluated.
Meta-analysis
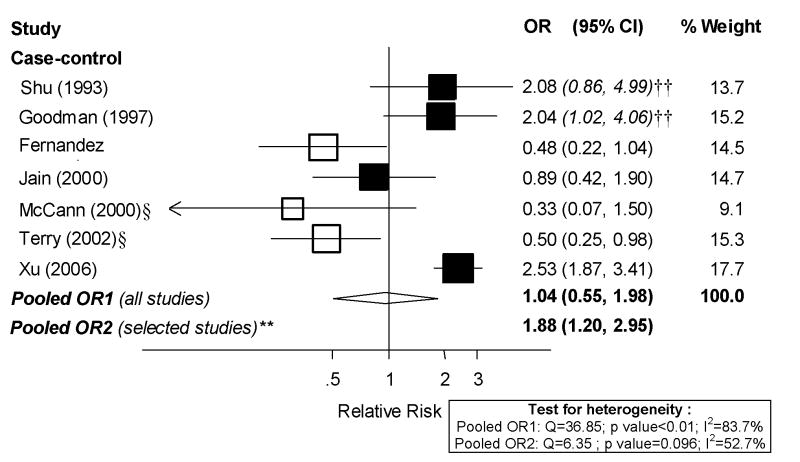
The available data only allowed meta-analyses for total fish. One cohort study [29] and one case-control study [7] could not be included in dose-response analyses because exposure levels were not presented. Also, the study by Hirose et al. [40] was not included because adjusted results were presented in only two categories of fish consumption. The remaining seven case-control studies were included in the dose-response analysis, presented in Figure 4. There was moderate to high heterogeneity among studies (I2: 83.7%, p value<0.01) and, overall, not much evidence of an association. The random-effects pooled OR was 1.04 (95% CI: 0.55, 1.98). Given the high heterogeneity and little support for an association, we decided not to pursue high vs. low analyses. We repeated analyses excluding selected studies that did not meet our quality criteria. After excluding the three studies that did not adjust for total energy intake, the pooled summary estimate became much stronger (OR: 1.88; 95% CI: 1.20–2.98) and heterogeneity among studies was no longer significant (Figure 4).
Figure 4. Random-effects meta-analysis of fish intake and endometrial cancer risk (per 100 g/day).
**Excluding studies for the following reasons: * Hospital-based; † Less than 200 cases; ‡ exclusion of hysterectomies not clearly specified; § not adjusted for total energy intake, || not adjusted for BMI/weight.
†† estimated confidence interval.
Eggs
Only a few studies have evaluated egg consumption and endometrial cancer risk, with inconsistent results (Table 6). The Iowa Women’s Health Study [29] suggested a possible increased risk, with a RR of 1.3 (no confidence limits presented) comparing high to low egg intake. Out of the five case-control studies reporting on this association [7, 9, 34, 37, 40], three reported increased risk associated with higher levels of egg consumption [7, 9, 34]. The other two studies found little evidence of an association.
Table 6.
Studies evaluating egg intake.
| Reference | Country | Age | Cases/ Controls or total cohort | Type of study | Exposure | Contrast | OR (95% CI) | P for trend | Covariates considered* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | E | S | H | R | |||||||||
| Zheng et al., 1995[29] | United States | 55–69 | 216/23,070 | Cohort | Eggs | Q3 vs. Q1 | 1.3 | >0.05 | 1 | 1 | 1 | 2 | ||
| Shu et al., 1993 [9] | China | 18–74 | 268/268 | Population- based cc | Eggs | >300 vs. <50 g/week | 2.1 | <0.01 | 1 | 1 | 1 | 1 | ||
| Goodman et al., 1997 [34] | United States | 18–84 | 332/511 | Population based cc | Eggs | >23.1 vs. <6 g/day | 1.6 | 0.06 | (A) | 1 | 1 | 1 | ||
| Xu et al., 2006 [37] | China | 30–69 | 1204/1212 | Population- based cc | Eggs | >43.7 vs. <12.5 g/day | 0.9 (0.7–1.2) | 1 | 1 | 1 | 1 | |||
| Levi et al., 1993 [7] | Italy and Switzerland | 30–75 | 274/572 | Hospital- based cc | Eggs | Q3 vs. Q1 | 2.13 | <0.01 | 1 | 1 | ||||
| Hirose et al., 1996[40] | Japan | >20 | 145/26,751 | Hospital- based cc | Eggs | >3 vs. <1–2 servings/week | 0.85 (0.54–1.34) | 1 | 1 | 1 | 2 | |||
Adjustment columns: A = Age; B = BMI/weight; E = Total Energy; S = Smoking; H = HRT/ERT use; R = Reproductive factors; (A): matched on age. Numbers in columns refer to the number of covariates adjusted for under that grouping. Abbreviations: Q: quantile; T: tertile; cc: case-control.
Meta-analysis
The one cohort study [29] and five case-control studies [7, 9, 34, 37, 40] identified were included in meta-analyses. However, because the cohort study [29] and one of the case-control studies [7] did not present exposure levels, we only conducted high vs. low meta-analyses, shown in Figure 5. Also, we estimated confidence intervals for the cohort study [29] and three of the case-control studies [7, 9, 34] so that they could be included in meta-analyses. Although there was some suggestion of an increased risk of endometrial cancer risk associated with egg consumption, given the data limitations, we decided not to conduct dose-response analyses or sensitivity analyses.
Figure 5. Random-effects meta-analysis of eggs intake and endometrial cancer risk (highest vs. lowest category).
*Hospital-based; † Less than 200 cases; ‡ exclusion of hysterectomies not clearly specified; § not adjusted for total energy intake, || not adjusted for BMI/weight, †† estimated confidence interval.
Dairy products
Studies evaluating dairy products either as a group or specific dairy products (e.g., milk) have generally provided little support for an association with risk of endometrial cancer (Table 7). Zheng et al. [29] examined the association between total dairy intake and endometrial cancer risk in the Iowa Women’s Health Study and did not find an association. Eight case-control studies [20, 30, 31, 33–36, 38] examined the association of dairy foods as a group, with inconsistent results. ORs of 1.2 were found in two of these studies [31, 33], while three studies suggested an inverse association [34, 35, 38] and the remaining three studies [20, 30, 36] no association. All reported confidence intervals that included the null.
Table 7.
Studies evaluating dairy products intake.
| Reference | Country | Age | Cases/ Controls or total cohort | Type of study | Exposure | Contrast | OR (95% CI) | P for trend | Covariates considered* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | E | S | H | R | |||||||||
| Zheng et al., 1995[29] | United States | 55–69 | 216/23,070 | Cohort | Dairy products | Q3 vs. Q1 | 1.1 | >0.05 | 1 | 1 | 1 | 2 | ||
| Potischman et al., 1993 [33] | United States | 20–74 | 399/296 | Population- based cc | Dairy foods | >17.6 vs. <6 times/month | 1.2 (0.7–2.0) | 1 | 1 | 1 | 1 | 1 | 1 | |
| Goodman et al., 1997 [34] | United States | 18–84 | 332/511 | Population- based cc | Dairy products | >301 vs. <60 g/day | 0.7 | 0.28 | (A) | 1 | 1 | 1 | ||
| McCann et al., 2000 [35] | United States | 40–85 | 232/639 | Population- based cc | Dairy | >56 vs. <32 times/month | 0.8 (0.5–1.3) | 0.25 | 1 | 1 | 1 | 1 | 3 | |
| Littman et al., 2001[36] | United States | 45–74 | 679/944 | Population- based cc | Dairy products | >2.4 vs. <1.2 servings/day | 1 (0.78–1.4) | 0.78 | 1 | 1 | 1 | 1 | 1 | |
| Terry et al., 2002[20] | Sweden | 50–74 | 709/2,877 | Population- based cc | Dairy products | Q4 vs. Q1 (35 vs. 5 median consumption per week) | 0.9 (0.7–1.2) | 0.3 | 1 | 1 | 1 | |||
| Tzonou et al., 1996[30] | Greece | 145/298 | Hospital- based cc | Dairy products | Per quartile | 0.94 (0.74–1.19) | 1 | 1 | 1 | 1 | 1 | 5 | ||
| Petridou et al., 2002 [31] | Greece | 84/84 | Hospital- based cc | Dairy products | Per quartile | 1.21 (0.86–1.69) | (A) | 1 | 1 | 1 | ||||
| Salazar-Martinez et al., 2005 [38] | Mexico | 18–81 | 85/629 | Hospital- based cc | Dairy products | T3 vs. T1 | 0.5 (0.23–1.13) | 0.23 | 1 | 1 | 1 | 1 | ||
Adjustment columns: A = Age; B = BMI/weight; E = Total Energy; S = Smoking; H = HRT/ERT use; R = Reproductive factors; (A): matched on age. Numbers in columns refer to the number of covariates adjusted for under that grouping. Abbreviations: Q: quantile; T: tertile; cc: case-control.
The relationship with milk intake was evaluated in a cohort study and seven case-control studies (data not shown) and, overall, the results are inconsistent. The cohort study [41], conducted in Norway, suggested elevated risk with milk consumption (age-adjusted RR: 2.16, no confidence limits reported; p for trend: 0.29; based on 11 cases of endometrial cancer). Seven case-control studies evaluated the association between milk consumption and endometrial cancer [7, 34, 37, 39, 40, 42, 43]. Five of them examined “milk” without specifying fat content; Goodman et al. [34] reported an OR of 0.8 (no confidence interval reported) for higher levels of milk consumption, while Levi et al. [7] reported an elevated OR (1.62, no confidence intervals shown). The other three found little indication of an association [37, 39, 40]. Confidence intervals were presented for only three of these estimates and they included one. Another hospital-based case-control study evaluated the separate roles of daily consumption of whole milk, 2% milk, and skim milk, compared to none [42]. An increased risk was reported only for whole milk, with an OR of 1.5 (95% CI: 1.0, 2.4). No association was found with the other two types of milk. In contrast, another case-control study [43] suggested a decreased endometrial cancer risk for those who reported consuming skim milk once a month or more often, compared to those consuming it less frequently (OR: 0.6, 95% CI: 0.3,1.0).
Studies evaluating the role of other dairy products offered conflicting results (data not shown). Cheese was evaluated in four case-control studies, with the two conducted in the U.S. suggesting an inverse relationship [34, 43], a study conducted in Italy and Switzerland suggesting a positive association [7], and the fourth, conducted in Canada [39], finding no association. One study conducted in the U.S. [43], also reported that consuming sour cream and yogurt at least once a month was associated with decreased endometrial cancer risk, with OR’s of 0.4 and 0.3, respectively. Both CI’s did not include one. Butter was evaluated in two case-control studies. Levi et al. [7] found elevated risk for those in the highest tertile of butter consumption compared to the lowest (OR: 3.29, no confidence limits reported). In contrast, Goodman et al. [34] suggested decreased risk (OR: 0.8, no confidence limits reported).
Meta-analysis
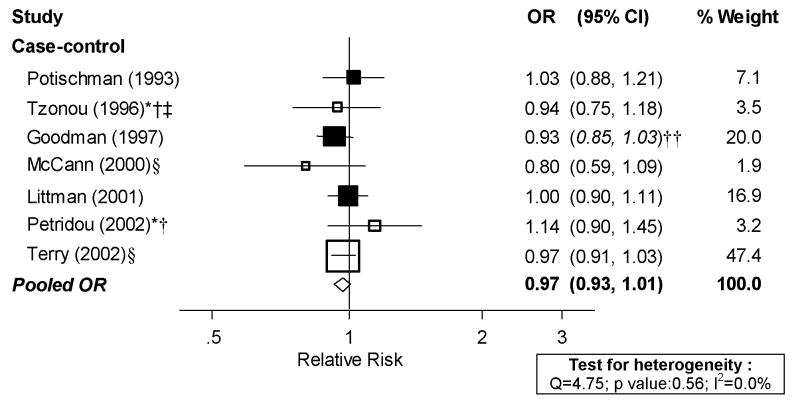
The data available on dairy products allowed meta-analysis only on total dairy product consumption. Although, as previously mentioned, there were five studies evaluating milk consumption, given the heterogeneous results and the fact that two of them only presented a qualitative assessment (i.e., “ever/never” or “daily vs. occasional use”) we decided not to pursue a meta-analysis of milk. For total dairy products, we identified one cohort study [29] and eight case-control studies [20, 30, 31, 33–36, 38]. However, the cohort study [29] and one of the case-control studies [38] could not be included in dose-response analyses because exposure levels were not presented. Dose-response analyses for dairy products are presented in Figure 6. There was no evidence of heterogeneity among studies (I2: 0.0%, p value: 0.562), with a random effects pooled OR of 0.97 per serving/day of dairy product (95% CI: 0.93, 1.01). The excluded cohort study also failed to find an association. We did not repeat analyses excluding studies not meeting our quality criteria because the remaining studies (indicated in Figure 6 with solid squares for the point estimates) clearly did not show an association.
Figure 6. Random-effects meta-analysis of dairy products intake and endometrial cancer risk (per 1 serving/day).
*Hospital-based; † Less than 200 cases; ‡ exclusion of hysterectomies not clearly specified; § not adjusted for total energy intake, || not adjusted for BMI/weight, †† estimated confidence interval.
DISCUSSION
This systematic literature review and meta-analysis, including 22 manuscripts from three cohort studies and 16 case-control studies evaluating several animal foods, suggested an increased endometrial cancer risk with meat consumption, particularly with red meat. Women in the highest category of meat or red meat consumption had a 39% and 48%, respectively, higher endometrial cancer risk, compared to the lowest category of consumption. Dose-response analyses indicated a 26% increase in risk per 100g/day of meat intake (95 % CI: 1.03–1.54), based on eight case-control studies and a 51% increase in risk per 100 g/day of red meat intake (95% CI: 1.19–1.93), based on seven case-control studies. These findings should be viewed with caution, given the high and significant heterogeneity among studies and because the only cohort study, the Iowa Women’s Health Study, [29] evaluating the association with meat and red meat intake found no association. However, it should be pointed out that analyses from this cohort also failed to find an association between red meat and colorectal cancer, which has generally been supported by other cohort studies [5]. The current epidemiologic literature does not support an association between dairy products and endometrial cancer. The evidence for poultry, fish, and eggs is limited and inconsistent. As for red meat, more studies, particularly cohort studies, are needed.
To our knowledge, this is the first comprehensive systematic literature review and meta-analysis evaluating the evidence for animal products and endometrial cancer risk. A narrative review of the literature of diet and endometrial cancer was included in the 1997 WCRF/AICR Report [4]. Meat consumption was not mentioned in this report. The evidence for fish consumption was found to be inconsistent and insufficient, based on three studies [7–9]. We excluded one of these studies [8] because it did not show risk estimates, only a comparison of mean fish intake in cases and controls. We identified one cohort [29] and nine case-control studies [7, 9, 21, 24, 34, 35, 37, 39, 40] reporting on fish and found little evidence of an association. However, when we excluded the studies that did not adjust for total energy intake, there was an indication of an increased risk associated with fish consumption. Three case-control studies [7–9] reporting on eggs were mentioned in the 1997 WCRF/AICR report, but again, the evidence was deemed insufficient. We identified one cohort [29] and five case-control studies [7, 9, 34, 37, 40] reporting on eggs, which, overall, provided inconsistent results. More studies are needed to make any conclusions regarding egg consumption and risk. Dairy products were not mentioned in the 1997 WCRF/AICR report. We found one cohort study [29] and eight case-control studies [20, 30, 31, 33–36, 38], and little support for a role of dairy food consumption on endometrial cancer risk.
There is a growing body of evidence linking red meat with certain cancers, particularly colorectal cancer [44]. Several mechanisms have been proposed for a carcinogenic effect of red meat. It is known that the processing and preparation of meat may result in the generation of carcinogenic n-nitroso compounds and heterocylic amines [45]. High red meat intake may result in higher pro-oxidant load from consumption of readily-absorbed heme iron, resulting in greater oxidative stress and potential for DNA damage [46, 47].
It has been postulated that higher meat consumption may also increase cancer risk because it may be associated with generally unhealthier dietary patterns, for example, lower fruit and vegetable and higher fat intakes. Its relatively high concentration of food energy may also be associated with higher total energy intake and higher body mass. Most of the studies took body mass index and total energy intake, but not all of them did. This should be kept in mind in both the interpretation of the current evidence and in the planning of future studies evaluating the role of meat intake on endometrial cancer risk.
Animal foods are also known to contain estradiol and its metabolites in various concentrations depending on several factors, including type of food, species, gender, physiological stage, and age [6]. In addition, the administration of exogenous sex steroids for growth promotion in meat-producing animals has been a common agricultural practice in the United States for decades [48]. However, the use of hormonal drugs in animal meat production is highly controversial, scientifically and politically. While most beef cattle in the US receive exogenous sex steroids, their use and import of meat from such animals is banned by the European Union [49]. The European Commission’s Scientific Committee on Veterinary Measures relating to Public Health stated that estradiol 17β is a likely complete carcinogen, both initiating and promoting carcinogenesis, as partial justification of this ban [50]. Unfortunately, the literature on red meat intake and endometrial cancer does not provide much insight into whether this is an important reason for the observed association, as we identified only one study conducted in Europe [23] that examined this association.
Concerns have also been raised regarding cow’s milk as an important source of food estrogens [13, 51]; although sex hormone treatments are not used in dairy animals in the United States [48], milk today is produced from pregnant cows [13]. Interestingly, we did not find much evidence for a relationship between dairy product consumption and endometrial cancer. Fish may also contain environmental pollutants including endocrine disrupting chemicals with estrogenic activity, such as organochlorines residues or polychlorinated biphenyls (PCBs) [37].
A limitation of the current body of literature is the lack of a clear definition of what foods should be included in the meat group. As shown in the Appendix Table, some studies have included composite meals, such as stews, which may contain vegetables and counteract the potential detrimental effect of meat. Fish was included in the meat group in some studies but not in others. In many studies what was included in the meat group was not specified. Clearly, future studies should aim towards more standardized definitions of meat, red meat, and other food groups.
Our meta-analyses were limited by the relatively small number of studies examining a given exposure, which precluded the evaluation of publication bias, or the conduct of more sophisticated sensitivity analyses and metaregression to ascertain possible causes of heterogeneity among studies. However, our systematic review and meta-analyses point to general trends in the data and underscore the need for additional population-based studies, and particularly prospective cohort studies, evaluating the relationship between animal product consumption and endometrial cancer risk.
In summary, the current epidemiologic literature, although limited, points to an increased endometrial cancer risk associated with meat and red meat intake. Because the current evidence is based mostly on case-control data, which may be more prone to selection and recall bias, no firm conclusions can be drawn at the present time. We encourage the evaluation of the role of red meat and other animal foods such as fish or eggs on endometrial cancer risk in cohort studies. Such analyses should control for the effects of body mass index and total energy intake, as well as other well-known risk factors for the disease. Although the most definitive nutrition-related factor to target to reduce endometrial cancer risk is obesity prevention, understanding the role of individual dietary factors may provide etiologic clues and additional strategies to prevent this disease.
Acknowledgments
We would like to thank James Thomas for his valuable help with the data extraction Access® program.
Funding: This work was funded in part by the WCRF and NIH-K07 CA095666. Although this work was funded in part by WCRF, interpretation of the evidence may not represent the views of WCRF and our conclusions may differ from those in the 2007 WCRF report summarizing evidence related to food, nutrition, physical activity, and cancer risk.
Abbreviations
- WCRF
World Cancer Research Fund International
- AICR
American Institute for Cancer Research
- SLR
Systematic Literature Review
- OR
Odds Ratio
- RR
Relative Risk
- CI
Confidence Interval
- FFQ
food frequency questionnaire
- BMI
body mass index
- HRT
hormone replacement therapy
APPENDIX 1
WCRF - PUBMED SEARCH STRATEGY
#1 diet therapy[MeSH Terms] OR nutrition[MeSH Terms]
#2 diet[tiab] OR diets[tiab] OR dietetic[tiab] OR dietary[tiab] OR eating[tiab] OR intake[tiab] OR nutrient*[tiab] OR nutrition[tiab] OR vegetarian*[tiab] OR vegan*[tiab] OR “seventh day adventist”[tiab] OR macrobiotic[tiab] OR breastfeed*[tiab] OR breast feed*[tiab] OR breastfed[tiab] OR breast fed[tiab] OR breastmilk[tiab] OR breast milk[tiab]
#3 food and beverages[MeSH Terms]
#4 food*[tiab] OR cereal*[tiab] OR grain*[tiab] OR granary[tiab] OR wholegrain[tiab] OR wholewheat[tiab] OR roots[tiab] OR plantain*[tiab] OR tuber[tiab] OR tubers[tiab] OR vegetable*[tiab] OR fruit*[tiab] OR pulses[tiab] OR beans[tiab] OR lentils[tiab] OR chickpeas[tiab] OR legume*[tiab] OR soy[tiab] OR soya[tiab] OR nut[tiab] OR nuts[tiab] OR peanut*[tiab] OR groundnut*[tiab] OR seeds[tiab] OR meat[tiab] OR beef[tiab] OR pork[tiab] OR lamb[tiab] OR poultry[tiab] OR chicken[tiab] OR turkey[tiab] OR duck[tiab] OR fish[tiab] OR fat[tiab] OR fats[tiab] OR fatty[tiab] OR egg[tiab] OR eggs[tiab] OR bread[tiab] OR oils[tiab] OR shellfish[tiab] OR seafood[tiab] OR sugar[tiab] OR syrup[tiab] OR dairy[tiab] OR milk[tiab] OR herbs[tiab] OR spices[tiab] OR chilli[tiab] OR chillis[tiab] OR pepper*[tiab] OR condiments[tiab]
#5 fluid intake[tiab] OR water[tiab] OR drinks[tiab] OR drinking[tiab] OR tea[tiab] OR coffee[tiab] OR caffeine[tiab] OR juice[tiab] OR beer[tiab] OR spirits[tiab] OR liquor[tiab] OR wine[tiab] OR alcohol[tiab] OR alcoholic[tiab] OR beverage*[tiab] OR ethanol[tiab] OR yerba mate[tiab] OR ilex paraguariensis[tiab]
#6 pesticides[MeSH Terms] OR fertilizers[MeSH Terms] OR “veterinary drugs”[MeSH Terms]
#7 pesticide*[tiab] OR herbicide*[tiab] OR DDT[tiab] OR fertiliser*[tiab] OR fertilizer*[tiab] OR organic[tiab] OR contaminants[tiab] OR contaminate*[tiab] OR veterinary drug*[tiab] OR polychlorinated dibenzofuran*[tiab] OR PCDF*[tiab] OR polychlorinated dibenzodioxin*[tiab] OR PCDD*[tiab] OR polychlorinated biphenyl*[tiab] OR PCB*[tiab] OR cadmium[tiab] OR arsenic[tiab] OR chlorinated hydrocarbon*[tiab] OR microbial contamination*[tiab]
#8 food preservation[MeSH Terms]
#9 mycotoxin*[tiab] OR aflatoxin*[tiab] OR pickled[tiab] OR bottled[tiab] OR bottling[tiab] OR canned[tiab] OR canning[tiab] OR vacuum pack*[tiab] OR refrigerate*[tiab] OR refrigeration[tiab] OR cured[tiab] OR smoked[tiab] OR preserved[tiab] OR preservatives[tiab] OR nitrosamine[tiab] OR hydrogenation[tiab] OR fortified[tiab] OR additive*[tiab] OR colouring*[tiab] OR coloring*[tiab] OR flavouring*[tiab] OR flavoring*[tiab] OR nitrates[tiab] OR nitrites[tiab] OR solvent[tiab] OR solvents[tiab] OR ferment*[tiab] OR processed[tiab] OR antioxidant*[tiab] OR genetic modif*[tiab] OR genetically modif*[tiab] OR vinyl chloride[tiab] OR packaging[tiab] OR labelling[tiab] OR phthalates[tiab]
#10 cookery[MeSH Terms]
#11 cooking[tiab] OR cooked[tiab] OR grill[tiab] OR grilled[tiab] OR fried[tiab] OR fry[tiab] OR roast[tiab] OR bake[tiab] OR baked[tiab] OR stewing[tiab] OR stewed[tiab] OR casserol*[tiab] OR broil[tiab] OR broiled[tiab] OR boiled[tiab] OR microwave[tiab] OR microwaved[tiab] OR re-heating[tiab] OR reheating[tiab] OR heating[tiab] OR re-heated[tiab] OR heated[tiab] OR poach[tiab] OR poached[tiab] OR steamed[tiab] OR barbecue*[tiab] OR chargrill*[tiab] OR heterocyclic amines[tiab] OR polycyclic aromatic hydrocarbons[tiab]
#12 dietary carbohydrates[MeSH Terms] OR dietary proteins[MeSH Terms] OR sweetening agents[MeSH Terms]
#13 salt[tiab] OR salting[tiab] OR salted[tiab] OR fiber[tiab] OR fibre[tiab] OR polysaccharide*[tiab] OR starch[tiab] OR starchy[tiab] OR carbohydrate*[tiab] OR lipid*[tiab] OR linoleic acid*[tiab] OR sterols[tiab] OR stanols[tiab] OR sugar*[tiab] OR sweetener*[tiab] OR saccharin*[tiab] OR aspartame[tiab] OR acesulfame[tiab] OR cyclamates[tiab] OR maltose[tiab] OR mannitol[tiab] OR sorbitol[tiab] OR sucrose[tiab] OR xylitol[tiab] OR cholesterol[tiab] OR protein[tiab] OR proteins[tiab] OR hydrogenated dietary oils[tiab] OR hydrogenated lard[tiab] OR hydrogenated oils[tiab]
#14 vitamins[MeSH Terms]
#15 supplements[tiab] OR supplement[tiab] OR vitamin*[tiab] OR retinol[tiab] OR carotenoid*[tiab] OR tocopherol[tiab] OR folate*[tiab] OR folic acid[tiab] OR methionine[tiab] OR riboflavin[tiab] OR thiamine[tiab] OR niacin[tiab] OR pyridoxine[tiab] OR cobalamin[tiab] OR mineral*[tiab] OR sodium[tiab] OR iron[tiab] OR calcium[tiab] OR selenium[tiab] OR iodine[tiab] OR magnesium[tiab] OR potassium[tiab] OR zinc[tiab] OR copper[tiab] OR phosphorus[tiab] OR manganese[tiab] OR chromium[tiab] OR phytochemical[tiab] OR allium[tiab] OR isothiocyanate*[tiab] OR glucosinolate*[tiab] OR indoles[tiab] OR polyphenol*[tiab] OR phytoestrogen*[tiab] OR genistein[tiab] OR saponin*[tiab] OR coumarin*[tiab]
#16 physical fitness[MeSH Terms] OR exertion[MeSH Terms] OR physical endurance[MeSH Terms] or walking[MeSH Terms]
#17 recreational activit*[tiab] OR household activit*[tiab] OR occupational activit*[tiab] OR physical activit*[tiab] OR physical inactivit*[tiab] OR exercise[tiab] OR exercising[tiab] OR energy intake[tiab] OR energy expenditure[tiab] OR energy balance[tiab] OR energy density[tiab]
#18 growth[MeSH Terms] OR anthropometry[MeSH Terms] OR body composition[MeSH Terms] OR body constitution[MeSH Terms]
#19 weight loss[tiab] or weight gain[tiab] OR anthropometry[tiab] OR birth weight[tiab] OR birthweight[tiab] OR birth-weight[tiab] OR child development[tiab] OR height[tiab] OR body composition[tiab] OR body mass[tiab] OR BMI[tiab] OR obesity[tiab] OR obese[tiab] OR overweight[tiab] OR over-weight[tiab] OR over weight[tiab] OR skinfold measurement*[tiab] OR skinfold thickness[tiab] OR DEXA[tiab] OR bio-impedence[tiab] OR waist circumference[tiab] OR hip circumference[tiab] OR waist hip ratio*[tiab]
#20 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19
KEY:
[tiab] searches the title and abstract fields only
[MeSH Terms] searches the Medical Subject Headings field only NB - explosion of MeSH terms is automatic
Footnotes
truncation symbol - searches all words with this combination of letters at the beginning
References
- 1.Cancer Facts and Figures: American Cancer Society; 2006.
- 2.Persson I, Adami H-O. Endometrial cancer. In: Adami H-O, Hunter D, Trichopoulos D, editors. Textbook of Cancer Epidemiology. New York, NY: Oxford University Press; 2002. [Google Scholar]
- 3.IARC Handbooks of Cancer Prevention. Weight Control and Physical Activity. Vol. 6. Lyon: International Agency for Research on Cancer, World Health Organization; 2002. [Google Scholar]
- 4.World Cancer Research Fund, American Institute for Cancer Research. Food, Nutrition and the Prevention of Cancer: A Global Perspective. Washington, DC: American Cancer Institute for Cancer Research; 1997. [DOI] [PubMed] [Google Scholar]
- 5.Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer. 2006;119:2657–64. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 6.Daxenberger A, Ibarreta D, Meyer HH. Possible health impact of animal oestrogens in food. Hum Reprod Update. 2001;7:340–55. doi: 10.1093/humupd/7.3.340. [DOI] [PubMed] [Google Scholar]
- 7.Levi F, Franceschi S, Negri E, La Vecchia C. Dietary factors and the risk of endometrial cancer. Cancer. 1993;71:3575–81. doi: 10.1002/1097-0142(19930601)71:11<3575::aid-cncr2820711119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.La Vecchia C, Decarli A, Fasoli M, Gentile A. Nutrition and diet in the etiology of endometrial cancer. Cancer. 1986;57:1248–53. doi: 10.1002/1097-0142(19860315)57:6<1248::aid-cncr2820570631>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 9.Shu XO, Zheng W, Potischman N, et al. A population-based case-control study of dietary factors and endometrial cancer in Shanghai, People’s Republic of China. Am J Epidemiol. 1993;137:155–65. doi: 10.1093/oxfordjournals.aje.a116655. [DOI] [PubMed] [Google Scholar]
- 10.Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Second Report on Food, Nutrition, Physical Activity and the Prevention of Cancer. World Cancer Research Fund International/American Institute for Cancer Research; 2007. The association between food, nutrition, and physical activity and the risk of endometrial cancer and underlying mechanisms. In Press. [Google Scholar]
- 11.Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Fruits and vegetables and endometrial cancer risk: a systematic literature review and meta-analysis. Nutr Cancer. 58 doi: 10.1080/01635580701307929. In Press. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975;15:617–31. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- 13.Ganmaa D, Sato A. The possible role of female sex hormones in milk from pregnant cows in the development of breast, ovarian and corpus uteri cancers. Med Hypotheses. 2005;65:1028–37. doi: 10.1016/j.mehy.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Tao MH, Xu WH, Zheng W, et al. A case-control study in Shanghai of fruit and vegetable intake and endometrial cancer. Br J Cancer. 2005;92:2059–64. doi: 10.1038/sj.bjc.6602609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lissner L, Kroon UB, Bjorntorp P, Blosk S, Wilhelmsen L, Silverstolpe G. Adipose tissue fatty acids and dietary fat sources in relation to endometrial cancer: a retrospective study of cases in remission, and population-based controls. Acta Obstet Gynecol Scand. 1993;72:481–7. doi: 10.3109/00016349309021139. [DOI] [PubMed] [Google Scholar]
- 16.Weiderpass E, Adami HO, Baron JA, et al. Organochlorines and endometrial cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9:487–93. [PubMed] [Google Scholar]
- 17.Kinlen LJ. Meat and fat consumption and cancer mortality: A study of strict religious orders in Britain. Lancet. 1982;1:946–9. doi: 10.1016/s0140-6736(82)91943-2. [DOI] [PubMed] [Google Scholar]
- 18.Zemla B, Guminski S, Banasik R. Study of risk factors in invasive cancer of the corpus uteri. Neoplasma. 1986;33:621–9. [PubMed] [Google Scholar]
- 19.Zemla B, Guminski S, Franek K, Kolosza Z, Banasik R. Etiological factors in invasive corpus uteri carcinoma. Neoplasma. 1991;38:157–63. [PubMed] [Google Scholar]
- 20.Terry P, Vainio H, Wolk A, Weiderpass E. Dietary factors in relation to endometrial cancer: a nationwide case-control study in Sweden. Nutr Cancer. 2002;42:25–32. doi: 10.1207/S15327914NC421_4. [DOI] [PubMed] [Google Scholar]
- 21.Terry P, Wolk A, Vainio H, Weiderpass E. Fatty fish consumption lowers the risk of endometrial cancer: a nationwide case-control study in Sweden. Cancer Epidemiol Biomarkers Prev. 2002;11:143–5. [PubMed] [Google Scholar]
- 22.La Vecchia C, Decarli A, Negri E, Parazzini F. Epidemiological aspects of diet and cancer: a summary review of case-control studies from northern Italy. Oncology. 1988;45:364–70. doi: 10.1159/000226642. [DOI] [PubMed] [Google Scholar]
- 23.Tavani A, La Vecchia C, Gallus S, et al. Red meat intake and cancer risk: a study in Italy. Int J Cancer. 2000;86:425–8. doi: 10.1002/(sici)1097-0215(20000501)86:3<425::aid-ijc19>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez E, Chatenoud L, La Vecchia C, Negri E, Franceschi S. Fish consumption and cancer risk. Am J Clin Nutr. 1999;70:85–90. doi: 10.1093/ajcn/70.1.85. [DOI] [PubMed] [Google Scholar]
- 25.Rothman KJ. Modern epidemiology. 1. Boston: Little, Brown; 1986. pp. 174–5. [Google Scholar]
- 26.Chene G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol. 1996;144:610–21. doi: 10.1093/oxfordjournals.aje.a008971. [DOI] [PubMed] [Google Scholar]
- 27.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 28.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 29.Zheng W, Kushi LH, Potter JD, et al. Dietary intake of energy and animal foods and endometrial cancer incidence. The Iowa Women’s Health Study. Am J Epidemiol. 1995;142:388–94. doi: 10.1093/oxfordjournals.aje.a117646. [DOI] [PubMed] [Google Scholar]
- 30.Tzonou A, Lipworth L, Kalandidi A, et al. Dietary factors and the risk of endometrial cancer: a case--control study in Greece. Br J Cancer. 1996;73:1284–90. doi: 10.1038/bjc.1996.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petridou E, Kedikoglou S, Koukoulomatis P, Dessypris N, Trichopoulos D. Diet in relation to endometrial cancer risk: a case-control study in Greece. Nutr Cancer. 2002;44:16–22. doi: 10.1207/S15327914NC441_3. [DOI] [PubMed] [Google Scholar]
- 32.Knekt P, Steineck G, Jarvinen R, Hakulinen T, Aromaa A. Intake of fried meat and risk of cancer: a follow-up study in Finland. Int J Cancer. 1994;59:756–60. doi: 10.1002/ijc.2910590608. [DOI] [PubMed] [Google Scholar]
- 33.Potischman N, Swanson CA, Brinton LA, et al. Dietary associations in a case-control study of endometrial cancer. Cancer Causes Control. 1993;4:239–50. doi: 10.1007/BF00051319. [DOI] [PubMed] [Google Scholar]
- 34.Goodman MT, Hankin JH, Wilkens LR, et al. Diet, body size, physical activity, and the risk of endometrial cancer. Cancer Res. 1997;57:5077–85. [PubMed] [Google Scholar]
- 35.McCann SE, Freudenheim JL, Marshall JR, Brasure JR, Swanson MK, Graham S. Diet in the epidemiology of endometrial cancer in western New York (United States) Cancer Causes Control. 2000;11:965–74. doi: 10.1023/a:1026551309873. [DOI] [PubMed] [Google Scholar]
- 36.Littman AJ, Beresford SA, White E. The association of dietary fat and plant foods with endometrial cancer (United States) Cancer Causes Control. 2001;12:691–702. doi: 10.1023/a:1011292003586. [DOI] [PubMed] [Google Scholar]
- 37.Xu WH, Dai Q, Xiang YB, et al. Animal food intake and cooking methods in relation to endometrial cancer risk in Shanghai. Br J Cancer. 2006 doi: 10.1038/sj.bjc.6603458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salazar-Martinez E, Lazcano-Ponce E, Sanchez-Zamorano LM, Gonzalez-Lira G, Escudero de los Rios P, Hernandez-Avila M. Dietary factors and endometrial cancer risk. Results of a case-control study in Mexico. Int J Gynecol Cancer. 2005;15:938–45. doi: 10.1111/j.1525-1438.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 39.Jain MG, Howe GR, Rohan TE. Nutritional factors and endometrial cancer in Ontario, Canada. Cancer Control. 2000;7:288–96. doi: 10.1177/107327480000700312. [DOI] [PubMed] [Google Scholar]
- 40.Hirose K, Tajima K, Hamajima N, et al. Subsite (cervix/endometrium)-specific risk and protective factors in uterus cancer. Jpn J Cancer Res. 1996;87:1001–9. doi: 10.1111/j.1349-7006.1996.tb02132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ursin G, Bjelke E, Heuch I, Vollset SE. Milk consumption and cancer incidence: a Norwegian prospective study. Br J Cancer. 1990;61:456–9. doi: 10.1038/bjc.1990.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mettlin CJ, Schoenfeld ER, Natarajan N. Patterns of milk consumption and risk of cancer. Nutr Cancer. 1990;13:89–99. doi: 10.1080/01635589009514049. [DOI] [PubMed] [Google Scholar]
- 43.Barbone F, Austin H, Partridge EE. Diet and endometrial cancer: a case-control study. Am J Epidemiol. 1993;137:393–403. doi: 10.1093/oxfordjournals.aje.a116687. [DOI] [PubMed] [Google Scholar]
- 44.Kushi LH, Byers T, Doyle C, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56:254–81. doi: 10.3322/canjclin.56.5.254. quiz 313–4. [DOI] [PubMed] [Google Scholar]
- 45.Bingham SA. High-meat diets and cancer risk. Proc Nutr Soc. 1999;58:243–8. doi: 10.1017/s0029665199000336. [DOI] [PubMed] [Google Scholar]
- 46.Weinberg ED. The role of iron in cancer. Eur J Cancer Prev. 1996;5:19–36. [PubMed] [Google Scholar]
- 47.McCord JM. Iron, free radicals, and oxidative injury. Semin Hematol. 1998;35:5–12. [PubMed] [Google Scholar]
- 48.Andersson AM, Skakkebaek NE. Exposure to exogenous estrogens in food: possible impact on human development and health. Eur J Endocrinol. 1999;140:477–85. doi: 10.1530/eje.0.1400477. [DOI] [PubMed] [Google Scholar]
- 49.Matthews KH, Bernstein JJCB. International trade of meat/poultry products and food safety issues. In: Buzby JC, editor. International trade and food safety: economic theory and case studies. Economic Research Service/USDA: Agricultural Economic Report No. AER828; 2003. pp. 48–73. http://www.ers.usda.gov/publications/aer828/aer828f.pdf. [Google Scholar]
- 50.European Commission, Health and Consumer Protection Directorate-General. Opinion of the Scientific Committee on Veterinary Measures Relating to Public Health on Review of previous SCVPH opinions of 30 April 1999 and 3 May 2000 on the potential risks to human health from hormones residues in bovine meat and meat products; (Adopted on 10 April 2002).
- 51.Malekinejad H, Scherpenisse P, Bergwerff AA. Naturally Occurring Estrogens in Processed Milk and in Raw Milk (from Gestated Cows) J Agric Food Chem. 2006;54:9785–91. doi: 10.1021/jf061972e. [DOI] [PubMed] [Google Scholar]