Abstract
Thrombin-Activatable Fibrinolysis Inhibitor (TAFI) is a protein that attenuates fibrinolysis potently. A considerable proportion of its variability levels is genetically determined. It has been associated with arterial and venous thrombosis. We conducted a genomewide linkage scan for genes affecting variation in plasma TAFI levels in 398 subjects from 21 extended Spanish families. The data were analyzed by a variance-component linkage method.
A strong linkage was found on the long arm of Chromosome 13, near the DNA marker D13S156, where the structural gene encoding for TAFI is located. In addition, other new linkage signals were detected on chromosome regions 5p and 7q. More importantly, we performed another multipoint linkage analysis of functional TAFI conditioned on TAFI antigen levels. We detected a strong linkage signal on Chromosome 19 (LOD = 3.0, p = 0.0001) suggesting a novel QTL in this region involved in the specific functional activity of TAFI, regardless of the TAFI antigen levels.
One notable aspect of this study is the identification of new QTLs that reveal a clearer picture of the genetic determinants responsible for variation in TAFI levels. Another is the replication of the linkage signal of the CPB2 gene, which confirms an important genetic determinant for TAFI antigen levels. These results strongly suggest an oligogenic mode of inheritance for TAFI, in which CPB2 gene accounts for a proportion of the variation of the phenotype together with other unknown genes that may represent potential risk factors for thrombotic disease.
Keywords: TAFI, Genome Wide Scan, QTL, Thrombotic risk, Complex Diseases
Introduction
The thrombin-activatable fibrinolysis inhibitor (TAFI) (Bajzar et al. 1995) as an inhibitor of fibrinolysis. It represents a crucial link between the coagulation system and fibrinolysis. TAFI is also known as procarboxypeptidase U. It is a zymogen secreted by the liver, following activation by the complex thrombin-thrombomodulin. It attenuates fibrinolysis potently by removing the fibrin C-terminal lysine and arginine residues that are important to bind plasminogen (Bajzar et al. 1995; Bouma et al. 2001). The relevance of TAFI levels in venous and arterial thrombotic disease is not absolutely clear. Several studies have investigated the role of funcional (TAFIf) and antigen TAFI (TAFIag) levels in arterial and venous thrombosis. Unfortunately, some of them present contradictory results (de Bruijne et al. 2007; Eichinger et al. 2004; Juhan-Vague and Morange 2003; Juhan-Vague et al. 2002; Ladenvall et al. 2007; Montaner et al. 2003; Schroeder et al. 2002; van Tilburg et al. 2000).
Some studies have demonstrated that a significant proportion of the variation in TAFI plasma levels is genetic. Twin studies in different European populations estimated TAFI heritabilities ranging from 71% to 82% (Ariens et al. 2002; Bladbjerg et al. 2006; Peetz et al. 2004). Also, a recent study of extended Mexican Americans families estimated the additive genetic heritability of TAFI levels as 53% (Warren et al. 2006) and found significant evidence of a Quantitative Trait Locus (QTL) located near the locus of the gene coding for TAFI (CPB2). However, the results concerning the genetic basis of this variation have been equivocal.
The gene encoding TAFI in humans (CPB2, MIM 603101) has 11 exons and measures 48 Kb. It is localized on Chromosome 13q14.11 (Boffa et al. 1999; Tsai and Drayna 1992; Vanhoof et al. 1996). Different polymorphisms in this gene have been described and some of them have been associated with TAFI levels and with the risk of arterial and venous thrombotic disease (Boffa et al. 2008; Brouwers et al. 2001; de Bruijne et al. 2007; Franco et al. 2001; Henry et al. 2001; Juhan-Vague et al. 2002). Nevertheless, the results in different populations are contradictory (Morange et al. 2005) suggesting that the genetic determinants of TAFI levels are still largely unknown. In addition, Tregouet et al. used a combined segregation-linkage analysis and provided evidence for two TAFI-linked QTLs still undetermined that explained 78% of the TAFI variation (Tregouet et al. 2001). Also, evidence for linkage apart from the structural gene have been obtained from a whole genome scan (Warren et al. 2006) meaning that other potential candidate genes influencing TAFI levels have yet to be identified.
To our knowledge, ours is the first study to describe the genetic factors that influence either TAFIf and TAFIag levels in a Spanish population. We hope that our results will help to clarify the genetic factors that determine TAFI levels and, more importantly, that they will help to understand the genetic factors that affect the risk of cardiovascular disease.
Materials and Methods
Subjects and Phenotypes
The recruitment, sampling and phenotyping used in the GAIT Project have been extensively described previously (Souto et al. 2000a; Souto et al. 2000b). Briefly, our sample included 398 individuals in three- to five-generation extended pedigrees. Twelve families were selected through a proband with idiopathic thrombophilia and the remaining 9 families were randomly selected without regard to phenotype. Antigen determination of TAFI levels was performed by an ELISA test, using a commercial kit from Hyphen, Andrésy. TAFIf levels were measured following the method described by Mosnier et al. (Mosnier et al. 1998).
All protocols were reviewed by the Institutional Review Board of the Hospital de la Santa Creu i Sant Pau (Barcelona). Adult subjects gave informed consent for themselves and for their minor children.
Genotypes
DNA was extracted using a standard method (Miller et al. 1988). The genome scan has been described previously (Souto et al. 2000a; Souto et al. 2000b).
In addition, we genotyped 2 Single Nucleotide Polymorphisms (SNPs) in the TAFI structural gene (CPB2), one non-synonymous coding polymorphism located in exon 6 and causing an Ala147Thr variation (rs3742264) and one located in the 3′UTR region of CPB2 gene (rs940).
The genotypic data were entered into a database and were analyzed for discrepancies (i.e. violations of Mendelian inheritance), using the INFER (PEDSYS) program (Dyke 1995). Discrepancies were checked for mistyping, and markers for discrepant individuals were either corrected or excluded from the analysis.
Linkage Analysis
Standard oligogenic multipoint variance component linkage methods, as implemented in the SOLAR software program, were used for the genome scan (Almasy and Blangero 1998). Levels of TAFIag and TAFIf in the GAIT sample exhibited a kurtosis of 0.68 and 1.54 respectively. Recent statistical genetic theory demonstrated that this level of kurtosis does not affect the distribution of LOD scores and that the standard nominal p-values for these LOD scores are appropriate (Blangero et al. 2000). Marker maps for multipoint analyses were obtained from ABI-Prism and from the Marshfield Medical Research Organization. As 12 of the families were ascertained through thrombophilic probands, all analyses included an ascertainment correction achieved by conditioning the likelihood of these pedigrees on the likelihoods of their respective probands (Boehnke and Lange 1984). Genome-wide p-values were calculated using the method of Feingold et al. (Feingold et al. 1993).
Quantitative trait association analyses were performed using the measured genotype approach (Hopper and Mathews 1982) by testing for genotype-specific differences in the means of the trait while allowing for the non-independence among family members. To assess linkage and association simultaneously (Almasy and Blangero 2004) an extension of the variance component-based linkage tests was performed by simultaneously incorporating the genotype-specific means of the measured genotype test. These analyses were performed using the SOLAR software (Almasy and Blangero 1998).
Results
We used a variance component method to analyze TAFIag and TAFIf levels. We introduced sex, age, age squared, smoking behavior and contraceptive use as fixed effects in the linea model.
The TAFIag levels were significantly affected by age and age-squared, but that only accounted for 5% of the variability. Additive genetic effects (heritability) accounted for 40.9% and the remaining 59.1% represented environmental effects and error measurements.
For TAFIf levels, only smoking behavior was significant, accounting for 2% of the variability. Heritability represented 15.6% of the variance, household 22.7% and the remaining 61.6% was due to the error term.
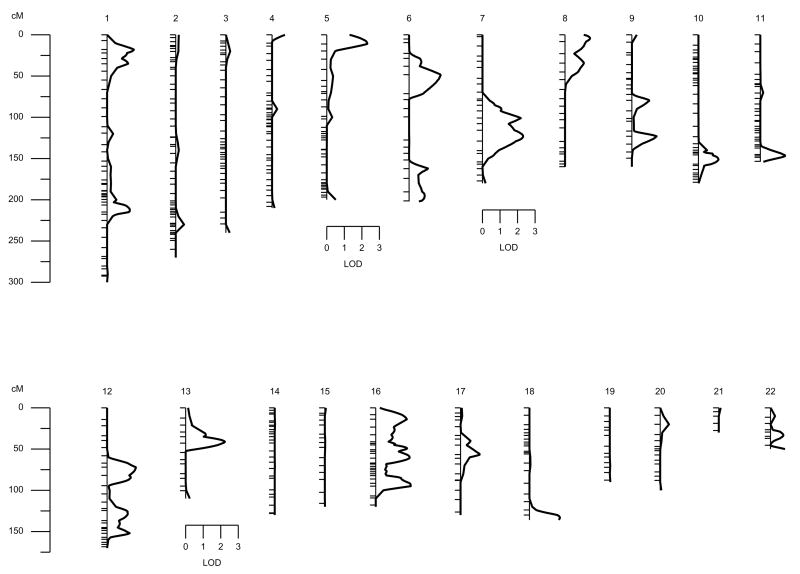
The results of the multipoint linkage analysis over the 22 autosomal chromosomes for TAFIag levels are shown in Table 1 and in Figure 1. A suggestive linkage signal was found on Chromosome 13 (LOD = 2.28, p = 0.0006) in marker D13S156, the area where the structural gene coding for TAFI is located (Figure 1). However, other regions with LODs greater than two appeared on Chromosomes 5p and 7q. In silico search for candidate genes was performed in these chromosomal regions, although no obvious candidate genes were found (Table 1, Figure 1). Thus, our results suggest an oligogenic model of inheritance for TAFIag levels where several genes may be contributing to the TAFI variability.
Table 1.
Chromosome Locations and Lod Scores of the Linkage Signals of TAFIag Levels Analyses.
| Chromosome | Location | Marker | LOD | Nominal p | Wide p |
|---|---|---|---|---|---|
| 5 | 5p15.32 | D5S406 | 2.31 | 0.0005 | 0.26 |
| 7 | 7q31.2 | D7S486 | 2.32 | 0.0005 | 0.26 |
| 13 | 13q14.2 | D13S153 | 2.28 | 0.0006 | 0.28 |
Figure 1.
Results from the initial autosomal multipoint genome scan for TAFIag levels. Hatch marks along chromosomes indicate marker positions.
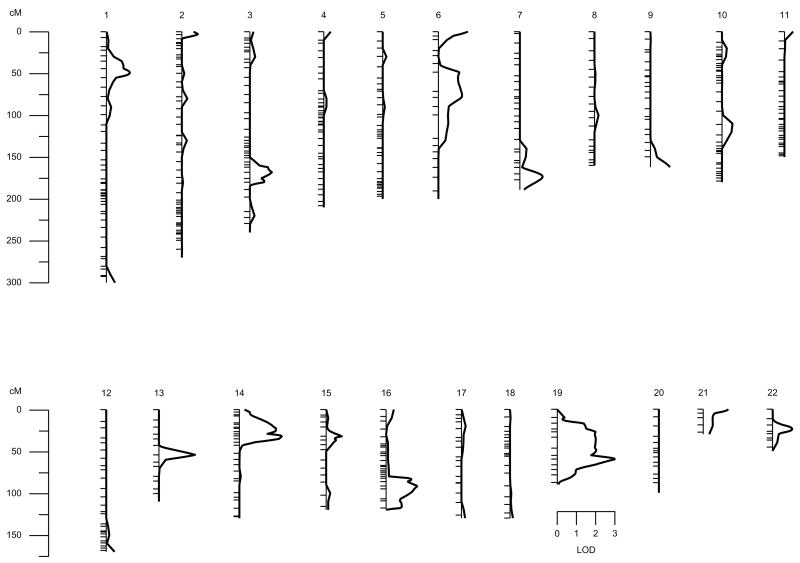
In addition, we also performed an analysis intended to measure only the specific functional activity of TAFI, regardless of the TAFIag levels. With this aim, we performed a multipoint linkage analysis of TAFIag-independent component of functional TAFI levels, i.e. considering the TAFIag levels as a controlled covariable in the linkage analyses. There was a clear evidence of linkage on chromosome region 19q13.3, near the marker D19S420 (LOD = 3.0, p = 0.0001) (Figure 2).
Figure 2.
Results from the autosomal multipoint genome scan for the new phenotype TAFIf conditional on TAFI. Hatch marks along chromosomes indicate marker positions.
Finally, we genotyped 2 polymorphisms of CPB2, located at chromosomal positions 13:45525481 C/T (rs3742264) and 13:45546095 G/C (rs940). The former is located in exon 6 and causes an Ala147Thr variation, and the later is located in the 3′UTR region of the gene. These two polymorphisms have been associated repeatedly with variation in TAFI levels (de Bruijne et al. 2007; Frere et al. 2005; Juhan-Vague et al. 2002; Knoefler et al. 2003; Kostka et al. 2003; Morange et al. 2005). In our sample, the measured genotype analysis revealed a highly significant association of these 2 SNPs (p < 0.0001 for both SNPs) and the TAFIag levels –altogether explained 32% of the variability in TAFIag levels- supporting the role of the CPB2 gene on the variation of TAFI levels. Specifically, rs3742264 explained 18% of the variability, and rs940 explained 28%. When we tested for a combined linkage-association analysis in this area with a model including the two polymorphisms as controlled covariables, the evidence of linkage dropped to 0.95. The fact that linkage did not drop to zero means that these two polymorphisms do not explain all of the linkage signal in this chromosomal region, and after controlling them as covariables, there is still an unexplained variability in the phenotype at this locus. Thus, it is likely that other yet undetermined polymorphic variants in CPB2 are causing the phenotypic variation in TAFIag levels.
Discussion
Activated TAFI down-regulates fibrinolysis by the removal of carboxi-terminal lysines from fibrin. Elimination of these lysines impaires the binding of plasminogen and its activators, resulting in a decreased generation rate of plasmin. Accordingly, many groups have tried to find an association between variation in TAFIag concentration or genotypes and thrombotic disorders (de Bruijne et al. 2007; Eichinger et al. 2004; Ladenvall et al. 2007; Montaner et al. 2003; Schroeder et al. 2002; van Tilburg et al. 2000). Moreover, many allelic variants in the gene enconding TAFI (CPB2) have been genotyped in hopes of finding functional allelic variants that lead to an increased risk of thrombosis (Boffa et al. 2008; Brouwers et al. 2001; de Bruijne et al. 2007; Franco et al. 2001; Henry et al. 2001; Juhan-Vague et al. 2002; Morange et al. 2005). Some studies have estimated the heritability of the TAFIag. For example, twin studies in different European populations estimated TAFI heritabilities ranging from 71% to 82% for TAFI (Ariens et al. 2002; Bladbjerg et al. 2006; Peetz et al. 2004). However, classical twin studies tend to be biased because of environmental effects leading to familiar correlations (Hopper 2002).
Also, a recent study based on extended Mexican Americans families estimated the additive genetic heritability of TAFIag levels as 53% (Warren et al. 2006). These studies found significant evidence of a QTL located near the locus of the TAFI coding gene. It is important to consider that heritability estimates cannot be compared between different populations and thus should be viewed qualitatively (Falconer 1996). In our study, TAFIag showed a heritability of 0.41, which is similar to the estimate by the SAFHS Project based on a subset of healthy Mexican American families (Warren et al. 2006). The heritability was much smaller than that obtained in the twin studies. In any case, the evidence is convincing that genes play a significant role in the variability of TAFIag levels. Therefore, there is a high probability that QTLs will be found that are responsible for this variability.
In contrast, TAFIf levels exhibited a low heritability (0.16) due, in part, to the significant household effect (0.25). The differences in heritaibility between TAFIag and TAFIf could be due in part to the different methodologies used in the measurement of the phenotypes.
From the genome wide scan, we found a highly suggestive signal on Chromosome 13, in the area where the structural gene coding for TAFI is located (LOD = 2.28, p = 0.0006). This result, in combination with the association of the 2 polymorphisms of the TAFI levels, confirms that some polymorphisms in the CPB2 gene are responsible for part of the variability of TAFIag levels. However, the evidence of linkage remained greater than zero (0.95) after including the 2 polymorphisms as covariates, which suggests that they cannot explain all of the evidence of linkage. Moreover, other signals greater than 2 (Table 1) suggest an oligogenic model of inheritance controlling the variability of TAFIag levels. Based on these results, we hypothesize that other genes, in addition to CPB2 on Chromosome 13, are responsible for the variability of TAFIag levels.
Apart from 2 new suggestive signals on Chromosomes 5p and 7q, our results show a strong significant linkage signal on Chromosome 19, near the marker D19S420 (LOD = 3.0, p = 0.0001) (Figure 2). These results are relevant, as it identifies a new QTL on Chromosome 19 involved in the specific functional activity of TAFI, regardless of the TAFIag levels.
The In silico search in these chromosomal regions did not reveal any obvious candidate genes on Chromosome 5. It is interesting that a member of the TFE3 subfamily of transcription factors is located within the linkage signal found on Chromosome 7. Finally, some biologically plausible candidate genes were found on Chromosome 19, such as the transcription factors (USF2) and other genes that may be important for the proper glycosilation of TAFI (G6PI).
Clearly, further studies are needed to fine-map these regions on Chromosomes 5, 7 and 19 to clearly elucidate the implication of these candidate genes. Ultimately, the search for new candidate genes affecting TAFI levels should continue in hopes of finding hallmarks for the risk of cardiovascular disease.
In conclusion, our results confirm the importance of CPB2 gene on TAFIag levels, and emphasize the need to study this gene in more depth. Moreover, we discovered a highly significant linkage signal on Chromosome 19 and two suggestive linkage signals on Chromosomes 5 and 7. Our hope is that our study will stimulate additional studies on this linkage signal that could represent potential new risk factors for thrombotic disease. Further analyses are needed to replicate these signals, and to confirm these provocative results that could be relevant in giving a clearer picture of the genetic determinants that are responsible for TAFI activity and its antigen levels.
Acknowledgments
We would like to acknowledge the advice and helpful discussion of Professor W.H. Stone. This study was supported partially by grants No. 2 R01 HL070751-05 from the USA NIH, PI-05/1361, PI-05/1382 and Redes Temáticas de Investigación Cooperativa (RETIC) Cardiovascular (RECAVA: Exp-06/0014/0016RD Ministerio de Sanidad y Consumo, Spain) and SAF2005-04738 from Ministerio de Ciencia y Tecnología and FEDER (Spain). J.M. Soria was supported by “Programa d'Estabilització d'Investigadors de la Direcció d'Estrategia i Coordinació del Departament de Salut” (Generalitat de Catalunya). Finally, we are indebted to all of the families who participated in the GAIT Project.
References
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Exploring positional candidate genes: linkage conditional on measured genotype. Behav Genet. 2004;34:173–7. doi: 10.1023/B:BEGE.0000013731.03827.69. [DOI] [PubMed] [Google Scholar]
- Ariens RA, de Lange M, Snieder H, Boothby M, Spector TD, Grant PJ. Activation markers of coagulation and fibrinolysis in twins: heritability of the prethrombotic state. Lancet. 2002;359:667–71. doi: 10.1016/S0140-6736(02)07813-3. [DOI] [PubMed] [Google Scholar]
- Bajzar L, Manuel R, Nesheim ME. Purification and characterization of TAFI, a thrombin-activable fibrinolysis inhibitor. J Biol Chem. 1995;270:14477–84. doi: 10.1074/jbc.270.24.14477. [DOI] [PubMed] [Google Scholar]
- Bladbjerg EM, de Maat MP, Christensen K, Bathum L, Jespersen J, Hjelmborg J. Genetic influence on thrombotic risk markers in the elderly--a Danish twin study. J Thromb Haemost. 2006;4:599–607. doi: 10.1111/j.1538-7836.2005.01778.x. [DOI] [PubMed] [Google Scholar]
- Blangero J, Williams JT, Almasy L. Robust LOD scores for variance component-based linkage analysis. Genet Epidemiol. 2000;19(Suppl 1):S8–14. doi: 10.1002/1098-2272(2000)19:1+<::AID-GEPI2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Boehnke M, Lange K. Ascertainment and goodness of fit of variance component models for pedigree data. Prog Clin Biol Res. 1984;147:173–92. [PubMed] [Google Scholar]
- Boffa MB, Maret D, Hamill JD, Bastajian N, Crainich P, Jenny NS, Tang Z, Macy EM, Tracy RP, Franco RF, Nesheim ME, Koschinsky ML. Effect of single nucleotide polymorphisms on expression of the gene encoding thrombin-activatable fibrinolysis inhibitor: a functional analysis. Blood. 2008;111:183–9. doi: 10.1182/blood-2007-03-078543. [DOI] [PubMed] [Google Scholar]
- Boffa MB, Reid TS, Joo E, Nesheim ME, Koschinsky ML. Characterization of the gene encoding human TAFI (thrombin-activable fibrinolysis inhibitor; plasma procarboxypeptidase B) Biochemistry. 1999;38:6547–58. doi: 10.1021/bi990229v. [DOI] [PubMed] [Google Scholar]
- Bouma BN, Marx PF, Mosnier LO, Meijers JC. Thrombin-activatable fibrinolysis inhibitor (TAFI, plasma procarboxypeptidase B, procarboxypeptidase R, procarboxypeptidase U) Thromb Res. 2001;101:329–54. doi: 10.1016/s0049-3848(00)00411-4. [DOI] [PubMed] [Google Scholar]
- Brouwers GJ, Vos HL, Leebeek FW, Bulk S, Schneider M, Boffa M, Koschinsky M, van Tilburg NH, Nesheim ME, Bertina RM, Gomez Garcia EB. A novel, possibly functional, single nucleotide polymorphism in the coding region of the thrombin-activatable fibrinolysis inhibitor (TAFI) gene is also associated with TAFI levels. Blood. 2001;98:1992–3. doi: 10.1182/blood.v98.6.1992. [DOI] [PubMed] [Google Scholar]
- de Bruijne EL, Murad SD, de Maat MP, Tanck MW, Haagsma EB, van Hoek B, Rosendaal FR, Janssen HL, Leebeek FW. Genetic variation in thrombin-activatable fibrinolysis inhibitor (TAFI) is associated with the risk of splanchnic vein thrombosis. Thromb Haemost. 2007;97:181–5. [PubMed] [Google Scholar]
- Dyke B. PEDSYS: a pedigree data management system. User’s manual. Southwest Foundation for Biomedical Research; San Antonio,Texas: 1995. [Google Scholar]
- Eichinger S, Schonauer V, Weltermann A, Minar E, Bialonczyk C, Hirschl M, Schneider B, Quehenberger P, Kyrle PA. Thrombin-activatable fibrinolysis inhibitor and the risk for recurrent venous thromboembolism. Blood. 2004;103:3773–6. doi: 10.1182/blood-2003-10-3422. [DOI] [PubMed] [Google Scholar]
- Falconer M. Introduction to quantitative genetics. Longman Group Ltd; Essex: 1996. [Google Scholar]
- Feingold E, Brown PO, Siegmund D. Gaussian models for genetic linkage analysis using complete high-resolution maps of identity by descent. Am J Hum Genet. 1993;53:234–51. [PMC free article] [PubMed] [Google Scholar]
- Franco RF, Fagundes MG, Meijers JC, Reitsma PH, Lourenco D, Morelli V, Maffei FH, Ferrari IC, Piccinato CE, Silva WA, Jr, Zago MA. Identification of polymorphisms in the 5'-untranslated region of the TAFI gene: relationship with plasma TAFI levels and risk of venous thrombosis. Haematologica. 2001;86:510–7. [PubMed] [Google Scholar]
- Frere C, Morange PE, Saut N, Tregouet DA, Grosley M, Beltran J, Juhan-Vague I, Alessi MC. Quantification of thrombin activatable fibrinolysis inhibitor (TAFI) gene polymorphism effects on plasma levels of TAFI measured with assays insensitive to isoform-dependent artefact. Thromb Haemost. 2005;94:373–9. doi: 10.1160/TH04-08-0497. [DOI] [PubMed] [Google Scholar]
- Henry M, Aubert H, Morange PE, Nanni I, Alessi MC, Tiret L, Juhan-Vague I. Identification of polymorphisms in the promoter and the 3' region of the TAFI gene: evidence that plasma TAFI antigen levels are strongly genetically controlled. Blood. 2001;97:2053–8. doi: 10.1182/blood.v97.7.2053. [DOI] [PubMed] [Google Scholar]
- Hopper JL. The Australian Twin Registry. Twin Res. 2002;5:329–36. doi: 10.1375/136905202320906048. [DOI] [PubMed] [Google Scholar]
- Hopper JL, Mathews JD. Extensions to multivariate normal models for pedigree analysis. Ann Hum Genet. 1982;46:373–83. doi: 10.1111/j.1469-1809.1982.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Juhan-Vague I, Morange PE. Very high TAFI antigen levels are associated with a lower risk of hard coronary events: the PRIME Study. J Thromb Haemost. 2003;1:2243–4. doi: 10.1046/j.1538-7836.2003.00398.x. [DOI] [PubMed] [Google Scholar]
- Juhan-Vague I, Morange PE, Aubert H, Henry M, Aillaud MF, Alessi MC, Samnegard A, Hawe E, Yudkin J, Margaglione M, Di Minno G, Hamsten A, Humphries SE. Plasma thrombin-activatable fibrinolysis inhibitor antigen concentration and genotype in relation to myocardial infarction in the north and south of Europe. Arterioscler Thromb Vasc Biol. 2002;22:867–73. doi: 10.1161/01.atv.0000015445.22243.f4. [DOI] [PubMed] [Google Scholar]
- Knoefler R, Ludwig K, Kostka H, Kuhlisch E, Siegert G, Suttorp M. The impact of single nucleotide polymorphisms of the thrombin activatable fibrinolysis inhibitor (TAFI) gene on TAFI antigen levels in healthy children and pediatric oncology patients. Semin Thromb Hemost. 2003;29:575–83. doi: 10.1055/s-2004-815625. [DOI] [PubMed] [Google Scholar]
- Kostka H, Kuhlisch E, Schellong S, Siegert G. Polymorphisms in the TAFI gene and the risk of venous thrombosis. Clin Lab. 2003;49:645–7. [PubMed] [Google Scholar]
- Ladenvall C, Gils A, Jood K, Blomstrand C, Declerck PJ, Jern C. Thrombin activatable fibrinolysis inhibitor activation peptide shows association with all major subtypes of ischemic stroke and with TAFI gene variation. Arterioscler Thromb Vasc Biol. 2007;27:955–62. doi: 10.1161/01.ATV.0000259354.93789.a6. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner J, Ribo M, Monasterio J, Molina CA, Alvarez-Sabin J. Thrombin-activable fibrinolysis inhibitor levels in the acute phase of ischemic stroke. Stroke. 2003;34:1038–40. doi: 10.1161/01.STR.0000063139.06585.45. [DOI] [PubMed] [Google Scholar]
- Morange PE, Tregouet DA, Frere C, Luc G, Arveiler D, Ferrieres J, Amouyel P, Evans A, Ducimetiere P, Cambien F, Tiret L, Juhan-Vague I. TAFI gene haplotypes, TAFI plasma levels and future risk of coronary heart disease: the PRIME Study. J Thromb Haemost. 2005;3:1503–10. doi: 10.1111/j.1538-7836.2005.01486.x. [DOI] [PubMed] [Google Scholar]
- Mosnier LO, von dem Borne PA, Meijers JC, Bouma BN. Plasma TAFI levels influence the clot lysis time in healthy individuals in the presence of an intact intrinsic pathway of coagulation. Thromb Haemost. 1998;80:829–35. [PubMed] [Google Scholar]
- Peetz D, Victor A, Adams P, Erbes H, Hafner G, Lackner KJ, Hoehler T. Genetic and environmental influences on the fibrinolytic system: a twin study. Thromb Haemost. 2004;92:344–51. doi: 10.1160/TH04-01-0001. [DOI] [PubMed] [Google Scholar]
- Schroeder V, Chatterjee T, Mehta H, Windecker S, Pham T, Devantay N, Meier B, Kohler HP. Thrombin activatable fibrinolysis inhibitor (TAFI) levels in patients with coronary artery disease investigated by angiography. Thromb Haemost. 2002;88:1020–5. [PubMed] [Google Scholar]
- Souto JC, Almasy L, Borrell M, Blanco-Vaca F, Mateo J, Soria JM, Coll I, Felices R, Stone W, Fontcuberta J, Blangero J. Genetic susceptibility to thrombosis and its relationship to physiological risk factors: the GAIT study. Genetic Analysis of Idiopathic Thrombophilia. Am J Hum Genet. 2000a;67:1452–9. doi: 10.1086/316903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto JC, Almasy L, Borrell M, Gari M, Martinez E, Mateo J, Stone WH, Blangero J, Fontcuberta J. Genetic determinants of hemostasis phenotypes in Spanish families. Circulation. 2000b;101:1546–51. doi: 10.1161/01.cir.101.13.1546. [DOI] [PubMed] [Google Scholar]
- Tregouet DA, Aubert H, Henry M, Morange P, Visvikis S, Juhan-Vague I, Tiret L. Combined segregation-linkage analysis of plasma thrombin activatable fibrinolysis inhibitor (TAFI) antigen levels with TAFI gene polymorphisms. Hum Genet. 2001;109:191–7. doi: 10.1007/s004390100558. [DOI] [PubMed] [Google Scholar]
- Tsai SP, Drayna D. The gene encoding human plasma carboxypeptidase B (CPB2) resides on chromosome 13. Genomics. 1992;14:549–50. doi: 10.1016/s0888-7543(05)80268-x. [DOI] [PubMed] [Google Scholar]
- van Tilburg NH, Rosendaal FR, Bertina RM. Thrombin activatable fibrinolysis inhibitor and the risk for deep vein thrombosis. Blood. 2000;95:2855–9. [PubMed] [Google Scholar]
- Vanhoof G, Wauters J, Schatteman K, Hendriks D, Goossens F, Bossuyt P, Scharpe S. The gene for human carboxypeptidase U (CPU)--a proposed novel regulator of plasminogen activation--maps to 13q14.11. Genomics. 1996;38:454–5. doi: 10.1006/geno.1996.0656. [DOI] [PubMed] [Google Scholar]
- Warren DM, Cole SA, Dyer TD, Soria JM, Souto JC, Fontcuberta J, Blangero J, Maccluer JW, Almasy L. A locus on chromosome 13 influences levels of TAFI antigen in healthy Mexican Americans. Hum Biol. 2006;78:329–39. doi: 10.1353/hub.2006.0049. [DOI] [PubMed] [Google Scholar]