Abstract
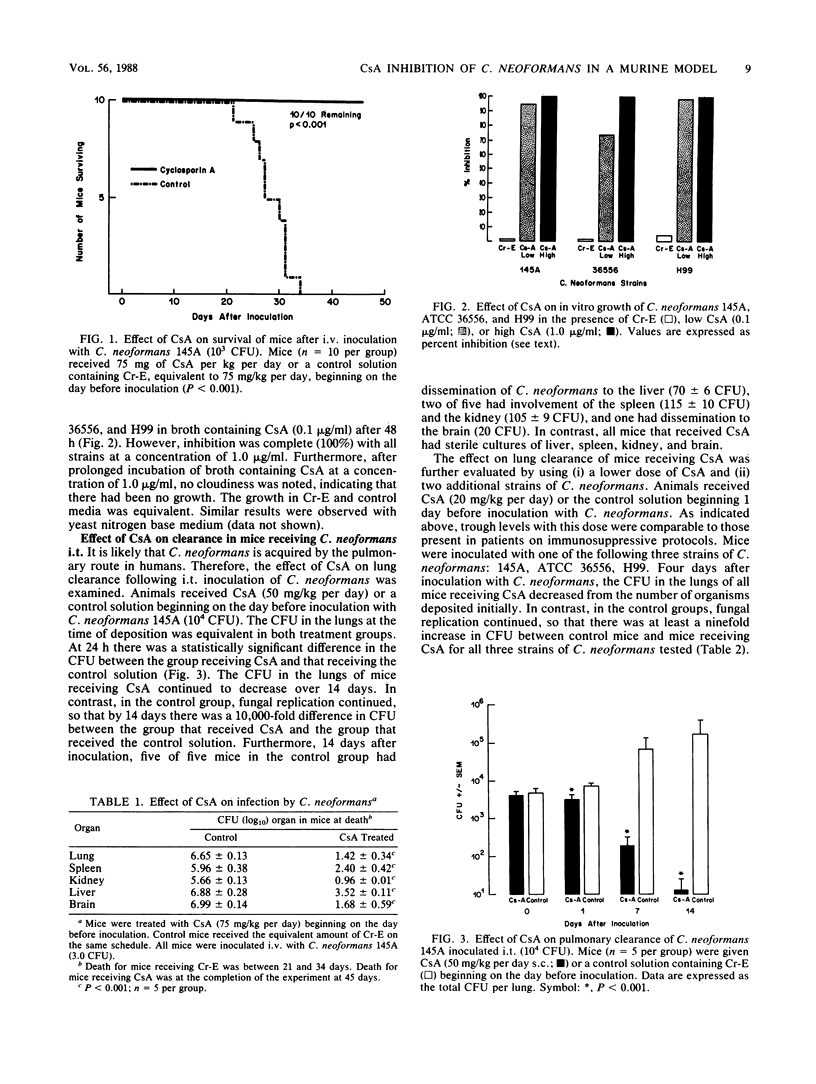
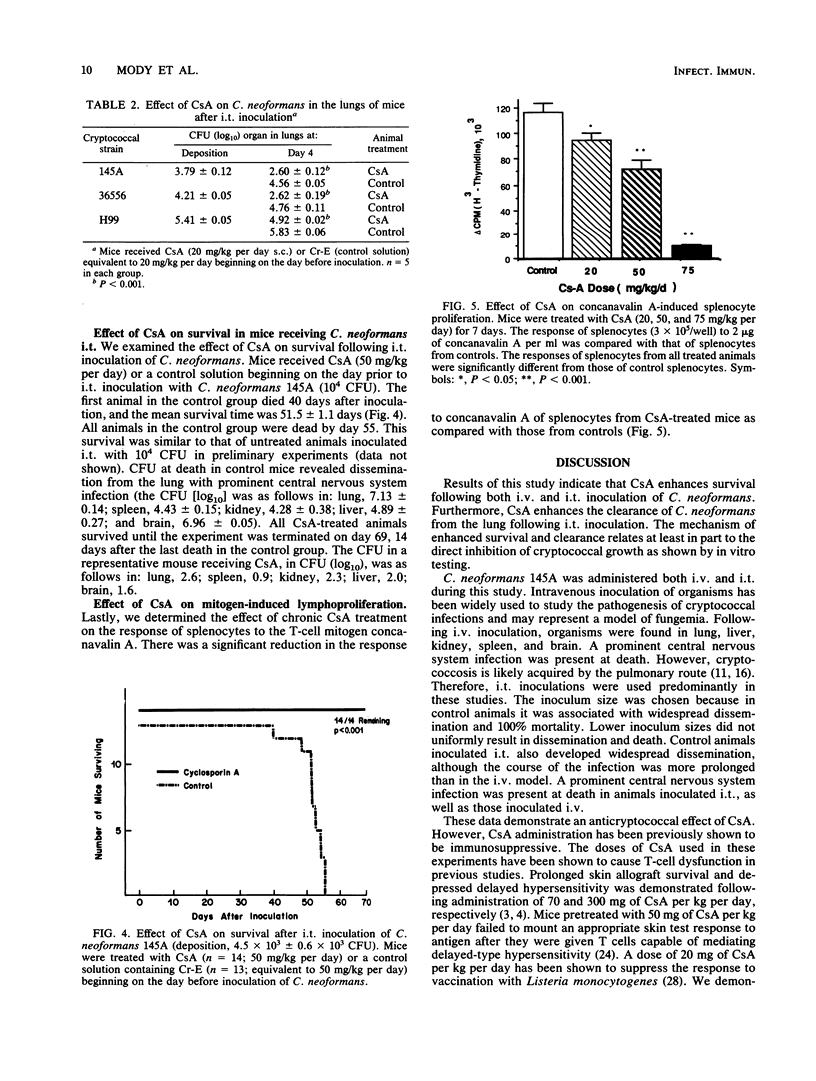
Cryptococcus neoformans is a frequent opportunistic infectious agent in patients with decreased T-lymphocyte-mediated immune function, including those with acquired immune deficiency syndrome. Cyclosporin A (CsA), a potent inhibitor of T-lymphocyte function, was administered subcutaneously to mice to study the pathogenesis of C. neoformans infections in the setting of impaired T-cell function. Surprisingly, survival was prolonged indefinitely in animals that received immunosuppressive doses of CsA following either intratracheal or intravenous inoculations of C. neoformans. Furthermore, following intratracheal inoculation, mice treated with CsA cleared C. neoformans from their lungs more rapidly than did control mice. CsA directly inhibited the growth of C. neoformans when it was added to cultures in vitro at concentrations comparable to the blood levels achieved in experimental mice. Thus, CsA inhibited both in vitro and in vivo growth of C. neoformans. While these results must be extended to studies in humans, these data suggest that patients who now receive CsA-immunosuppressive therapy may be fortuitously protected against infections with C. neoformans. Furthermore, research into cyclosporin derivatives may yield compounds with less immunosuppressive properties and enhanced antifungal activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behforouz N. C., Wenger C. D., Mathison B. A. Prophylactic treatment of BALB/c mice with cyclosporine A and its analog B-5-49 enhances resistance to Leishmania major. J Immunol. 1986 Apr 15;136(8):3067–3075. [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Gubler H. U., Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976 Jul;6(4):468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Bout D., Deslèe D., Capron A. Antischistosomal effect of cyclosporin A: cure and prevention of mouse and rat schistosomiasis mansoni. Infect Immun. 1986 Jun;52(3):823–827. doi: 10.1128/iai.52.3.823-827.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bout D., Haque A., Capron A. Filaricidal effects of cyclosporin-A against Dipetalonema viteae in Mastomys natalensis. Trans R Soc Trop Med Hyg. 1984;78(5):670–671. doi: 10.1016/0035-9203(84)90236-0. [DOI] [PubMed] [Google Scholar]
- Bowers L. D., Canafax D. M. Cyclosporine: experience with therapeutic monitoring. Ther Drug Monit. 1984;6(2):142–147. [PubMed] [Google Scholar]
- Bunjes D., Hardt C., Röllinghoff M., Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981 Aug;11(8):657–661. doi: 10.1002/eji.1830110812. [DOI] [PubMed] [Google Scholar]
- Cohen D. J., Loertscher R., Rubin M. F., Tilney N. L., Carpenter C. B., Strom T. B. Cyclosporine: a new immunosuppressive agent for organ transplantation. Ann Intern Med. 1984 Nov;101(5):667–682. doi: 10.7326/0003-4819-101-5-667. [DOI] [PubMed] [Google Scholar]
- Eng R. H., Bishburg E., Smith S. M., Kapila R. Cryptococcal infections in patients with acquired immune deficiency syndrome. Am J Med. 1986 Jul;81(1):19–23. doi: 10.1016/0002-9343(86)90176-2. [DOI] [PubMed] [Google Scholar]
- Hess A. D., Tutschka P. J., Santos G. W. Effect of cyclosporin A on human lymphocyte responses in vitro. III. CsA inhibits the production of T lymphocyte growth factors in secondary mixed lymphocyte responses but does not inhibit the response of primed lymphocytes to TCGF. J Immunol. 1982 Jan;128(1):355–359. [PubMed] [Google Scholar]
- Kirkland T. N., Fierer J. Cyclosporin A inhibits Coccidioides immitis in vitro and in vivo. Antimicrob Agents Chemother. 1983 Dec;24(6):921–924. doi: 10.1128/aac.24.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs J. A., Kovacs A. A., Polis M., Wright W. C., Gill V. J., Tuazon C. U., Gelmann E. P., Lane H. C., Longfield R., Overturf G. Cryptococcosis in the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Oct;103(4):533–538. doi: 10.7326/0003-4819-103-4-533. [DOI] [PubMed] [Google Scholar]
- Lillehoj H. S., Malek T. R., Shevach E. M. Differential effect of cyclosporin A on the expression of T and B lymphocyte activation antigens. J Immunol. 1984 Jul;133(1):244–250. [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W. Transfer of immunity to cryptococcosis by T-enriched splenic lymphocytes from Cryptococcus neoformans-sensitized mice. Infect Immun. 1980 Oct;30(1):5–11. doi: 10.1128/iai.30.1.5-11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack D. G., McLeod R. New micromethod to study the effect of antimicrobial agents on Toxoplasma gondii: comparison of sulfadoxine and sulfadiazine individually and in combination with pyrimethamine and study of clindamycin, metronidazole, and cyclosporin A. Antimicrob Agents Chemother. 1984 Jul;26(1):26–30. doi: 10.1128/aac.26.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer T. P., Johnson P., Faynor S. M., Sterioff S. Cyclosporine: a review of drug monitoring problems and presentation of a simple, accurate liquid chromatographic procedure that solves these problems. Clin Biochem. 1986 Apr;19(2):83–89. doi: 10.1016/s0009-9120(86)80053-4. [DOI] [PubMed] [Google Scholar]
- Onofrio J. M., Toews G. B., Lipscomb M. F., Pierce A. K. Granulocyte-alveolar-macrophage interaction in the pulmonary clearance of Staphylococcus aureus. Am Rev Respir Dis. 1983 Mar;127(3):335–341. doi: 10.1164/arrd.1983.127.3.335. [DOI] [PubMed] [Google Scholar]
- Perfect J. R., Durack D. T. Effects of cyclosporine in experimental cryptococcal meningitis. Infect Immun. 1985 Oct;50(1):22–26. doi: 10.1128/iai.50.1.22-26.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpff S. C., Bennett J. E. Abnormalities in cell-mediated immunity in patients with Cryptococcus neoformans infection. J Allergy Clin Immunol. 1975 Jun;55(6):430–441. doi: 10.1016/0091-6749(75)90082-2. [DOI] [PubMed] [Google Scholar]
- Schuller-Levis G. B., Kozlowski P. B., Wisniewski H. M. Cyclosporin A treatment of an induced attack in a chronic relapsing model of experimental allergic encephalomyelitis. Clin Immunol Immunopathol. 1986 Aug;40(2):244–252. doi: 10.1016/0090-1229(86)90027-9. [DOI] [PubMed] [Google Scholar]
- Shidani B., Milon G., Marchal G., Truffa-Bachi P. Cyclosporin A inhibits the delayed-type hypersensitivity reaction: impaired production of early pro-inflammatory mediator(s). Eur J Immunol. 1984 Apr;14(4):314–318. doi: 10.1002/eji.1830140407. [DOI] [PubMed] [Google Scholar]
- Solbach W., Forberg K., Kammerer E., Bogdan C., Röllinghoff M. Suppressive effect of cyclosporin A on the development of Leishmania tropica-induced lesions in genetically susceptible BALB/c mice. J Immunol. 1986 Jul 15;137(2):702–707. [PubMed] [Google Scholar]
- Thommen-Scott K. Antimalarial activity of cyclosporin A. Agents Actions. 1981 Dec;11(6-7):770–773. doi: 10.1007/BF01978803. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., Moon D. K., Geczy C. L., Nelson D. S. Cyclosporin A inhibits lymphokine production but not the responses of macrophages to lymphokines. Immunology. 1983 Feb;48(2):291–299. [PMC free article] [PubMed] [Google Scholar]
- van den Bosch J. F., Kanis I. Y., Antonissen A. C., Buurman W. A., van Boven C. P. T-cell-independent macrophage activation in mice induced with rRNA from Listeria monocytogenes and dimethyldioctadecylammonium bromide. Infect Immun. 1986 Sep;53(3):611–615. doi: 10.1128/iai.53.3.611-615.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


