Opinion statement
The rates of depression, anxiety, and sleep disturbance (suicide risk factors) are greater in patients with allergic rhinitis than in the general population. The rate of allergy is also greater in patients with depression. Preliminary data suggest that patients with a history of allergy may have an increased rate of suicide. Clinicians should actively inquire to diagnose allergy in patients with depression and depression in patients with allergy.
Spring peaks of suicide are highly replicated, but their origin is poorly understood. Preliminary epidemiologic data suggest that seasonal spring peaks in aeroallergens are associated with seasonal spring peaks in suicide. Our research in Brown Norway rats demonstrates that sensitization and exposure to aeroallergens induces anxiety-like and aggressive behaviors as well as allergy-related helper T-cell type 2 (Th2) cytokine gene expression in the prefrontal cortex. Thus, it is possible that sensitization and exposure to aeroallergens, which peak in spring, may be conducive to seasonal exacerbation of suicide risk factors such as anxiety, depression, hostility/ aggression, and sleep disturbance. Connecting allergy with suicide and suicide risk factors adds to previous neurologic literature connecting allergy with migraines and seizure disorders.
Our recent report of Th2 (allergy-mediating) cytokine expression in the orbito-frontal cortex of suicide victims should lead to future studies to test the hypothesis that mediators of allergic inflammation in the nasal cavities may result in Th2 cytokine expression in the brain, influencing affect and behavioral modulation.
Certain medications used to treat allergy can exacerbate suicide risk factors, potentially worsening suicide risk and even triggering suicide. Systemic (but not topical) corticosteroids have been associated with manic and depressive episodes and mixed mood states. Recently, the US Food and Drug Administration started investigating the possibility that montelukast may trigger suicide. Although this association requires further exploration and confirmation, clinicians should err on the side of caution, inquiring about past suicide attempts; hopelessness; reasons for living; and suicidal ideation, intent, or plan; and referring the patient to a mental health professional for evaluation if appropriate.
Introduction
ALLERGY, DEPRESSION, AND SUICIDE
In 2004, suicide was the 11th leading cause of death in the United States, accounting for 32,439 deaths. For every suicide death, there are an estimated 8 to 25 attempted suicides. Most individuals who complete suicide are depressed [1, Class II]. Many short-term and long-term risk factors have been proposed, including socioeconomic factors and psychiatric symptoms and syndromes including depression, anxiety, sleep loss, and aggression. Asthma (with its treatment) is among the many chronic illnesses proposed to increase the risk of suicide, but allergic rhinitis has not made this list in any review or chapter known to us. The stress-diathesis model, currently one of the most accepted models of suicide vulnerabilities and triggering [1, Class II], proposes that a stressor leads to the exacerbation of a preexisting psychiatric disorder, triggering suicide. A highly replicated finding in epidemiology research is the spring peak in suicide from April to June with a mirror image in the Southern Hemisphere [2•, Class II]. There is a smaller and less consistent peak of suicide in late summer and early fall. Many factors have been implicated in contributing to the spring peak in depression and suicide, ranging from environmental (light) to social factors, but no single explanation has yet proven satisfactory [2•, Class II].
One of the most dramatic environmental changes, which coincides with physical symptoms, is the robust increase in pollen during the spring and the corresponding increased incidence of upper respiratory inflammation. Pollen is the most important and the most seasonal of the aeroallergens [3, Class III]. Every year, the air is flooded with pollen from wind-pollinated plants, and the development of allergies due to human exposure is an unfortunate consequence of this process. Tree pollination occurs in the spring and represents the largest source of airborne pollen (∼ 75% of the yearly total). As an example of the time window of pollen peaks, we refer to the weekly mean values reported for total pollen production in the Baltimore, MD–Washington, DC area [4, Class I]. In April, the value typically increases from less than 100 grains/m3 in the first week of the month to more than 1000 to 1300 grains/m3 in the third week. The levels of pollen return to below 100 grains/m3 by the first week of June. The dramatic increase in atmospheric concentration of tree pollen is highly relevant to human health. Another increase in atmospheric pollen occurs during the fall, mainly represented by the pollen of ragweed. Even though ragweed pollen amounts to only about 15% of the yearly pollen total, it is highly allergenic.
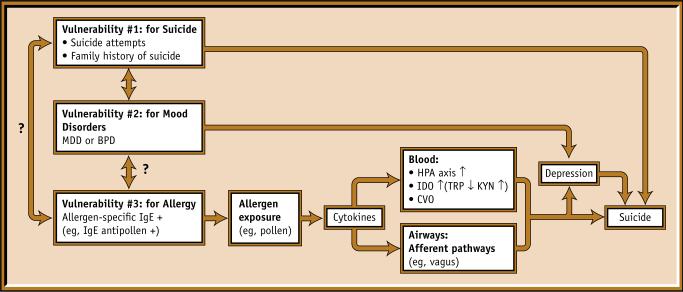
Consistent with the hypothesis that allergens trigger suicide in those with a triple vulnerability (ie, for suicide, mood disorders, and allergic reactions) (Fig. 1), we have found a twofold increase in the rate of nonviolent completed suicides among women younger than 65 years during the spring tree allergy season in the high-exposure period compared with the pre-exposure period (95% CI, 1.3−3.0) [5•, Class II]. This finding is consistent with the findings of Timonen et al. [6, Class II], who reported a greater seasonality of suicide in those with a history of allergy than in those without such a history. One possible explanation for this phenomenon is that one or more of the known suicide risk factors—depression, anxiety, aggression, or sleep impairment—may be affected by exposure to allergens in vulnerable individuals. Marshall et al. [7, Class II] used the Multi-Dimensional Fatigue Inventory and the Positive Affect–Negative Affect mood rating scales in patients sensitized to ragweed and reported higher levels of general and mental fatigue (but not physical fatigue), reduced motivation, increased sadness, and reduced pleasurable engagement during the ragweed pollen season as compared with the off-season. Our own work in a student population has shown that seasonality of mood is directly related to self-reported sensitivity to pollen counts [8, Class II]. Finally, we have shown that changes in allergy symptoms from low to high tree-pollen seasons correlate with changes in depression scores [9, Class II] and anxiety scores [10, Class II] in patients with recurrent mood disorders.
Figure 1.
Triple vulnerability for suicide, mood disorders, and allergic sensitization with an environmental trigger (allergen). Mechanisms by which cytokines affect the brain include activation of the hypothalamo-pituitary-adrenal (HPA) axis and the actions of indoleamine deoxygenase (IDO), an enzyme involved in the catabolism of tryptophan (TRP), stealing it from the TRP hydroxylase pathway and serotonin synthesis and producing potentially neurotoxic compounds such as kynurenine (KYN). In addition, several small areas of the brain (the circumventricular organs, CVO) lack a blood-brain barrier and allow larger molecules such as cytokines to interact with neurons, their terminals, and glial cells. BPD—bipolar disorder; MDD—major depressive disorder.
Increasing evidence suggests an association between recurrent depression and allergic diseases [6,11-14, Class II]. In addition, a shared risk for allergic and depressive symptoms has been described among adult Finnish twins [15, Class II].
NEUROPSYCHIATRIC SYMPTOMS ASSOCIATED WITH ALLERGEN EXPOSURE
When exposed to pollen, subjects with allergic rhinitis had decrements in reaction time, attention, and vigilance [16, Class I]. Others have reported an association of increased fatigue and depressed mood in individuals with sensitivity to ragweed during the ragweed season [1, Class II]. Another suicide risk factor, anxiety, has been associated with allergy in women [17,18, Class II].
Immune function may play a role in depression and suicide [19-25,26•, Class II]. It is known that treatment of hepatitis C with the cytokine interferon-α, resulting in the reduction of viral titers, induces depression in a significant proportion of patients [27-29, Class II], as does treatment with the cytokine interleukin (IL)-2 in melanoma patients [30,31, Class II]. A causal relationship between cytokine treatment and depression is further suggested by the cessation of depressive symptoms when cytokine treatments are discontinued [22,32, Class II].
Moreover, several studies have reported the presence of elevated proinflammatory cytokines in depressed patients [33,34, Class II] and a reduction in these mediators of inflammation after treatment with antidepressants [35, Class II]. However, because cytokines induce sickness behavior, the validity of these studies has been limited by the overlap with the sickness syndrome. (For studies and reviews on this issue, see references 36-38, Class I.) Although the degree to which sickness, rather than depression, is involved in these effects is under debate, studies show that healthy individuals receiving low doses of lipopolysaccharides (LPS), components of bacterial cell walls, had elevated anxiety and depressive scores in the absence of sickness behavior [39, Class I]. In animal studies, behaviors that resembled depression [40, Class I] and anxiety [41, Class I] were induced by activation of the immune system with LPS. These effects have been shown to be mediated by the actions of cytokines in the brain, likely as an adaptive mechanism to cope with potentially lethal infectious agents. Although the involvement of the immune system and cytokines in the pathophysiology of depression has not been fully addressed, it is an active area of research that may contribute to fundamental advances in the understanding of mood disorders.
BRAIN CYTOKINES
We have recently reported an elevated expression of helper T-cell type 2 (Th2) cytokines in the human orbito-frontal cortex in suicide victims [42••, Class II]. We have also reported an increased tumor necrosis factor-α (TNF-α) and IL-6 cytokine expression in the hippocampus of rats after intranasal immune challenge with LPS [43•, Class I], anxiety-like behaviors in Brown Norway rats sensitized and exposed to tree pollen [44, Class II], and aggression (seen in the resident-intruder test) in rats sensitized and exposed to allergens after a swim stress [45, Class II]. The anxiety-like behaviors and aggression-like behaviors have been replicated using mice and with a different allergen, ovalbumin (Tonelli, personal communication).
Although no data show conclusively that peripheral cytokines can cross the blood-brain barrier, these cytokines can signal cells within the brain through several mechanisms. These include direct actions of circulating cytokines via circumventricular organs, cytokine signaling through the vagus nerve [46, Class II], de novo transcription of cytokine genes in astrocytes, and microglia induced by inflammatory mediators produced by endothelial cells of the cerebrovasculature [47-52, Class II].
Several mechanisms have been proposed to explain how cytokines may affect brain function and behavior. Two of the most important are interactions with the hypothalamic-pituitary-adrenal (HPA) axis and with the indoleamine deoxygenase enzyme. Cytokines can induce activation of the HPA axis [53, Class III], resulting in altered levels of corticotropin-releasing hormone and cortisol with detrimental effects for neurons. Activation of the indoleamine deoxygenase enzyme steals tryptophan from serotonin synthesis toward production of kynurenines and reduces serotonin production. Moreover, direct interactions between brain cells and cytokines also have been proposed [54, Class II].
Thus, one important mechanism of neuroimmune interactions is the brain's capacity to produce cytokines in response to peripheral immune challenge [47,55-57, Class II]. This mechanism may change the balance of cytokine expression in the brain and, in turn, may affect neuronal function in response to inflammatory processes initiated in the periphery.
SLEEP IMPAIRMENT
Insomnia, hypersomnia, nightmares, and sleep panic attacks are important suicide risk factors [58, Class III]. Correcting them may reduce suicide risk relatively swiftly [59, Class III]. Proinflammatory cytokines, such as TNF-α, IL-1, IL-6, and interferons, tend to induce sleepiness and sleep [60-64, Class II]. In contrast, “anti-inflammatory cytokines” have been reported to disrupt sleep. For instance, it has been reported that IL-4 [65, Class II] and IL-10 [66, Class II] inhibit spontaneous sleep. Sleep, suicide, and cytokines could also be interconnected via HPA axis activation [67, Class II].
Moreover, in addition to the effects of cytokines, mechanical obstruction secondary to mucosal edema and secretions in the upper respiratory tract could be a direct cause of sleep disturbance and daytime sleepiness and may exacerbate or make manifest an obstructive sleep apnea. Intranasal corticosteroids are effective agents in improving sleep in patients with allergy [68, Class II].
GENDER EFFECTS
A stronger association between allergy, depression, and suicide in women may be explained in part by an increased incidence of allergy and depression in women, or by increased anxiety associated with depression in women [18, Class II]. To a certain degree, the effects could be hormonal. Specifically, beta estradiol and progesterone significantly increase the production of IL-4 and IL-13 [69, Class II], whereas testosterone inhibits histamine release and eosinophil degranulation [70, Class II]. Estrogens favor a Th2 IgE–mediated response, which may explain, in part, the increased prevalence of allergic disease in women of reproductive age as compared with men [71, Class II].
INTRANASAL-INTRACRANIAL COMMUNICATION
As much as the brain is (incorrectly) considered an “immune-privileged organ,” the relationship between the nasal cavity and the brain is also a “privileged” and “intimate” one. First, the nasal cavity is the only place in which central nervous tissue (ie, the olfactory neurons) is directly exposed to air carrying chemicals, pathogens, pollutants, and allergens. Second, the transfer of large molecules and pathogens into the brain via the intranasal pathway has been reported [72-75, Class II]. It is important to mention that the intranasal pathways may, via olfactory and trigeminal pathways, deliver large molecules into brain areas that play a key role in mood regulation and dysregulation more efficiently than intravascular administration [73, Class II]. In addition, it has been shown that intra-nasal instillation of recombinant cytokines, including IL-6 and IL-12, affects the course of experimentally induced neurologic disorders in rats [76,77, Class II].
ALLERGIC RHINITIS: CLINICAL CONSIDERATIONS
Allergic diseases affect 10% to 30% of adults and nearly 40% of children [78,79, Class II] and are increasing in prevalence for unknown reasons [80, Class II]. Symptoms of allergy are occasionally life-threatening, sometimes debilitating, and frequently very bothersome. In the lungs, allergy is manifested by bronchoconstriction or asthma; in the nose, by rhinitis; in the skin, by urticaria; and in the eyes, by conjunctivitis. Anaphylaxis—the systemic and life-threatening manifestations of allergy—requires immediate medical attention. For several reasons, this article focuses on allergic rhinitis. First, in contrast to allergic rhinitis, asthma is often treated with systemic corticosteroids, which may themselves cause neuropsychiatric symptoms. Second, because of anatomic proximity, inflammation of the nasal cavities may have a closer relationship with the brain than inflammation of the skin, lungs, or digestive tract. The distinction between asthma and nasal inflammation is somewhat illusory, however, because more than 60% of individuals with rhinitis also have asthma, and allergic rhinitis frequently progresses toward allergic asthma [81, Class II].
Rhinitis symptoms include rhinorrhea, nasal congestion, sneezing, itching of the nose, postnasal drainage, or a combination of these. Allergic rhinitis also is often associated with symptoms of allergic conjunctivitis, including tearing, redness, ocular itch, and sometimes swelling and photophobia. In addition to allergic variables, irritant, infectious, hormonal, occupational, and other factors may predispose individuals to rhinitis or may trigger or aggravate it [82, Class II].
Peak presentations of allergic rhinoconjunctivitis occur between ages 10 and 25 years. Rhinitis has traditionally been classified as “seasonal” or “perennial,” but recently there has been a tendency to classify rhinitis as intermittent (≤ 4 days per week or, if > 4 days per week, then total duration of < 4 weeks) or persistent [83, Class III]. Allergic rhinitis is classified as moderate/severe rather than mild if symptoms are severe or if they result in impairment of sleep, daily activities, work, or school.
RESEARCH DIRECTIONS
Animal studies, as an immediate priority, can test the effect of medications used to treat allergy in behavioral analogues of suicide risk factors (eg, aggression, behaviors resembling depression or anxiety, impulsivity, sleep impairment) at baseline and after sensitization and exposure to antigens. Animal studies also can elucidate mechanisms of transmission of inflammation from the nasal cavity to the brain and test mechanisms of interventions. Larger postmortem studies with better controls are needed to localize the cellular substrate of Th2 inflammation in the brain using in situ hybridization. Large-scale clinical trials could compare the effect on suicide risk factors of intranasal corticosteroids, antihistamines, and leukotriene inhibitors in patients with recurrent mood disorders (preferably those with a history of suicide attempts). Functional neuroimaging studies are needed to determine brain activity changes with exposure to aeroallergens in sensitized individuals stratified by diagnosis of mood disorders and suicide attempt history (number, severity). Epidemiologic studies are needed to study completed suicide; for replication, it is essential to confirm a relationship between suicide (completed and attempted) and prior history of allergy and timing of exposure to allergens, ideally with adjustment for history of mental and medical illness and socioeconomic factors previously implicated in suicide.
The relationships described in this article add to previously reported associations between allergy and neurologic conditions such as migraines [84,85, Class II] and epilepsy [86, Class II], between mast cells as central cells in both allergy and migraine pathophysiology [87, Class II], and between intranasal administration of cytokines and seizure thresholds [76]. To move our knowledge further, from association toward causation, it is important to study (probably at multiple levels) the effects of allergen exposure (intensity and timing), allergic sensitization, and nasal inflammation in general (allergy-induced, viral, bacterial, cold-induced, or idiopathic) on brain function and dysfunction. In parallel, clinicians should be familiar with the current standards of treatment of allergic rhinitis. Animal studies are needed to systematically examine the effects on suicide risk factors. of medications used to treat allergy. Understanding the interface between allergic inflammation and brain function requires multilevel observational—and especially interventional—approaches (epidemiologic, treatment trials, imaging, animal, postmortem).
PATHOPHYSIOLOGY
Certain individuals with an atopic predisposition synthesize IgE antibodies upon initial exposure to allergen. IgE binding to mast cells and basophils sets the stage for the allergic response. Upon re-exposure, allergen binds and crosslinks IgE bound to the cell surface, resulting in the release of inflammatory mediators. Early response mediators include lipid mediators (leukotrienes, prostaglandins) and granule mediators (histamine, tryptase). Minutes to hours later, cytokines such as TNF-α, IL-4, IL-5, and IL-6 and chemokines (IL-8, monocyte chemotactic protein-1, macrophage inflammatory protein-1α) are released [88, Class II]. The type of allergen, the degree and length of exposure, and the atopic tendency of the individual determine the manifestation of symptoms. Allergen contact occurs via the air (seasonal aeroallergens tree, grass, and weed pollen and perennial dust mite protein, animal dander, and mold), food, drugs, and skin (stinging insects).
Allergic rhinitis pathophysiology is characterized by edema of the nasal mucosa as a result of IgE-mediated release of early-phase and late-phase mediators and Th2 cytokines, which promote the infiltration of mucosa with inflammatory cells: eosinophils, neutrophils, basophils, T cells, and macrophages [79, Class I].
DIAGNOSIS
In addition to the symptoms of allergy, in vivo and laboratory-based testing for allergen sensitivity may objectively support the diagnosis of allergic disease [79,82,89,90, Class II]. Skin prick and intradermal testing measure IgE-mediated hypersensitivity in the skin, quantifying the wheal and flare response within 15 minutes of allergen contact. The skin prick and intradermal testing lose their value if skin response is decreased through treatment with antihistamines, antihistaminic effects of other medications (including many psychotropic agents), or increased skin sensitivity from dermatographism. In that case, specific IgE antibodies may be detected in serum with comparable sensitivity and specificity [91, Class I].
Treatment
There are no current data on the treatment of allergic rhinitis in individuals with a history of neuropsychiatric conditions. Nevertheless, good management of nasal inflammation may be very important for the general management of neuropsychiatric conditions, specifically recurrent mood disorders with an increased risk for suicide. The general principles of treatment are given here. Future studies ideally will successfully focus on the treatment of allergic rhinitis in patients with depression, the treatment of depression in patients with allergic rhinitis, and the effects on specific suicide risk factors of each medication from the antiallergy arsenal.
Diet and lifestyle
Minimize contact with allergens. Avoid outdoor exposure when aeroallergen counts are high. For seasonal aeroallergens, a useful resource is the National Allergy Bureau, which provides the most accurate and reliable pollen and mold counts from approximately 75 counting stations throughout the United States and two counting stations in Canada.
Reduce the aeroallergen load to the mucosa. Saline nasal irrigation is a safe and effective method of cleansing the nasal mucosa of allergens and improving sinus symptoms [92, Class II].
Reduce allergen load in microclimates. Vacuum cleaning, furnace filters, dehumidifiers, “allergy bedding,” and washing bed linens in hot water may reduce exposure to dust mites (the most important perennial allergen) and other perennial allergens (Class III).
Pharmacologic treatment
Choice of medications
Drug therapy for allergic rhinitis has many aims: stabilizing mast cells, reducing the effects of histamine, reducing inflammation, reducing secretions, reducing the production and effects of inflammatory mediators, reducing activation of prostaglandin pathways, maintaining the patency of sinuses and lacrimal channels, treating pain from sinus infection, and desensitization.
Clinicians should ask about depression, anxiety, and sleep disturbance, and treat them if present.
Clinicians also should ask about suicidal ideation and consider whether medications the patient is taking are increasing the risk of suicide. Referral for evaluation of suicide risk and preventive treatment may be appropriate.
Drugs used to treat allergic rhinitis (Table 1) include decongestants (oral and intranasal), antihistamines (oral and intranasal) (Table 2), corticosteroids (intranasal, rarely oral or parenteral) (Table 3), cromolyn (intranasal), anticholinergics (intranasal), and some newer combinations.
Debate on the sequence of use or combination of these agents has generated many studies. Weiner et al. [93, Class I] completed a worldwide meta-analysis of intranasal corticosteroids versus oral antihistamines, including 2267 patients with allergic rhinitis in 16 studies. End points were nasal blockage, nasal discharge, sneezing, nasal itch, postnasal drip, nasal discomfort, total nasal symptoms, nasal resistance, eye symptoms, and global ratings. The results demonstrate a superiority of intranasal corticosteroids over antihistamines. This conclusion was confirmed by Yáñez and Rodrigo [94, Class I], who showed the superiority of intra-nasal corticosteroids for ameliorating nasal blockage, sneezing, nasal discharge, nasal itch, and total nasal symptoms.
Antileukotrienes have been shown to be effective for treating daytime symptoms of allergic rhinitis but not for night symptoms [95, Class I].
Decongestants such as pseudoephedrine and phenylpropanolamine are effective in reducing nasal congestion from rhinitis, but they can cause insomnia, loss of appetite, and excessive nervousness [96, Class II]. Topical alpha-adrenergic nasal decongestants are commonly used but may cause rhinitis medicamentosa, a syndrome of rebound nasal congestion, when overused.
Antihistamines are commonly used to treat symptoms of allergic rhinoconjunctivitis. Antihistamines have long been the standard therapy for allergic rhinitis and are effective in relieving symptoms of itching, sneezing, and rhinorrhea, as well as allergic conjunctivitis. They have been shown to relieve symptoms of seasonal and perennial rhinitis [97, Class I].
A recent investigation by the US Food and Drug Administration (FDA) of the possibility that montelukast (Singulair; Merck & Co., White-house Station, NJ) may trigger suicide awaits additional data and conclusions. As montelukast neither improves sleep in allergic rhinitis (sleep disruption being a suicide risk factor) nor reduces production of cytokines (as would an intranasal corticosteroid), it may be possible that montelukast does not directly trigger suicide but rather fails to reduce suicide risk mediators elevated by the allergic inflammation. This question will require additional longitudinal studies. In the meantime, Merck has updated the prescribing information and patient information for Singulair to include the following postmarketing adverse events: depression (April 2007), suicidality (suicidal thinking and behavior) (October 2007), and anxiousness (February 2008). The FDA [98, Class III] currently recommends monitoring patients taking Singulair for suicidality and changes in mood.
Table 1.
Pharmacologic treatment of allergic diseases related to mental health
| Drug | Mode of action | Uses | Comments | Suicide risk considerations |
|---|---|---|---|---|
| Antihistamines | Histamine antagonist at HI receptor site | Allergic rhinitis and conjunctivitis, urticaria, allergic asthma | First-line therapy for mild or intermittent symptoms | 1st generation: somnolence, cognitive dysfunction |
| Oral, 1st generation | Often combined with oral decongestant | 2nd generation: less prominent, although sedation possible | ||
| Oral, 2nd generation | For symptoms rhinorrhea, sneezing, nasal and ocular itch | Attention to combinations; propensity to potentiation of ethanol and other sedating medications | ||
| Intranasal | 2nd generation: less significant CNS side effects | |||
| Topical (optical) | Not very effective for congestion (may require addition of decongestant); minimal effect on cytokine production | |||
| Corticosteroids | Anti-inflammatory | Treatment of many allergic diseases: allergic rhinitis and conjunctivitis, asthma, urticaria | First-line therapy for moderate/severe and persistent symptoms | Systemic: may cause depression, mania, psychosis |
| Oral | Potent anti-inflammatory; reduces cytokine production and effects | Local (eg, intranasal): compliance often is not very good; some mild systemic effects with local administration | ||
| Inhaled | Intranasal first-line therapy for moderate to severe symptoms of rhinitis | |||
| Intranasal | Oral: severe exacerbations of asthma, urticaria | |||
| Topical (skin) | ||||
| Decongestants | Alpha adrenergic agonist causes nasal vasoconstriction | Rhinitis | Reduce nasal congestion | Phenylephrine, phenylpropanolamine, pseudoephedrine, and synephrine may cause agitation, restlessness, insomnia, anxiety |
| Oral | Intranasal may cause rebound nasal congestion (rhinitis medicamentosa) | |||
| Intranasal | ||||
| Cromolyn/nedocromil sodium | Mast cell stabilizer; inhibits degranulation | Asthma, allergic rhinitis | Nonsteroidal anti-inflammatory | Few, if any, CNS effects |
| Oral | Minimal side effects | |||
| Inhaled | Mild efficacy | |||
| Intranasal | Requires frequent administration (daily dosing, poor compliance) | |||
| Anticholinergics | Muscarinic receptor antagonist | Asthma, rhinitis | Effectively reduces rhinorrhea | May add to anticholinergic effects of psychiatric medications |
| Inhaled | Role in acute bronchospasm | |||
| Intranasal | ||||
| Leukotriene inhibitors | Phospholipid metabolism inhibition | Asthma | Effective for daytime symptoms | Depression, anxiety, suicidal ideation |
| Oral (montelukast, zafirlukast, zileuton) | Steroid-sparing | Ongoing investigation of possibility of increased suicide risk with montelukast (check FDA website) | ||
| Adjust for liver impairment, monitor liver enzymes | ||||
| Zafirlukast and zileuton inhibit metabolism of warfarin | ||||
| Allergen immunotherapy | Th2 response suppression, Th1 response stimulation | Allergic rhinitis and conjunctivitis, asthma | Clinical efficacy confirmed in allergic rhinitis and allergic asthma (SCIT) | Unknown, caution recommended considering possible immune connections |
| Subcutaneous (SCIT) | Emerging evidence efficacy in allergic rhinitis (SLIT) | |||
| Sublingual (SLIT) | ||||
| Anti-IgE antibody | Reduces serum IgE | Severe asthma, possibly chronic urticaria and other IgE-mediated diseases | Approved for severe asthma | Unknown |
| IM injection | Requires multiple IM injections | |||
| Expensive |
CNS—central nervous system; FDA—US Food and Drug Administration; IM—intramuscular; Th1—T helper cell type 1; Th2—T helper cell type 2.
(Modified from Komarow HD, Postolache TT: Seasonal allergy and seasonal decrements in athletic performance. Clin Sports Med 2005, 24:e35—e50, and Postolache et al.: Allergy, depression, and suicide. Directions of Psychiatry 2005, 25:59−70.)
Table 2.
Dosage and administration of antihistamines for allergic rhinitis
| Generic name | Trade name | Available with decongestant? | Dosage |
|---|---|---|---|
| Diphenhydramine HCl | Benadryl* | No | Adults: 25−50 mg tid |
| Children 6−11 years: 5 mg/kg/d | |||
| Chlorpheniramine maleate | Chlor-Trimeton† | Yes | Adults: 8−12 mg bid |
| Children: 6−11 y, 0.35 mg/kg/d; 2−5 y, 1.25 mg bid | |||
| Hydroxyzine HCl | Atarax* | No | Adults: 25−50 mg bid |
| Children: 2 mg/kg/d | |||
| Loratadine | Claritin† | Yes | Adults: 10 mg qd |
| Children 6−11 y: 10 mg qd | |||
| Desloratidine | Clarinex† | Yes | Adults: 5 mg qd |
| Children: 6−11 y, 2.5 mg qd; 1−5 y, 1.25 mg qd | |||
| Cetirizine HCl | Zyrtec‡ | No | Adults: 5−10 mg qd |
| Children: 6−11 y, 5−10 mg qd; 2−5 y, 2.5−5 mg qd | |||
| Fexofenadine | Allegra§ | Yes | Adults: 60 mg bid, 120 mg qd |
| Azelastine HCl | Astelin¶ | No | Adults: 2 sprays/nostril bid |
| Children 5−11 y: 1 spray/nostril bid (0.1% solution) |
Pfizer.
Schering-Plough.
McNeil-PPC.
Aventis.
AstraZeneca.
bid—twice a day; qd—every day; tid—three times a day.
Table 3.
Dosage and administration of topical corticosteroids for allergic rhinitis
| Generic name | Trade name | Delivery | Dosage |
|---|---|---|---|
| Beclomethasone | Beconase,* Vancenase,* Beconase AQ,* Vancenase AQ* | Fluorocarbon aerosol, liquid spray | 1 spray = 42 μg; 1−2 sprays/nostril bid to tid |
| Triamcinolone | Nasacort,† Nasacort AQ† | Fluorocarbon aerosol, liquid spray | 1 spray = 55 μg; 1−2 sprays/nostril qd to bid |
| Flunisolide | Nasalide,‡ Nasarel‡ | Liquid spray | 1 spray = 25 μg; 1−2 sprays/nostril bid |
| Mometasone | Nasonex§ | Liquid spray | 1 spray = 50 μg; 2 sprays/nostril qd |
| Budesonide | Rhinocort,¶ Rhinocort AQ¶ | Fluorocarbon aerosol, liquid spray | 1 spray = 32 μg; 1−4 sprays/nostril qd |
| Fluticasone | Flonase* | Liquid spray | 1 spray = 50 μg; 1−2 sprays/nostril qd |
GlaxoSmithKline.
Aventis.
Ivax Laboratories.
Schering-Plough.
AstraZeneca.
AQ—aqueous; bid—twice a day; qd—every day; tid—three times a day.
Sequence of treatment
- Recent recommendations [99, Class II] suggest a stepwise approach:
- Mild, intermittent allergic rhinitis can be treated with 1) avoidance of allergens and irritants, 2) an oral or local nonsedating H1 histamine blocker, or 3) an intranasal or oral decongestant, each for fewer than 10 days.
- Moderate to severe or persistent allergic rhinitis can be treated with intranasal corticosteroids as a first-line treatment. If major blockage occurs, add a short course of decongestant. Reassess in 2 to 4 weeks; if symptoms persist, add an oral antihistamine (with or without decongestants) and ipratropium.
- For persistent allergic rhinitis, regardless of severity, consider the addition of allergen immunotherapy.
For specific drugs, see Table 1.
For dosages, see Table 2 (antihistamines), Table 3 (intranasal corticosteroids), and Table 4 (leukotriene inhibitors).
Table 4.
Leukotriene inhibitors for the treatment of allergy and asthma
| Generic name | Trade name | Dosage | Therapeutic issues |
|---|---|---|---|
| Montelukast | Singulair* | Adults: 10 mg qhs | Renal adjustments: none |
| Children: 6−14 y, 5 mg qhs; 2−5 y, 4 mg qhs | Hepatic adjustments: in mild to moderate disease | ||
| Zafirlukast | Accolate† | Age ≥ 12 y: 20 mg bid | Renal adjustments: none |
| 5−11 y: 10 mg bid | Hepatic adjustments: not defined | ||
| Monitor hepatic enzymes every 2−3 mo | |||
| Administration with meals decreases bioavailability; take at least 1 h before meals or 2 h after | |||
| Inhibits metabolism of warfarin (Coumadin), increasing prothrombin time | |||
| Zileuton | Zyflo‡ | Patients > 12 y: 600 mg with meals and qhs | Can inhibit metabolism of warfarin, theophylline, and propanolol |
| Monitor hepatic enzymes every 2−3 mo |
Merck.
AstraZeneca.
Critical Therapeutics, Inc.
bid—twice a day; qhs—at bedtime.
Interventional procedures
Subcutaneous immunotherapy
For effectiveness, see reference 100•• (Class I).
Standard procedure
Patients with allergic rhinitis, allergic conjunctivitis, allergic asthma, or insect allergy who have evidence of specific IgE antibodies correlating with suspected environmental triggers, patient exposure, and clinical history may be candidates for subcutaneous immunotherapy (SCIT). Once the potential benefit is established (taking into consideration response to standard pharmacotherapy, convenience, cost, and risks of anaphylaxis), an extract of the specific allergens likely to cause allergic symptoms is made. The immunotherapy begins with a build-up phase of injections, with the ultimate goal of reaching a maintenance phase. The maintenance phase is the dose that achieves clinical efficacy. For inhalant allergens, it is generally administered every 2 to 4 weeks.
Contraindications
Medical conditions that may impair a patient's ability to survive an anaphylactic reaction or the necessary treatment of this reaction. Examples are severe asthma, cardiovascular disease, and β-blocker use.
Complications
Anaphylaxis, large local reactions, cellulitis.
Special points
Effect size with SCIT (standardized mean difference, 95%) is −0.73 (95% CI, −0.97 to −0.50). The comparable figures with medications are −0.57 (95% CI, −0.82 to −0.33) [100••, Class I].
Sublingual immunotherapy
Clinical efficacy has been supported by several studies [101, Class II]. However, the optimal maintenance dose, dosing schedule, and mechanism of action have not been established.
Standard procedure
Standard procedure has not been established. Current protocols include a sublingual tablet or liquid containing allergen extract that is ingested daily.
Contraindications
Same as for subcutaneous immunotherapy.
Complications
Anaphylaxis; gastrointestinal symptoms such as mouth, throat, and tongue irritation or swelling. Headaches have been reported [102, Class II].
Special points
The effect size (standardized mean difference, 95%) is −0.43 (95% CI, −0.57 to −0.28). With medications it is −0.41 (95% CI, −0.55 to −0.28) [97, Class II].
Emerging therapies
Anti-IgE antibodies
Given the central role of IgE in the pathogenesis of allergic disease, it stands to reason that inhibiting IgE responses by using anti-IgE antibodies would decrease its sensitizing effects on mast cells and basophils. Several studies indeed indicate that anti-IgE therapy is effective in treating asthma and allergic rhinitis [103, Class II].
Acknowledgments
Supported by the American Foundation for Suicide Prevention and NARSAD Independent Investigator Award to Dr. Postolache, R21 MH075891 (PI, Dr. Postolache; Co-PI, Dr. Tonelli), R21 MH075905 (PI, Dr. Tonelli, Co-PI, Dr. Postolache), and R01MH074891 (PI, Dr. Postolache). We thank Sarah Zimmerman for her general assistance.
Footnotes
Disclosures No potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 2•.Goodwin FK, Jamison KR. Manic Depressive Illness: Bipolar Disorders and Recurrent Depression. 2. Oxford University Press; New York: 2007. [Of importanceAuthoritative textbook on broadly understood manic-depressive illness, describing seasonality of suicide in recurrent mood disorders.] [Google Scholar]
- 3.American Academy of Allergy Asthma & Immunology: National Allergy Bureau [June 19, 2008]; Available at http://www.aaaai.org/nab/index.cfm.
- 4.Kosisky SE, Carpenter GB. Predominant tree aeroallergens of the Washington, DC area: a six year survey (1989−1994) Ann Allergy Asthma Immunol. 1997;78:381–392. doi: 10.1016/S1081-1206(10)63200-0. [DOI] [PubMed] [Google Scholar]
- 5•.Postolache TT, Stiller JW, Herrell R, et al. Tree pollen peaks are associated with increased nonviolent suicide in women. Mol Psychiatry. 2005;10:232–235. doi: 10.1038/sj.mp.4001620. [Of importanceFirst report on the relationship between seasonal peaks in tree pollen and peaks of suicide in spring.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timonen M, Jokelainen J, Hakko H, et al. Atopy and depression: results from the Northern Finland 1966 Birth Cohort Study. Mol Psychiatry. 2003;8:738–744. doi: 10.1038/sj.mp.4001274. [DOI] [PubMed] [Google Scholar]
- 7.Marshall PS, O'Hara C, Steinberg P. Effects of seasonal allergic rhinitis on fatigue levels and mood. Psychosom Med. 2002;64:684–691. doi: 10.1097/01.psy.0000021944.35402.44. [DOI] [PubMed] [Google Scholar]
- 8.Guzman A, Tonelli LH, Roberts D, et al. Mood-worsening with high-pollen-counts and seasonality: a preliminary report. J Affect Disord. 2007;101:269–274. doi: 10.1016/j.jad.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postolache TT, Lapidus M, Sander ER, et al. Changes in allergy symptoms and depression scores are positively correlated in patients with recurrent mood disorders exposed to seasonal peaks in aeroallergens. ScientificWorldJournal. 2007;7:1968–1977. doi: 10.1100/tsw.2007.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postolache TT, Langenberg PB, Zimmerman SA, et al. Changes in severity of allergy and anxiety symptoms are positively correlated in patients with recurrent mood disorders who are exposed to seasonal peaks of aeroallergens. Int J Child Health Hum Dev. (in press) [PMC free article] [PubMed] [Google Scholar]
- 11.Bell IR, Jasnoski ML, Kagan J, et al. Depression and allergies: survey of a nonclinical population. Psychother Psychosom. 1991;55:24–31. doi: 10.1159/000288404. [DOI] [PubMed] [Google Scholar]
- 12.Hashiro M, Okumura M. The relationship between the psychological and immunological state in patients with atopic dermatitis. J Dermatol Sci. 1998;16:231–235. doi: 10.1016/s0923-1811(97)00074-1. [DOI] [PubMed] [Google Scholar]
- 13.Timonen M, Jokelainen J, Silvennoinen–Kassinen S, et al. Association between skin test diagnosed atopy and professionally diagnosed depression: a Northern Finland 1966 Birth Cohort study. Biol Psychiatry. 2002;52:349–355. doi: 10.1016/s0006-3223(01)01364-6. [DOI] [PubMed] [Google Scholar]
- 14.Timonen M, Jokelainen J, Herva A, et al. Presence of atopy in first-degree relatives as a predictor of a female proband's depression: results from the Northern Finland 1966 Birth Cohort. J Allergy Clin Immunol. 2003;111:1249–1254. doi: 10.1067/mai.2003.1546. [DOI] [PubMed] [Google Scholar]
- 15.Wamboldt MZ, Hewitt JK, Schmitz S, et al. Familial association between allergic disorders and depression in adult Finnish twins. Am J Med Genet. 2000;96:146–153. doi: 10.1002/(sici)1096-8628(20000403)96:2<146::aid-ajmg4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.Wilken JA, Berkowitz R, Kane R. Decrements in vigilance and cognitive functioning associated with ragweed-induced allergic rhinitis. Ann Allergy Asthma Immunol. 2002;89:372–380. doi: 10.1016/S1081-1206(10)62038-8. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz EL, Morgenstern H. Cross-sectional associations of asthma, hay fever, and other allergies with major depression and low-back pain among adults aged 20−39 years in the United States. Am J Epidemiol. 1999;150:1107–1116. doi: 10.1093/oxfordjournals.aje.a009936. [DOI] [PubMed] [Google Scholar]
- 18.Addolorato G, Ancona C, Capristo E, et al. State and trait anxiety in women affected by allergic and vasomotor rhinitis. J Psychosom Res. 1999;46:283–289. doi: 10.1016/s0022-3999(98)00109-3. [DOI] [PubMed] [Google Scholar]
- 19.Prolo P, Licinio J. Cytokines in affective disorders and schizophrenia: new clinical and genetic findings. Mol Psychiatry. 1999;4:396. doi: 10.1038/sj.mp.4000555. [DOI] [PubMed] [Google Scholar]
- 20.Capuron L, Ravaud A, Gualde N, et al. Association between immune activation and early depressive symptoms in cancer patients treated with interleukin-2-based therapy. Psychoneuroendocrinology. 2001;26:797–808. doi: 10.1016/s0306-4530(01)00030-0. [DOI] [PubMed] [Google Scholar]
- 21.Dantzer R, Wollman EE, Yirmiya R. Cytokines and depression: an update. Brain Behav Immun. 2002;16:501–502. doi: 10.1016/s0889-1591(02)00002-8. [DOI] [PubMed] [Google Scholar]
- 22.Capuron L, Dantzer R. Cytokines and depression: the need for a new paradigm. Brain Behav Immun. 2003;17(Suppl 1):S119–S124. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 23.Anisman H, Merali Z, Poulter MO, et al. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11:963–972. doi: 10.2174/1381612053381701. [DOI] [PubMed] [Google Scholar]
- 24.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [Of importanceExcellent review on effects of cytokines on brain function.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: a review. Am J Psychiatry. 2000;157:867–876. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- 28.Crone CC, Gabriel GM, Wise TN. Managing the neuropsychiatric side effects of interferon-based therapy for hepatitis C. Cleve Clin J Med. 2004;71(Suppl 3):S27–S32. doi: 10.3949/ccjm.71.suppl_3.s27. [DOI] [PubMed] [Google Scholar]
- 29.Raison CL, Demetrashvili M, Capuron L, et al. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denicoff KD, Rubinow DR, Papa MZ, et al. The neuropsychiatric effects of treatment with interleukin-2 and lymphokine-activated killer cells. Ann Intern Med. 1987;107:293–300. doi: 10.7326/0003-4819-107-2-293. [DOI] [PubMed] [Google Scholar]
- 31.Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol. 2000;18:2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- 32.Capuron L, Dantzer R, Miller AH. Neuro-immune interactions in psychopathology with the example of interferon alpha-induced depression. J Soc Biol. 2003;197:151–156. [PubMed] [Google Scholar]
- 33.Frommberger UH, Bauer J, Haselbauer P, et al. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci. 1997;247:228–233. doi: 10.1007/BF02900219. [DOI] [PubMed] [Google Scholar]
- 34.Levine J, Barak Y, Chengappa KN, et al. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40:171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- 35.Yirmiya R, Weidenfeld J, Pollack Y, et al. Cytokines, “depression due to a general medical condition,” and anti-depressant drugs. Adv Exp Med Biol. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [DOI] [PubMed] [Google Scholar]
- 36.Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: What can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 37.Dunn AJ, Swiergiel AH. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol Biochem Behav. 2005;81:688–693. doi: 10.1016/j.pbb.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swiergiel AH, Dunn AJ. Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav. 2007;86:651–659. doi: 10.1016/j.pbb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reichenberg A, Yirmiya R, Schuld A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 40.De La Garza R. Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neurosci Biobehav Rev. 2005;29:761–770. doi: 10.1016/j.neubiorev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Engeland CG, Kavaliers M, Ossenkopp KP. Sex differences in the effects of muramyl dipeptide and lipopolysaccharide on locomotor activity and the development of behavioral tolerance in rats. Pharmacol Biochem Behav. 2003;74:433–447. doi: 10.1016/s0091-3057(02)01024-9. [DOI] [PubMed] [Google Scholar]
- 42••.Tonelli LH, Stiller J, Rujescu D, et al. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand. 2008;117:198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [Of major importanceFirst study reporting Th2 cytokine gene expression in the human brain, with elevation in suicide victims.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Tonelli LH, Holmes A, Postolache TT. Intranasal immune challenge induces sex-dependent depressive-like behavior and cytokine expression in the brain. Neuropsychopharmacology. 2008;33:1038–1048. doi: 10.1038/sj.npp.1301488. [Of importanceFirst study to show that the intranasal immune challenge (in rats) results in depression-like behaviors and cytokine activation in the brain.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tonelli LH, Virk G, Joppy B, et al. Experimentally induced allergy to tree pollen induces depressive-like behavior and mast cell activation in the brain of female rats.. Society of Biological Psychiatry Annual Meeting; Toronto, Canada. 2006. [Google Scholar]
- 45.Tonelli LH, Hoshino A, Katz M, et al. Acute stress promotes aggressive-like behavior in rats made allergic to tree pollen. Int J Child Health Hum Dev. (in press) [PMC free article] [PubMed] [Google Scholar]
- 46.Goehler LE, Gaykema RP, Hansen MK, et al. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 47.Quan N, Herkenham M. Connecting cytokines and brain: a review of current issues. Histol Histopathol. 2002;17:273–288. doi: 10.14670/HH-17.273. [DOI] [PubMed] [Google Scholar]
- 48.Pitossi F, del Ray A, Kabiersch A, et al. Induction of cytokine transcripts in the central nervous system and pituitary following peripheral administration of endotoxin to mice. J Neurosci Res. 1997;48:287–298. doi: 10.1002/(sici)1097-4547(19970515)48:4<287::aid-jnr1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 49.Molina-Holgado F, Toulmond S, Rothwell NJ. Involvement of interleukin-1 in glial responses to lipopolysaccharide: endogenous versus exogenous interleukin-1 actions. J Neuroimmunol. 2000;111:1–9. doi: 10.1016/s0165-5728(00)00344-1. [DOI] [PubMed] [Google Scholar]
- 50.Turrin NP, Plata-Salamán CR. Cytokine-cytokine interactions and the brain. Brain Res Bull. 2000;51:3–9. doi: 10.1016/s0361-9230(99)00203-8. [DOI] [PubMed] [Google Scholar]
- 51.Vitkovic L, Konsman JP, Bockaert J, et al. Cytokine signals propagate through the brain. Mol Psychiatry. 2000;5:604–615. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- 52.Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav Immun. 2003;17:13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 53.Turnbull AV, Rivier C. Regulation of the HPA axis by cytokines. Brain Behav Immun. 1995;9:253–275. doi: 10.1006/brbi.1995.1026. [DOI] [PubMed] [Google Scholar]
- 54.Tonelli LH, Postolache TT, Sternberg EM. Inflammatory genes and neural activity: involvement of immune genes in synaptic function and behavior. Front Biosci. 2005;10:675–680. doi: 10.2741/1562. [DOI] [PubMed] [Google Scholar]
- 55.Quan N, Zhang Z, Demetrikopoulos MK. Evidence for involvement of B lymphocytes in the surveillance of lung metastasis in the rat. Cancer Res. 1999;59:1080–1089. [PubMed] [Google Scholar]
- 56.Tonelli LH, Maeda S, Rapp KL, et al. Differential induction of interleukin-I beta mRNA in the brain parenchyma of Lewis and Fischer rats after peripheral injection of lipopolysaccharides. J Neuroimmunol. 2003;140:126–136. doi: 10.1016/s0165-5728(03)00171-1. [DOI] [PubMed] [Google Scholar]
- 57.Tonelli LH, Postolache TT. Tumor necrosis factor alpha, interleukin-1 beta, interleukin-6 and major histocompatibility complex molecules in the normal brain and after peripheral immune challenge. Neurol Res. 2005;27:679–684. doi: 10.1179/016164105X49463. [DOI] [PubMed] [Google Scholar]
- 58.Singareddy RK, Balon R. Sleep and suicide in psychiatric patients. Ann Clin Psychiatry. 2001;13:93–101. doi: 10.1023/a:1016619708558. [DOI] [PubMed] [Google Scholar]
- 59.Fawcett J, Scheftner WA, Fogg L, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147:1189–1194. doi: 10.1176/ajp.147.9.1189. [DOI] [PubMed] [Google Scholar]
- 60.Krueger JM, Walter J, Dinarello CA, et al. Sleep-promoting effects of endogenous pyrogen (interleukin-1). Am J Physiol. 1984;246:R994–R999. doi: 10.1152/ajpregu.1984.246.6.R994. [DOI] [PubMed] [Google Scholar]
- 61.Opp MR, Krueger JM. Interleukin 1-receptor antagonist blocks interleukin 1-induced sleep and fever. Am J Physiol. 1991;260:R453–R457. doi: 10.1152/ajpregu.1991.260.2.R453. [DOI] [PubMed] [Google Scholar]
- 62.Kapas L, Krueger JM. Tumor necrosis factor-beta induces sleep, fever, and anorexia. Am J Physiol. 1992;263:R703–R707. doi: 10.1152/ajpregu.1992.263.3.R703. [DOI] [PubMed] [Google Scholar]
- 63.Gemma C, Imeri L, de Simoni MG, et al. Interleukin-1 induces changes in sleep, brain temperature, and serotonergic metabolism. Am J Physiol. 1997;272:R601–R606. doi: 10.1152/ajpregu.1997.272.2.R601. [DOI] [PubMed] [Google Scholar]
- 64.Hogan D, Morrow JD, Smith EM, et al. Interleukin-6 alters sleep of rats. J Neuroimmunol. 2003;137:59–66. doi: 10.1016/s0165-5728(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 65.Kushikata T, Fang J, Wang Y, Krueger JM. Interleukin-4 inhibits spontaneous sleep in rabbits. Am J Physiol. 1998;275:R1185–R1191. doi: 10.1152/ajpregu.1998.275.4.R1185. [DOI] [PubMed] [Google Scholar]
- 66.Kushikata T, Fang J, Krueger JM. Interleukin-10 inhibits spontaneous sleep. J Interferon Cytokine Res. 1999;19:1025–1030. doi: 10.1089/107999099313244. [DOI] [PubMed] [Google Scholar]
- 67.Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31:15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 68.Hughes K, Glass C, Ripchinski M, et al. Efficacy of the topical nasal steroid budesonide on improving sleep and daytime somnolence in patients with perennial allergic rhinitis. Allergy. 2003;58:380–385. doi: 10.1034/j.1398-9995.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 69.Hamano N, Terada N, Maesako K, et al. Effect of sex hormones on eosinophilic inflammation in nasal mucosa. Allergy Asthma Proc. 1998;19:263–269. doi: 10.2500/108854198778557773. [DOI] [PubMed] [Google Scholar]
- 70.Vliagoftis H, Dimitriadou V, Boucher W, et al. Estradiol augments while tamoxifen inhibits rat mast cell secretion. Int Arch Allergy Immunol. 1992;98:398–409. doi: 10.1159/000236217. [DOI] [PubMed] [Google Scholar]
- 71.Osman M. Therapeutic implications of sex differences in asthma and atopy. Arch Dis Child. 2003;88:587–590. doi: 10.1136/adc.88.7.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56:3–17. doi: 10.1211/0022357022539. [DOI] [PubMed] [Google Scholar]
- 73.Thorne RG, Pronk GJ, Padmanabhan V. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 74.Mori I, Goshima F, Ito H, et al. The vomeronasal chemosensory system as a route of neuroinvasion by herpes simplex virus. Virology. 2005;334:51–58. doi: 10.1016/j.virol.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 75.Loftus LT, Li HF, Gray AJ, et al. In vivo protein transduction to the CNS. Neuroscience. 2006;139:1061–1067. doi: 10.1016/j.neuroscience.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 76.Kalueff AV, Lehtimaki KA, Ylinen A, et al. Intranasal administration of human IL-6 increases the severity of chemically induced seizures in rats. Neurosci Lett. 2004;365:106–110. doi: 10.1016/j.neulet.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 77.Pelidou SH, Zou LP, Deretzi G, et al. Enhancement of acute phase and inhibition of chronic phase of experimental autoimmune neuritis in Lewis rats by intranasal administration of recombinant mouse interleukin 17: potential immunoregulatory role. Exp Neurol. 2000;163:165–172. doi: 10.1006/exnr.2000.7357. [DOI] [PubMed] [Google Scholar]
- 78.Annesi-Maesano I. Epidemiological evidence of the occurrence of rhinitis and sinusitis in asthmatics. Allergy. 1999;54(Suppl 57):7–13. doi: 10.1111/j.1398-9995.1999.tb04401.x. [DOI] [PubMed] [Google Scholar]
- 79.Dykewicz MS, Fineman S, Skoner DP, et al. Diagnosis and management of rhinitis: complete guidelines of the Joint Task Force on Practice Parameters in Allergy, Asthma and Immunology. American Academy of Allergy, Asthma, and Immunology. Ann Allergy Asthma Immunol. 1998;81:478–518. doi: 10.1016/s1081-1206(10)63155-9. [DOI] [PubMed] [Google Scholar]
- 80.The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351:1225–1232. [PubMed] [Google Scholar]
- 81.Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5 Suppl):S147–S334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 82.Dykewicz MS. Rhinitis and sinusitis. J Allergy Clin Immunol. 2003;111(2 Suppl):S520–S529. doi: 10.1067/mai.2003.82. [DOI] [PubMed] [Google Scholar]
- 83.Demoly P, Allaert FA, Lecasble M, Bousquet J. Validation of the classification of ARIA (allergic rhinitis and its impact on asthma). Allergy. 2003;58:672–675. doi: 10.1034/j.1398-9995.2003.t01-1-00202.x. [DOI] [PubMed] [Google Scholar]
- 84.Ku M, Silverman B, Prifti N, et al. Prevalence of migraine headaches in patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2006;97:226–230. doi: 10.1016/S1081-1206(10)60018-X. [DOI] [PubMed] [Google Scholar]
- 85.Low NC, Merikangas KR. The comorbidity of migraine. CNS Spectr. 2003;8:433–434. 437–444. doi: 10.1017/s1092852900018745. [DOI] [PubMed] [Google Scholar]
- 86.Frediani T, Lucarelli S, Pelliccia A, et al. Allergy and childhood epilepsy: a close relationship? Acta Neurol Scand. 2001;104:349–352. doi: 10.1034/j.1600-0404.2001.00347.x. [DOI] [PubMed] [Google Scholar]
- 87.Theoharides TC, Donelan J, Kandere-Grzybowska, et al. The role of mast cells in migraine pathophysiology. Brain Res Rev. 2005;49:65–76. doi: 10.1016/j.brainresrev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 88.Boyce JA. Mast cells: beyond IgE. J Allergy Clin Immunol. 2003;111:24–32. doi: 10.1067/mai.2003.60. [DOI] [PubMed] [Google Scholar]
- 89.Berger WE. Treatment update: allergic rhinitis. Allergy Asthma Proc. 2001;22:191–198. [PubMed] [Google Scholar]
- 90.Borish L. Allergic rhinitis: systemic inflammation and implications for management. J Allergy Clin Immunol. 2003;112:1021–1031. doi: 10.1016/j.jaci.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 91.Hamilton RG, Adkinson NF., Jr. Clinical laboratory assessment of IgE-dependent hypersensitivity. J Allergy Clin Immunol. 2003;111(2 Suppl):S687–S701. doi: 10.1067/mai.2003.123. [DOI] [PubMed] [Google Scholar]
- 92.Tomooka LT, Murphy C, Davidson TM. Clinical study and literature review of nasal irrigation. Laryngoscope. 2000;110:1189–1193. doi: 10.1097/00005537-200007000-00023. [DOI] [PubMed] [Google Scholar]
- 93.Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ. 1998;317:1624–1629. doi: 10.1136/bmj.317.7173.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yáñez A, Rodrigo GJ. Intranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2002;89:479–484. doi: 10.1016/S1081-1206(10)62085-6. [DOI] [PubMed] [Google Scholar]
- 95.Rodrigo GJ, Yáñez A. The role of antileukotriene therapy in seasonal allergic rhinitis: a systematic review of randomized trials. Ann Allergy Asthma Immunol. 2006;96:779–786. doi: 10.1016/S1081-1206(10)61339-7. [DOI] [PubMed] [Google Scholar]
- 96.Chait LD. Factors influencing the reinforcing and subjective effects of ephedrine in humans. Psychopharmacology (Berl) 1994;113:381–387. doi: 10.1007/BF02245213. [DOI] [PubMed] [Google Scholar]
- 97.Meltzer EO, Weiler JM, Widlitz MD. Comparative outdoor study of the efficacy, onset and duration of action, and safety of cetirizine, loratadine, and placebo for seasonal allergic rhinitis. J Allergy Clin Immunol. 1996;97:617–626. doi: 10.1016/s0091-6749(96)70307-x. [DOI] [PubMed] [Google Scholar]
- 98.US Food and Drug Administration Early communication about an ongoing safety review of montelukast (Singulair). 2008 March 27; Available at http://www.fda.gov/cder/drug/ early_comm/montelukast.htm.
- 99.Brozek JL, Baena-Cagnani CE, Bonini S, et al. Methodology for development of the Allergic Rhinitis and its Impact on Asthma guideline 2008 update. Allergy. 2008;63:38–46. doi: 10.1111/j.1398-9995.2007.01560.x. [DOI] [PubMed] [Google Scholar]
- 100••.Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007:CD001936. doi: 10.1002/14651858.CD001936.pub2. [Of major importanceCochrane review on allergen immunotherapy via injection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Radulovic S, Calderon MA, Wilson DR, et al. Safety profile of sublingual immunotherapy (SLIT) for allergic rhinitis (AR). J Allergy Clin Immun. 2008;121:S142. [Google Scholar]
- 102.Didier A, Malling H, Worm M, et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;120:1338–1345. doi: 10.1016/j.jaci.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 103.Kaliner MA. Omalizumab and the treatment of allergic rhinitis. Curr Allergy Asthma Rep. 2004;4:237–244. doi: 10.1007/s11882-004-0032-2. [DOI] [PubMed] [Google Scholar]