Abstract
The median preoptic nucleus (MnPN) of the hypothalamus contains sleep-active neurons including sleep-active GABAergic neurons and is involved in the regulation of nonREM/REM sleep. The hypocretinergic (HCRT) neurons of the perifornical-lateral hypothalamic area (PF-LHA) and serotonergic (5-HT) neurons of the dorsal raphe nucleus (DRN) are mostly active during waking and have been implicated in the regulation of arousal. MnPN GABAergic neurons project to the PF-LHA and DRN. It is hypothesized that MnPN promotes sleep by inhibiting multiple arousal systems including HCRT and other wake-active neurons within the PF-LHA and 5-HT neurons in the DRN. We examined the effects of inactivation of MnPN neurons by locally microinjecting 0.2 µl of 1mM or 10mM solutions of a GABAA receptor agonist, muscimol, into the MnPN on Fos expression (Fos-IR) in the PF-LHA neurons including HCRT neurons and 5-HT neurons in the DRN in anesthetized rats. Compared to artificial cerebrospinal fluid control, microinjection of muscimol into the MnPN resulted in significantly higher percentages of HCRT and non-HCRT neurons in the PF-LHA and 5-HT neurons in the DRN that exhibited Fos-IR. The percentage of melanin-concentrating hormone (MCH)+/Fos+ neurons in the PF-LHA did not change after muscimol treatments. These results support a hypothesis that the activation of MnPN neurons contributes to the suppression of wake-promoting systems including HCRT and other unidentified neurons in the PF-LHA and 5-HT neurons in the DRN. These results also suggest that MCH neurons may not be under MnPN inhibitory control. These findings are consistent with a hypothesized role of MnPN in sleep regulation.
Keywords: Orexin, Melanin-concentrating hormone, Median preoptic nucleus, Posterior-lateral hypothalamus, Serotonin, Sleep
INTRODUCTION
Various lines of evidence support that the median preoptic nucleus (MnPN) of the hypothalamus is involved in the regulation of non-rapid eye movement (nonREM) and REM sleep [6,32,46]. Many MnPN neurons exhibit sleep-associated discharge activity, i.e., the lowest discharge during waking, which increases with the onset of sleep, and the highest discharge during non-REM and REM sleep[50]. The number of MnPN neurons exhibiting c-Fos protein immunoreactivity (Fos-IR), a marker of neuronal activation, increases with the amount of preceding sleep and with increasing homeostatic pressure for REM sleep [12,15,16].
The perifornical-lateral hypothalamic area (PF-LHA) has been implicated in the promotion and/or maintenance of behavioral arousal [20,22,43,45,48]. Evidence suggests that hypocretin (HCRT), also called as orexin, containing neurons within PF-LHA are wake-active, i.e., exhibit wake-associated discharge as well as Fos-IR [9,10,29,36]. A loss of HCRT neurons is associated with the pathogenesis of the disease narcolepsy, characterized by excessive sleepiness [40,54]. The HCRT level in cerebrospinal fluid is higher during active-waking and applications of HCRT into various brain regions, e.g., preoptic area, basal forebrain, tuberomammillary nucleus and locus coeruleus, promote waking [5,8,17,19,23,35,53]. On the other hand, the PF-LHA contains other cell types including melaninconcentrating hormone (MCH) and GABAergic neurons, that have been implicated in the regulation of sleep [27,37,60].
Evidence supports that serotonergic (5-HT) system of the dorsal raphe nucleus (DRN) constitutes an important component of the brainstem arousal system[32,57]. Putative 5-HT neurons exhibit wake-related discharge, become less active during non-REM and nearly cease firing during REM sleep[14,33,56]. The firing rates of DRN 5-HT neurons show a strong positive relationship with tonic level of motor activity and 5-HT levels in the DRN as well as at projection sites parallel the 5-HT neuronal discharge, i.e., are higher during waking and lower during sleep, particularly REM sleep [4,42].
Although it is well documented that most of the PF-LHA neurons including HCRT neurons as well as 5-HT neurons in the DRN are active during waking and quiescent in non-REM/REM sleep, the anatomical regions or factors that cause the suppression/inhibition of those neurons during sleep remains poorly understood[2,24,26,29,33,36]. Recent anatomical, immunohistochemical, and electrophysiological studies suggest that PF-LHA and DRN neurons may be under inhibitory control of the MnPN sleep promoting system. For example, a) MnPN sleep-active neurons are predominantly GABAergic and exhibit the state-dependent activities that are reciprocal to the PF-LHA and 5-HT neurons [2,11,33,50]; b) MnPN neurons constitute a source of afferents to PF-LHA and DRN [59]; c) a subset of MnPN neurons projecting to PF-LHA are GABAergic and express Fos-IR during sleep[11,44,58]; and d) MnPN electrical and chemical stimulations suppress whereas its chemical inactivation increases the discharge activity of the majority of PF-LHA neurons [49]. However, the neurotransmitter phenotypes of the PF-LHA neurons that are under MnPN inhibitory control are not known. It is also not known if 5-HT neurons are, in part, under MnPN inhibitory control.
In this study we determined the phenotypes of neurons exhibiting Fos-IR in the PF-LHA and in the DRN resulting from inactivation of MnPN neurons by local microinjection of a GABAA receptor agonist, muscimol, in anesthetized rats. We found that percentages of HCRT neurons in the PF-LHA and 5-HT neurons in the DRN exhibiting Fos-IR increased after inactivation of MnPN neurons by local microinjection of muscimol, whereas MCH neurons remained largely unaffected. These findings seem consistent with the hypothesis that MnPN promotes sleep by inhibiting multiple arousal systems including HCRT and other wake-active neurons within the PF-LHA and 5-HT neurons within the DRN.
RESULTS
1. Location of the microinjection sites
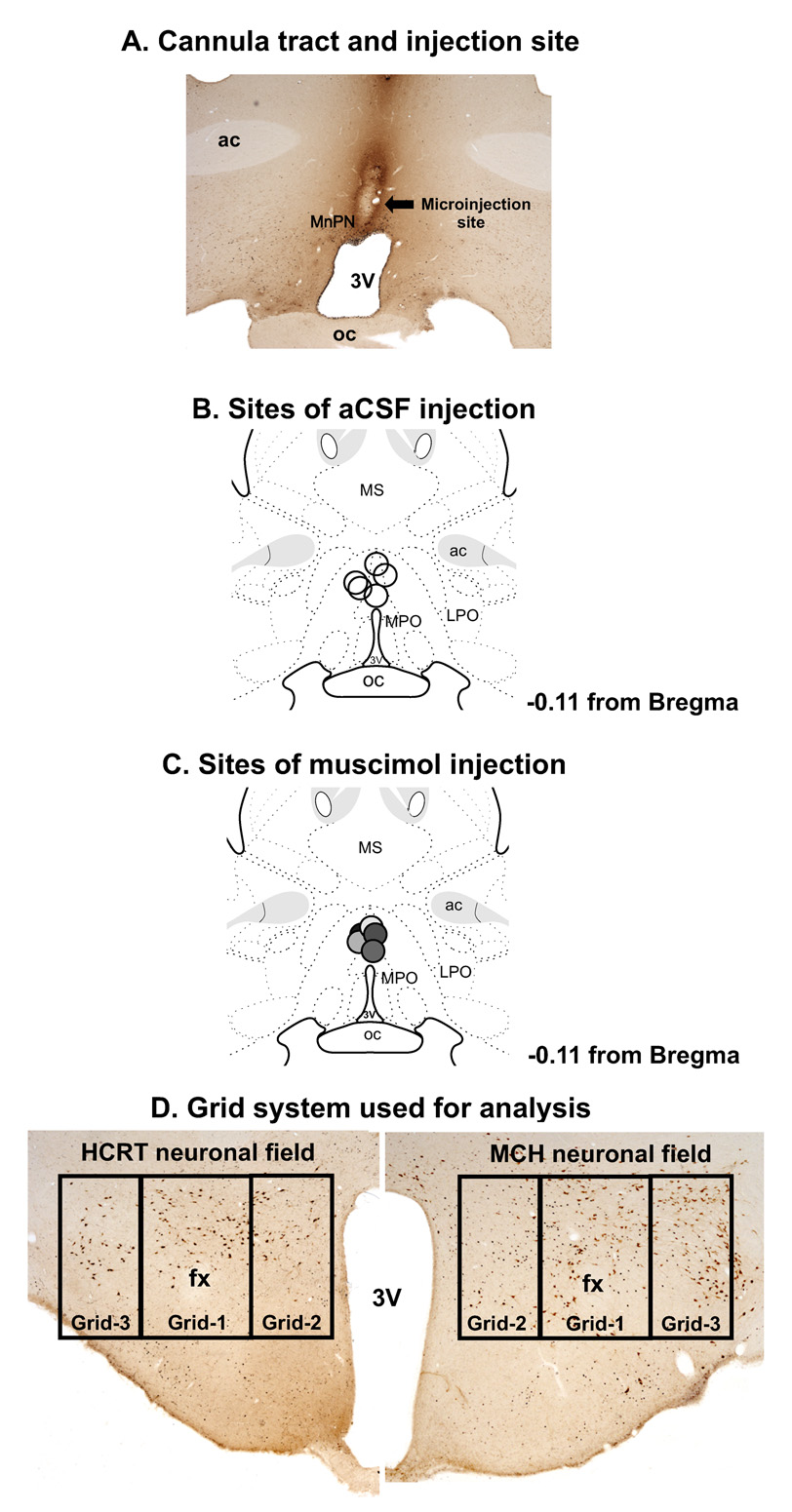
Figure-1 shows a representative histological section (A) as well as reconstruction diagrams through MnPN (B and C) showing the locations of the microinjection sites. There were typical tissue damages at the site of injections. The placements of injections were within and in close proximity of MnPN encompassing areas that constitute sources of afferents to PF-LHA and DRN and where clusters of sleep-active neurons including sleep-active GABAergic neurons are localized[11,12,50].
Figure 1. Locations of the microinjection sites.
Photomicrograph of a coronal section (20X magnification) through the MnPN of a rat, which was injected with aCSF, showing the tract of the injector cannula and the site of microinjection (A). Reconstruction diagrams through MnPN showing the locations of aCSF (B) and muscimol microinjection sites (C). D. Photomicrographs (40X) through the PF-LHA showing the anatomical distributions of the HCRT and MCH neurons and the grid system that used for the analyses. ac, anterior commissure; fx, fornix; LPO, lateral preoptic area; MnPN, median preoptic nucleus; MPO, medial preoptic area; mt, mammillothalamic tract; oc, optic chiasm; 3V, third ventricle.
2. Effects of muscimol microinjection into MnPN on Fos-IR in PF-LHA neurons
A. HCRT neurons
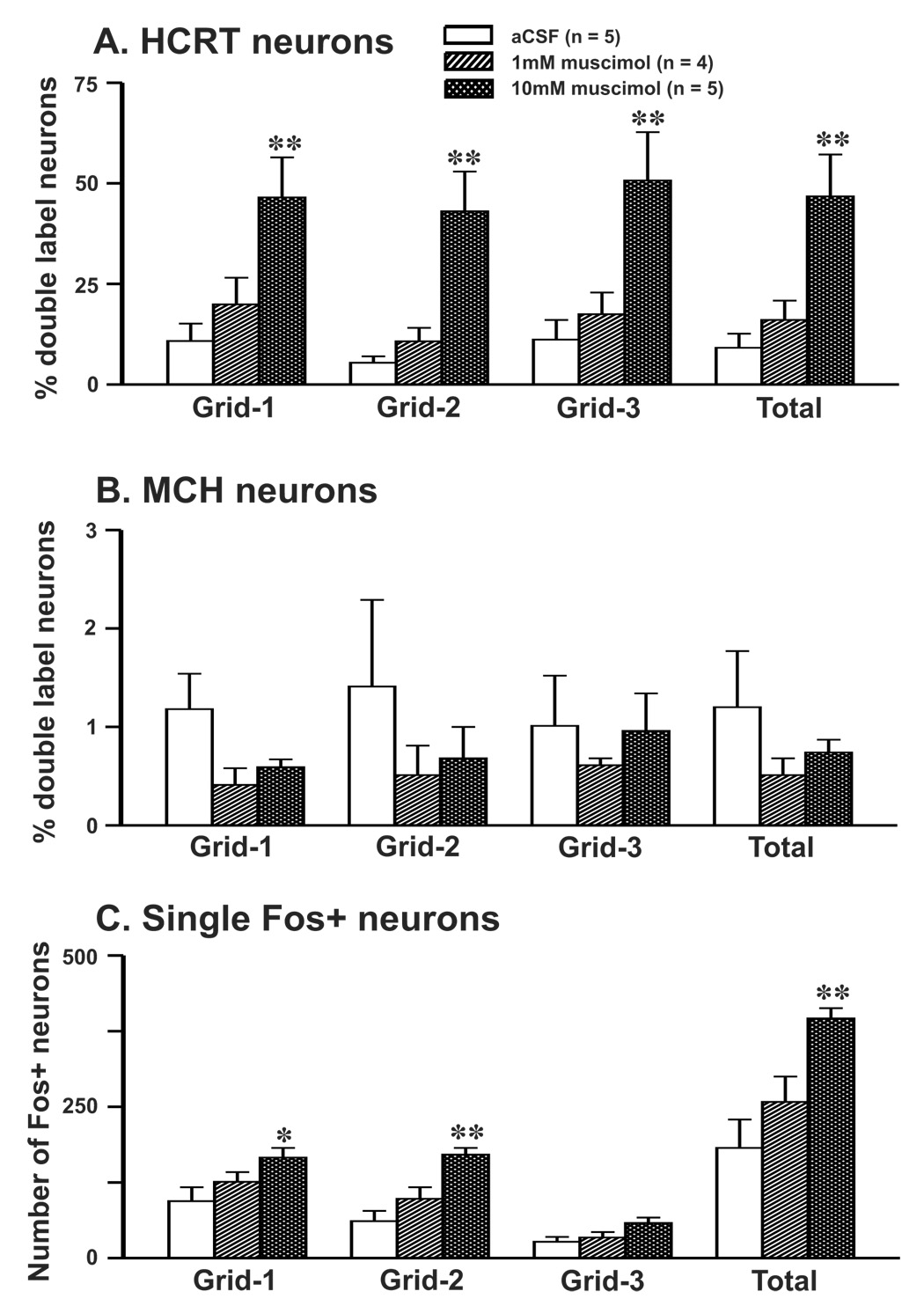
The effects of artificial cerebrospinal fluid (aCSF) and two doses of muscimol microinjections into the MnPN on HCRT+ neurons exhibiting Fos-IR in anesthetized rats are shown in figure 2&figure 3 and table-1. The number of HCRT+ neurons in the PF-LHA as a whole or its three sub-regions, i.e., perifornical area (grid-1), medial area (grid-2) and lateral area (grid-3) in both aCSF and muscimol treated rats were comparable (table-1, figure 1–figure 3). In rats injected with aCSF, small percentages of HCRT+ neurons expressing Fos-IR were observed in the PF-LHA as a whole (9 ± 3%) and each sub-region studied. However, as compared to aCSF treatment, the percentages of HCRT+/Fos+ neurons increased significantly in the PF-LHA as a whole (47 ± 10%) as well as each sub-region after 10 mM muscimol treatment (table-1, figure-3). The percentages of HCRT+/Fos+ neurons increased only marginally in rats injected with 1mM muscimol into the MnPN. The percentages of HCRT+/Fos+ neurons in various sub-regions of the HCRT field in muscimol treated rats were not significantly different. The percentages of HCRT+/Fos+ neurons in the HCRT field in 10mM muscimol treated rats were comparable to those found in rats that spent 74% of the time in spontaneous waking during lights-off period [47 ± 10 vs. 41 ± 03% (n = 5; unpublished data)].
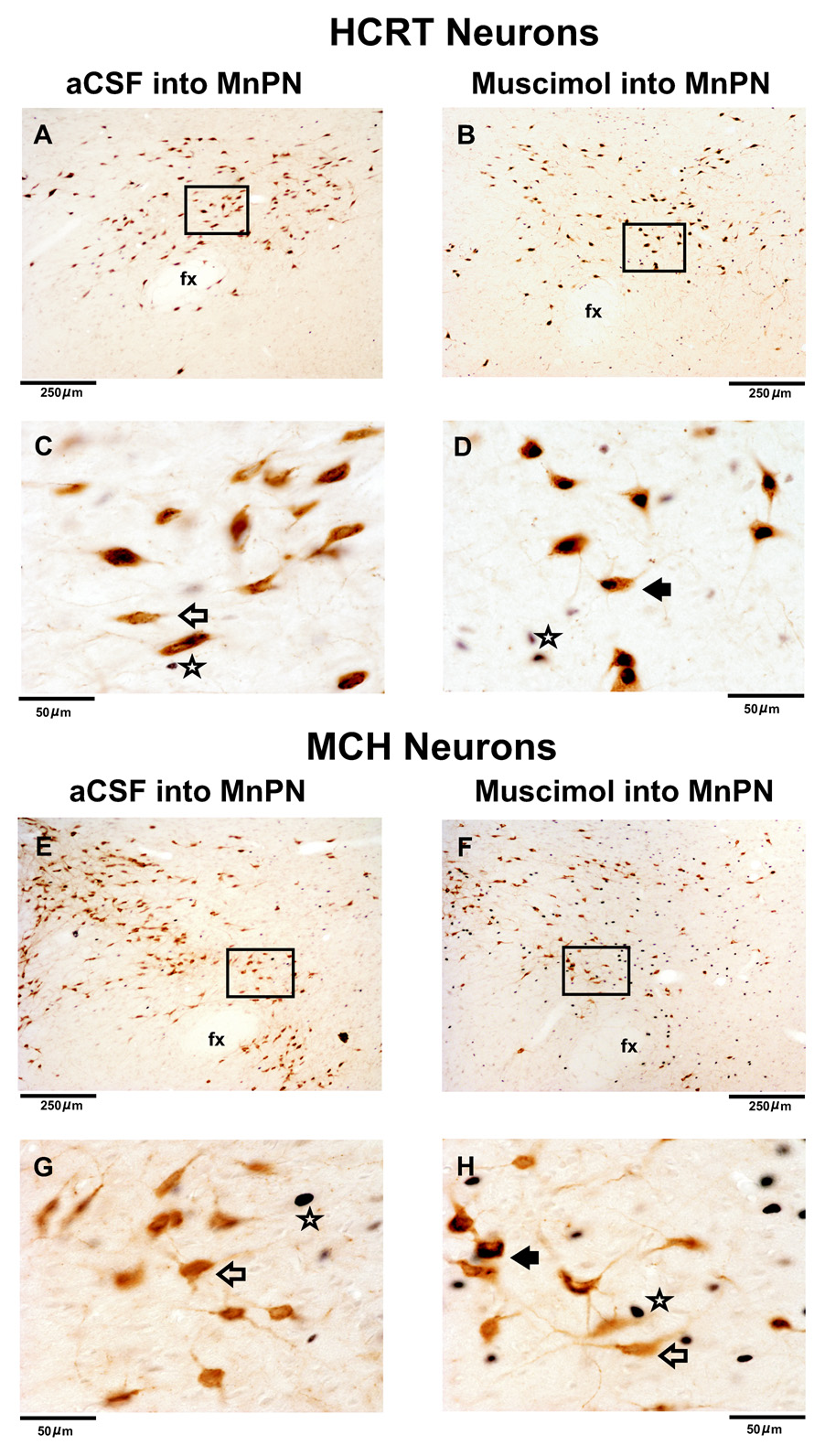
Figure 2. Effects of muscimol microinjection into the MnPN on Fos-IR in HCRT+ and MCH+ neurons.
Photomicrographs of coronal sections (100X magnification) from individual anesthetized rats microinjected with aCSF and 10 mM muscimol on HCRT neurons (A&B) and MCH neurons (E&F). The magnified images (600X) of the marked sections in figures A&B and E&F are shown in C&D and G&H, respectively. In aCSF treated animals, fewer Fos+ and HCRT+/Fos+ neurons were observed. In muscimol microinjected rats, although the numbers of HCRT+/Fos+ and single Fos+ neurons were higher, the numbers of MCH+/Fos+ neurons were similar to aCSF treated animals. Filled arrow, HCRT+/Fos+ or MCH+/Fos+ neuron; blank arrow, HCRT+/Fos- or MCH+/Fos- neuron; star, single Fos+ neuron.
Figure 3. Effects of muscimol microinjection into the MnPN on Fos-IR in PF-LHA neurons.
Mean (±SEM) percentages of HCRT+/Fos+ (A), MCH+/Fos+ neurons (B), and the number of single Fos+ neurons (C) in perifornical (grid-1), medial (grid-2) and lateral (grid-3) part of the HCRT and MCH neuronal fields after aCSF and 2 doses of muscimol microinjections into the MnPN. As compared to the aCSF control, the percentages of HCRT+/Fos+ and single Fos-IR neurons increased significantly after muscimol microinjection into the MnPN. As compared to the HCRT+ neurons, significantly lower percentages of MCH+ neurons expressed Fos-IR after aCSF or muscimol microinjections. *, as compared to the aCSF treatment; *, P < 0.05; **, P < 0.01 level of significance.
Table-1.
Effects of muscimol microinjection into the MnPN on Fos-IR in PF-LHA neurons
| Treatments | aCSF (n=5) | 1 mM muscimol (n=4) | 10 mM muscimol (n=5) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cell types /regions studied | Single | Double | Total | Single | Double | Total | Single | Double | Total | |
| HCRT neurons | PF | 137 ± 15 | 15 ± 05 | 152 ± 11 | 120 ± 19 | 27 ± 08 | 147 ± 15 | 72 ± 14 | 61 ± 12** | 134 ± 04 |
| Medial | 48 ± 05 | 03 ± 01 | 50 ± 05 | 53 ± 09 | 06 ± 01 | 58 ± 09 | 30 ± 06 | 23 ± 06** | 52 ± 05 | |
| Lateral | 53 ± 04 | 07 ± 03 | 60 ± 03 | 50 ± 07 | 10 ± 03 | 60 ± 05 | 30 ± 07 | 31 ± 07** | 61 ± 03 | |
| Total | 238 ± 17 | 24 ± 09 | 262 ± 09 | 222 ± 31 | 43 ± 12 | 265 ± 24 | 132 ± 26 | 115 ± 25** | 247 ± 04 | |
| MCH neurons | PF | 232 ± 26 | 02 ± 01 | 234 ± 26 | 267 ± 30 | 01 ± 01 | 268 ± 31 | 236 ± 15 | 01 ± 01 | 238 ± 15 |
| Medial | 55 ± 07 | 01 ± 01 | 55 ± 07 | 54 ± 09 | 01 ± 01 | 55 ± 09 | 49 ± 06 | 01 ± 01 | 49 ± 06 | |
| Lateral | 204 ± 29 | 02 ± 01 | 204 ± 28 | 213 ± 17 | 01 ± 01 | 214 ± 17 | 196 ± 14 | 02 ± 01 | 198 ± 14 | |
| Total | 489 ± 49 | 05 ± 02 | 494 ± 48 | 534 ± 50 | 03 ± 01 | 537 ± 50 | 482 ± 21 | 04 ± 01 | 486 ± 22 | |
| Single Fos+ | PF | 94 ± 23 | 94 ± 23 | 126 ± 16 | 126 ± 16 | 166 ± 16 | 166 ± 16* | |||
| Medial | 61 ± 17 | 61 ± 17 | 98 ± 19 | 98 ± 19 | 171 ± 11 | 171 ± 11** | ||||
| Lateral | 27 ± 08 | 27 ± 08 | 34 ± 09 | 34 ± 09 | 58 ± 09 | 58 ± 09 | ||||
| Total | 181 ± 47 | 181 ± 47 | 258 ± 42 | 258 ± 42 | 395 ± 17 | 395 ± 17** | ||||
| 5-HT neurons | DRN | 376 ± 19 | 08 ± 02 | 383 ± 21 | 382 ± 10 | 11 ± 02 | 393 ± 09 | 363 ± 10 | 17 ± 03* | 380 ± 13 |
| Single Fos+ | DRN | 96 ± 18 | 96 ± 18 | 123 ± 09 | 123 ± 09 | 139 ± 10 | 139 ± 10 | |||
as compared to aCSF; <0.05
<0.01 level of significance.
Counts (mean ± S.E.M.) and are based on average of three sections counted bilaterally per animal.
B. MCH neurons
The effects of aCSF and muscimol microinjections into the MnPN on Fos-IR in MCH+ neurons in anesthetized rats are shown in figure 2&figure 3 and table-1. The number of MCH+ neurons in the PF-LHA as a whole or in its three sub-regions in both aCSF and muscimol treated rats were comparable (figure 1–figure 3). As compared to HCRT+ neurons, very few MCH+ neurons expressed Fos-IR (about 1%) irrespective of the treatment conditions, even though the number of MCH+ neurons present in the PF-LHA was significantly higher (HCRT+, 238 ± 17 vs. MCH+, 489 ± 49, t = −4.814, P = 0.001). The percentages of MCH+/Fos+ neurons in the PF-LHA as a whole or in the three sub-regions after muscimol microinjections were not significantly different, although slightly reduced, from those found in aCSF treated rats (figure 2&figure 3).
C. Non-HCRT neurons
In anesthetized rats microinjected with aCSF into the MnPN, a moderate number of single Fos+ neurons were observed in the PF-LHA as a whole or in the three sub-regions (figure 2&figure 3, table-1). As compared to aCSF treatment, higher numbers of Fos+ neurons were observed in the PF-LHA as such or the respective three sub-regions after muscimol microinjections into the MnPN. Although, 1mM muscimol microinjection into the MnPN produced only marginal increase in the number of Fos+ neurons, significantly higher numbers of Fos+ neurons were observed in rats with 10mM muscimol injections (table-1, figure-3). The number of Fos+ neurons in the lateral part (area-3) was significantly lower as compared to the perifornical (grid-1) and medial part (grid-2) of the HCRT field (grid-1 vs. grid-3, t= 6.25, P= <0.01; grid-2 vs. grid-3, t = 6.53, P = <0.01; Holm-Sidak test) in muscimol treated rats. The number of single Fos+ neurons in the HCRT field in 10mM muscimol treated rats was comparable to that observed in rats that were sacrificed after spending 74% of the time in spontaneous waking during lightsoff period [396 ± 17 vs. 497 ± 54 (n=5; unpublished data)].
2. Effects of muscimol microinjection into the MnPN on Fos-IR in 5-HT neurons
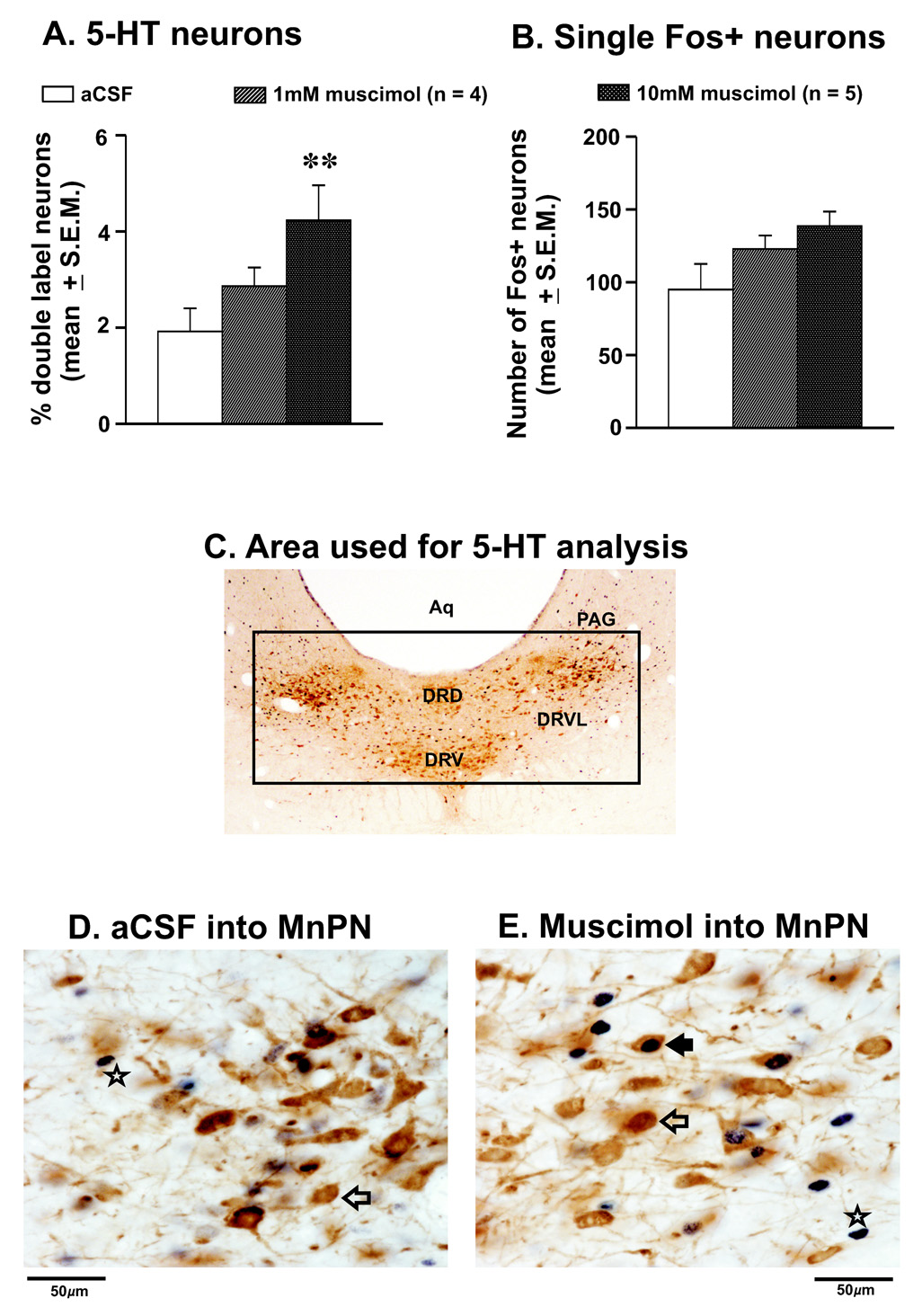
The effects of aCSF and muscimol microinjections into the MnPN on Fos-IR in DRN neurons in anesthetized rats are shown in figure-4 and table-1. The total number of 5-HT+ neurons in the DRN in both aCSF and muscimol treated rats were comparable (table-1). A small percentage of 5-HT+ neurons expressing Fos-IR (mean 1.9 ± 0.5%) were observed in the DRN in rats injected with aCSF. However, as compared to aCSF treatment, the percentage of 5-HT+/Fos+ neurons increased significantly in the DRN after microinjection of 10mM muscimol into the MnPN (4.4 ± 0.6%). The number of single Fos+ neurons in the DRN also increased after 10mM muscimol injection into the MnPN, although the changes were not significant (F = 3.62; p = 0.059). The microinjection of 1mM muscimol into the MnPN produced only marginal increases in 5-HT+/Fos+ and single Fos+ neurons.
Figure 4. Effects of muscimol microinjection into the MnPN on Fos-IR in 5-HT neurons.
Mean (±SEM) percentages of 5-HT+/Fos+ (A), and the number of single Fos+ neurons (B) in the DRN after aCSF and 2 doses of muscimol microinjections into the MnPN. As compared to aCSF, the percentages of 5-HT+/Fos+ increased significantly after muscimol microinjection into the MnPN. The number of single Fos+ neurons also increased but that was not to a significant level. C. Photomicrograph of a coronal section (40X magnification) through the DRN of a rat showing the anatomical distributions of the 5-HT neurons and the grid system that used for the analyses. Photomicrographs (600X magnification) of sections through DRN after aCSF and 10mM muscimol microinjection are shown in D and E, respectively. In aCSF treated animals, fewer Fos+ and 5HT+/Fos+ neurons were observed, whereas in muscimol microinjected rats the numbers of 5-HT+/Fos+ and single Fos+ neurons were higher. Filled arrow, 5-HT+/Fos+ neuron; blank arrow, 5-HT+/Fos- neuron; star, single Fos+ neuron; aq, aqueduct; DRD, dorsal raphe nucleus, dorsal part; DRV, dorsal raphe nucleus, ventral part; DRVL, dorsal raphe nucleus, ventrolateral part; PAG, periaqueductal gray.
DISCUSSION
This study demonstrates that local inactivation of MnPN neurons by a GABAA receptor agonist, muscimol, in anesthetized rats caused increased number of HCRT and other unidentified neurons exhibiting Fos-IR in the PF-LHA but produced no such changes in MCH neurons. Local MnPN inactivation also increased Fos-IR in 5-HT neurons in the DRN. Using c-Fos as a marker of neuronal activation, this is the first in vivo study providing quantitative evidence: a) that HCRT and 5-HT neurons are under MnPN inhibitory control; and b) that MnPN sleep-promoting system exerts differential influences on wake-promoting HCRT and sleep promoting MCH neurons in the PF-LHA. The findings suggest that the physiological outputs from MnPN to PF-LHA and DRN are predominantly inhibitory and are consistent with a hypothesis that MnPN promotes sleep by inhibiting multiple arousal systems including wake-promoting systems within PF-LHA and DRN.
The localized use of muscimol for reversible inactivation of specific brain regions/neuronal populations is well established [7,18,25,34,49]. Although the injection sites in this study were largely localized in the MnPN, it is possible that the injected muscimol diffused beyond MnPN and into adjacent regions including preoptic area, septum, and diagonal band. In this study the specific contributions of adjoining areas were not examined. Given the locations of the injection core, it is likely that the observed changes in Fos-IR in PF-LHA and DRN neurons were predominantly caused by the inactivation of MnPN neurons, although neurons in adjoining areas may have contributed to the observed effects as well. Furthermore, the findings that muscimol injected into MnPN produced differential effects on Fos-IR in wake-active HCRT and 5-HT neurons vs. REM sleep-active MCH neurons, a neuronal group that has overlapping distribution with HCRT neurons, suggest that the observed effects were physiological.
Evidence suggests that the sedative action of general anesthetics may involve some pathways that are activated and/or inhibited during sleep, although MnPN has not been implicated specifically [38]. Our findings that only small populations of HCRT and non-HCRT neurons in the PF-LHA or 5-HT neurons exhibited Fos-IR in aCSF treated anesthetized rats suggest that wake-active neurons exhibit minimal activation during anesthetic-induced sedation, similar to that observed during spontaneous sleep (see introduction). Furthermore, a much lower percentage of MCH+/Fos+ neurons (~ 1%) were observed in aCSF treated anesthetized rats. This is consistent with the earlier findings that a very small proportion of MCH neurons exhibit Fos-IR and that MCH neurons may not be affected by anesthetics[3,21].
Recently, we found that perfusion of muscimol into the MnPN in freely behaving animals produced arousal and activated many of the PF-LHA neurons[49]. In the present study EEG and EMG changes were not recorded, but rats injected with muscimol into the MnPN started showing signs of recovery from anesthesia or woke up 20–30 min earlier as compared to the controls. This accelerated recovery from anesthesia could be due to the activation of HCRT and other PF-LHA neurons as well as 5HT neurons in the DRN, as observed in this study. This finding is consistent with earlier in vivo studies showing: a) that HCRT decreased barbiturate-induced anesthesia time (loss of righting reflex) whereas SB-334867-A (HCRT-1 receptor antagonist) increased anesthesia time [28]; and b) that the genetic ablation of the HCRT neurons delayed emergence from anesthesia[21]. However, at the time of sacrifice animals in the present study remained partially sedated and exhibited only sporadic motor activity. On the other hand, as we report here, in muscimol treated rats about 47% of HCRT neurons expressed Fos-IR, which was comparable to animals sacrificed after spending nearly 75% time in spontaneous waking in the dark phase, a time of their maximal alertness. This evidence supports the hypothesis that the increased Fos-IR in the HCRT/PF-LHA and 5-HT neurons was predominantly caused by MnPN inactivation rather than behavioral changes during recovery from anesthesia. This explanation lends its support from an earlier study where unilateral perfusion of muscimol into the basal forebrain produced arousal and Fos-IR in 40% of the HCRT neurons ipsilateral to the perfusion site. However, only 10% of HCRT neurons on the contralateral side expressed Fos-IR in HCRT neurons which could be the reflection of the behavioral arousal [47].
The PF-LHA contains local GABAergic neurons as well as receives GABAergic inputs including those from MnPN, ventrolateral preoptic area and basal forebrain[1,11,13,58,61]. Much evidence supports a hypothesis that GABA inhibits the wake-promoting systems localized in the posterior hypothalamus including PF-LHA. For example, a) Local microinjection of muscimol into posterior hypothalamus produces sedation in cats and rats[31,38]; b) Injection of gabazine, a GABAA receptor antagonist, into the posterior hypothalamus attenuates the sedative effects of the GABAergic agents [38]; c) GABA release in posterior hypothalamus is higher during non-REM and REM sleep[39]; d) In an in vitro preparation, muscimol hyperpolarized whereas bicuculline blocked the muscimol-mediated hyperpolarization of the HCRT neurons[30], and e) Blockade of the local GABAergic transmission in the PF-LHA by a GABAA antagonist induced Fos-IR in PF-LHA neurons including HCRT neurons, and suppressed both non-REM and REM sleep[3]. The findings of this study that the numbers of HCRT and other PF-LHA neurons exhibiting Fos-IR increased after MnPN inactivation suggest that the physiological output from MnPN to PF-LHA arousal systems including HCRT neurons is predominantly inhibitory.
Evidence suggests that HCRT neurons also co-localize glutamate [55] and that the local glutamate release in the PF-LHA modulates the activation of HCRT neurons in vitro [30]. Therefore, it is likely that PF-LHA glutamatergic neurons are involved in the promotion/maintainenance of arousal both directly and/or indirectly via the activation of HCRT neurons. Although, the neurotransmitter phenotypes of non-HCRT neurons that exhibited Fos-IR in the PF-LHA as a result of MnPN inactivation could not be ascertained, it is likely that some of these neurons are wake-active glutamatergic neurons.
Unlike HCRT and other PF-LHA neurons, MnPN inactivation did not affect the number of MCH neurons expressing Fos-IR. This suggests that MCH neurons may not be under MnPN inhibitory control. However, it is pertinent to note that although MCH neurons exhibit REM sleep-associated Fos-IR, their percentage is very small [37,60] and possibly Fos-IR may not be a suitable parameter for the quantification of MCH neuronal activation. In our earlier extracellular neuronal recording study, MnPN stimulation, except for a subset of sleep-active neurons that were excited, inhibited most of the PF-LHA neurons[49]. Given that within PF-LHA, MCH neurons are intermingled with HCRT neurons and that their number is more than double to that of HCRT neurons, it is likely that in our earlier study at least some of the neurons that were inhibited by MnPN stimulation were MCH neurons and that at least some of the MCH neurons are wake- or wake-REM-active neurons.
Although in the DRN fewer 5-HT neurons expressed Fos-IR, their number was significantly higher after MnPN inactivation. Given the anatomical connectivity and reciprocal discharging patterns of the MnPN and 5-HT neurons, it is likely that increased Fos-IR in the 5-HT neurons after MnPN inactivation was partly caused by a direct withdrawal of MnPN inhibitory influences on 5-HT neurons. Furthermore, DRN receives afferents from PF-LHA including those from HCRT neurons and HCRT exerts excitatory influences on 5-HT neurons[41,52]. Therefore, possibly some of the Fos-IR in 5-HT neurons was the indirect consequences of HCRT neuronal activation and related behavioral changes.
In conclusion, the data presented here demonstrates that local inactivation of MnPN neurons by muscimol resulted in increased Fos-IR in PF-LHA neurons including HCRT neurons as well as 5-HT neurons in the DRN in anesthetized rats. Given the well documented role of PF-LHA/HCRT and 5-HT neurons in behavioral arousal vs. that of MnPN neurons in sleep, and their anatomical connectivity, these results suggest that the physiological outputs from MnPN to PF-LHA and DRN is predominantly inhibitory. These findings are consistent with a hypothesis that the activation of MnPN neurons promotes spontaneous sleep by inhibiting multiple arousal systems including wake-promoting HCRT and 5-HT systems.
EXPERIMENTAL PROCEDURE
All the experiments were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and were approved by Veteran Administration Greater Los Angeles Healthcare System’s Institutional Animal Research Committee. The rats were housed individually and maintained on 12:12 h light-dark cycle (lights on at 7:00 h) with food and water available ad libitum.
1. Experimental protocol
Experiments were conducted on a total of 19 anesthetized Sprague-Dawley male rats, weighing between 300–350 grams, during lights-on period (between 9.00 – 12.00 hr). Evidence suggests that the activity of majority of PF-LHA neurons as well as 5-HT neurons is higher during behavioral arousal, particularly during active movement, and is minimal during sleep[2,9,10,29,33,36]. Therefore, these experiments were conducted in anesthetized rats to achieve a stable predominantly synchronized state and minimal Fos-IR in the HCRT and 5-HT neurons.
Immediately following anesthetization (Ketamine + Xylazine: 80:10 mg/kg; i.p.) rats were secured in a Kopf stereotaxic frame. An injector cannula (30-G) of a glass Hamilton syringe (Hamilton, Reno, NV, US) mounted on a KD Scientific microdrive was positioned on to the bregma and its stereotaxic coordinates were noted. Then, a 2mm × 2mm window centered at bregma was made in the skull and the injector cannula was inserted into the MnPN according the following coordinates, AP, 0.0 to −0.3; L, 0.00, H, −6.5 to −7.0, from bregma [51]. Afterwards, a total volume of 0.2 µl of artificial cerebrospinal fluid (aCSF; composition in mM, 145 NaCl, 2.7 KCl, 1.3 MgSO4, 1.2 CaCl2, and 2 Na2HPO4; pH, 7.2) or aCSF containing either of the two doses of muscimol (1 mM, amount = 0.2nmoles, n=4; 10 mM, amount = 2nmoles, n=5) were pressure injected into the MnPN. aCSF or muscimol was microinjected in 10 min, i.e., very slowly to minimize the spread of the aCSF/muscimol into the neighboring sites. After injection, the cannula was kept in place for another 5 min to further allow the diffusion of the drugs into the tissue. The rats were then taken out of the stereotaxic frame, monitored for another 90 min in the home cage and sacrificed.
2. Immunohistochemistry
After 90 min of microinjection, rats were given a lethal dose of pentabarbital (100 mg/Kg) and heparin (500U, i.p.), and perfused transcardially with 30–50 ml of 0.1 M phosphate buffer (pH 7.2) followed by 300 ml of 4% paraformaldehyde in phosphate buffer containing 15% saturated picric acid solution. The brains were removed and equilibrated in 10%, 20% and finally 30% sucrose untill they sank. Coronal sections through MnPN, PF-LHA, and DRN were freeze-cut at 30 µm thickness and immunostained using previously described methods[11,26,59]. Sections through the microinjection needle tract encompassing MnPN were immunostained for glutamic acid decarboxylase (GAD). Alternate sections from the series of sections through the PF-LHA were immunostained for c-Fos and HCRT or c- Fos and MCH and every fourth section through DRN was immunostained for c-Fos and 5-HT.
a. c-Fos immunostaining
Sections were incubated in rabbit anti-Fos antibody (1: 20,000 Oncogene, California, USA) in diluent solution containing 4% goat serum and 0.2% triton in TBS for 40–48 hours at 4 °C. Sections were then incubated in biotinylated goat anti-rabbit IgG (1:1000 Vector Laboratories, California, USA) for 2 hours and incubated with avidin-biotin complex (ABC, 1:500, Vector Laboratories) for 2 hours. The sections were developed with nickel-3,3’-diaminobenzidine tetrahydrochloride (DAB, Sigma, USA) for visualization that produced black reaction product confined to the nuclei. After staining for Fos-IR, alternate sections were processed for HCRT-1 or MCH immunostaining.
b. HCRT-1 immunostaining
Sections were washed in TBS and then incubated in rabbit anti-HCRT-1 (orexin-A) antibody (1:1000, Oncogene) for 40–48 hours at 4 °C. The sections were then incubated in biotinylated goat anti-rabbit IgG (1:500 Vector Laboratories) for 2 hours, followed by incubation in ABC (1:250, Vector Laboratories) for 2 hours and then developed with DAB producing a brown reaction product for visualization.
c. MCH immunostaining
After washing in TBS, sections were incubated in rabbit anti-MCH antibody (1:20,000, Phoenix Pharmaceuticals, California) for 40–48 hours at 4 °C. The sections were then incubated in biotinylated goat anti-rabbit IgG (1:1000 Vector Laboratories) for 2 hours, reacted with ABC (1:500, Vector Laboratories) for 2 hours followed by DAB visualization.
d. GAD immunostaining
Sections through the microinjection cannula tracts were processed for GAD immunostaining. Sections were incubated in mouse anti-GAD67 monoclonal antibody (1:500, Chemicon, Temecula, California) for 40 – 48 hours at 4 °C. The sections were then incubated in biotinylated antimouse IgG (1:400, Vector Laboratories) for 2 hours, reacted with ABC (1:200, Vector Laboratories) for 2 hours followed by DAB visualization.
e. 5-HT immunostaining
After washing in TBS, sections were incubated in rabbit anti-5HT antibody (1:70,000, Immunostar, California) for 40–48 hours at 4 °C. The sections were then incubated in biotinylated goat anti-rabbit IgG (1:1000 Vector Laboratories) for 2 hours, reacted with ABC (1:500, Vector Laboratories) for 2 hours followed by DAB visualization.
Two sections from each brain areas were treated as above except for the omission of the Fos, HCRT-1, MCH, GAD, or 5-HT primary antibody to control for nonspecific staining.
3. Cell counting and analyses
A single person blind to the treatment conditions performed the counting and plotting of the immunoreactive neurons using the Neurolucida computer-aided plotting system (MicroBrightField). Three representative sections, ~120 µm apart and encompassing the regions of maximum numbers of HCRT+ or MCH+ neurons in the PF-LHA and 5-HT neurons in the DRN per animal were selected for counting. The identification and counting of different neuronal types, i.e., single Fos+, HCRT+, MCH+, or 5-HT+ neurons and double-labeled HCRT+/Fos+, MCH+/Fos+, or 5-HT+/Fos+ neurons were done manually under 400X magnification. Fos-IR was recognized by black stain localized to the nucleus, whereas neurons with brown-stained soma and dendrites were recognized as HCRT+, MCH+, or 5-HT+ neurons. Neurons having black nucleus and brown cytoplasm were identified as HCRT+/Fos+, MCH+/Fos+, or 5-HT+/Fos+ neurons in particular slides.
Evidence suggests that a lower percentage of HCRT neurons exhibit wake-associated Fos-IR along the lateral part as compared to the medial and perifornical region of the HCRT field[10]. Therefore, the area of interest was divided into three sub-regions, perifornical area (700µm wide and 1000µm long, grid-1), medial area (500µm wide and 1000µm long, grid-2) and lateral area (500µm wide and 1000µm long, grid-3) of the HCRT+ or MCH+ neuronal fields (figure-1). A 800µm wide and 1600µm long grid encompassing 5-HT neuronal population in dorsal raphe-dorsal, -ventral, -ventrolateral and part of ventrolateral periaqueductal gray, was used for quantifying the effects of muscimol microinjection into the MnPN on Fos-IR in 5-HT neurons. The MnPN is located in the midline. Small changes in the laterality of the microinjections could affect the laterality changes in Fos-IR. Therefore, to avoid hemispheric bias, cells were counted on both sides of the PF-LHA and counts from both sides were used for analyses.
4. Statistical analyses
The numbers of single-labeled Fos+, HCRT+, MCH+, and 5-HT+ and double-labeled HCRT+/Fos+, MCH+/Fos+, and 5-HT+/Fos+ neurons after muscimol microinjection were compared to those obtained after aCSF microinjection as well as the cell counts amongst three PF-LHA sub-regions were compared using one-way ANOVA followed by Bonferroni t-test for pair-wise multiple comparisons. In cases where equal variance test failed Kruskal-Wallis one-way ANOVA on ranks followed by Dunn’s method were used to determine the levels of significance amongst various treatment groups.
ACKNOWLEDGEMENTS
We thank Chris Angara for his excellent technical support. This work was supported by the US Department of Veteran Affairs Medical Research Service and US National Institutes of Health grants, NS-050939 (Alam); MH47480, HL60296 (McGinty); and MH63323 (Szymusiak).
ABBREVIATIONS
- aCSF
Artificial cerebrospinal fluid
- DRN
Dorsal raphe nucleus
- Fos-IR
c-fos protein immunoreactivity
- GABA
Gamma-aminobutyric acid
- GAD
Glutamic acid decarboxylase, a marker for GABAergic neurons
- HCRT
Hypocretin
- MCH
Melanin-concentrating hormone
- MnPN
Median preoptic nucleus
- PF-LHA
Perifornical-lateral hypothalamic area
- POA
Preoptic area
- TBS
Tris buffer saline
- VLPO
Ventrolateral preoptic area
- 5-HT
serotonin (5-hydroxytryptamine)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE REFERENCES
- 1.Abrahamson EE, Moore RY. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res. 2001;889:1–22. doi: 10.1016/s0006-8993(00)03015-8. [DOI] [PubMed] [Google Scholar]
- 2.Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–631. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol. 2005;563:569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auerbach SB, Minzenberg MJ, Wilkinson LO. Extracellular serotonin and 5-hydroxyindoleacetic acid in hypothalamus of the unanesthetized rat measured by in vivo dialysis coupled to high-performance liquid chromatography with electrochemical detection: dialysate serotonin reflects neuronal release. Brain Res. 1989;499:281–290. doi: 10.1016/0006-8993(89)90776-2. [DOI] [PubMed] [Google Scholar]
- 5.Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datta S, Maclean RR. Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: Reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci Biobehav Rev. 2007;31:775–824. doi: 10.1016/j.neubiorev.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lima MN, Luft T, Roesler R, Schroder N. Temporary inactivation reveals an essential role of the dorsal hippocampus in consolidation of object recognition memory. Neurosci Lett. 2006;405:142–146. doi: 10.1016/j.neulet.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 8.Espana RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 9.Espana RA, Valentino RJ, Berridge CW. Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience. 2003;121:201–217. doi: 10.1016/s0306-4522(03)00334-8. [DOI] [PubMed] [Google Scholar]
- 10.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong H, Szymusiak R, King J, Steininger T, McGinty D. Sleep-related c-Fos protein expression in the preoptic hypothalamus: effects of ambient warming. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2079–R2088. doi: 10.1152/ajpregu.2000.279.6.R2079. [DOI] [PubMed] [Google Scholar]
- 13.Gritti I, Mariotti M, Mancia M. GABAergic and cholinergic basal forebrain and preoptic-anterior hypothalamic projections to the mediodorsal nucleus of the thalamus in the cat. Neuroscience. 1998;85:149–178. doi: 10.1016/s0306-4522(97)00573-3. [DOI] [PubMed] [Google Scholar]
- 14.Guzman-Marin R, Alam MN, Szymusiak R, Drucker-Colin R, Gong H, McGinty D. Discharge modulation of rat dorsal raphe neurons during sleep and waking: effects of preoptic/basal forebrain warming. Brain Res. 200;875:23–34. doi: 10.1016/s0006-8993(00)02561-0. [DOI] [PubMed] [Google Scholar]
- 15.Gvilia I, Turner A, McGinty D, Szymusiak R. Preoptic area neurons and the homeostatic regulation of rapid eye movement sleep. J Neurosci. 2006;26:3037–3044. doi: 10.1523/JNEUROSCI.4827-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gvilia I, Xu F, McGinty D, Szymusiak R. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci. 2006;26:9426–9433. doi: 10.1523/JNEUROSCI.2012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardiman MJ, Ramnani N, Yeo CH. Reversible inactivations of the cerebellum with muscimol prevent the acquisition and extinction of conditioned nictitating membrane responses in the rabbit. Exp Brain Res. 1996;110:235–247. doi: 10.1007/BF00228555. [DOI] [PubMed] [Google Scholar]
- 19.Huang ZL, Qu WM, Li WD, Mochizuki T, Eguchi N, Watanabe T, Urade Y, Hayaishi O. Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci U S A. 2001;98:9965–9970. doi: 10.1073/pnas.181330998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Kelz MB, Sun Y, Chen J, Cheng Meng Q, Moore JT, Veasey SC, Dixon S, Thornton M, Funato H, Yanagisawa M. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A. 2008;105:1309–1314. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- 23.Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koyama Y, Takahashi K, Kodama T, Kayama Y. State-dependent activity of neurons in the perifornical hypothalamic area during sleep and waking. Neuroscience. 2003;119:1209–1219. doi: 10.1016/s0306-4522(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 25.Krupa DJ, Thompson RF. Reversible inactivation of the cerebellar interpositus nucleus completely prevents acquisition of the classically conditioned eye-blink response. Learn Mem. 1997;3:545–556. doi: 10.1101/lm.3.6.545. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Szymusiak R, Bashir T, Rai S, McGinty D, Alam MN. Effects of serotonin on perifornical-lateral hypothalamic area neurons in rat. Eur J Neurosci. 2007;25:201–212. doi: 10.1111/j.1460-9568.2006.05268.x. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Szymusiak R, Methippara MM, Rai S, Suntsova N, McGinty D, Alam MN. GABAergic and glutamatergic neurons in the perifornical lateral hypothalamic area exhibit differential Fos expression after sleep deprivation vs. recovery sleep. Sleep. 2005;29:A146. [Google Scholar]
- 28.Kushikata T, Hirota K, Yoshida H, Kudo M, Lambert DG, Smart D, Jerman JC, Matsuki A. Orexinergic neurons and barbiturate anesthesia. Neuroscience. 2003;121:855–863. doi: 10.1016/s0306-4522(03)00554-2. [DOI] [PubMed] [Google Scholar]
- 29.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 31.Lin JS, Sakai K, Vanni-Mercier G, Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479:225–240. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- 32.McGinty D, Szymusiak R. Hypothalamic regulation of sleep and arousal. Front Biosci. 2003;8:s1074–s1083. doi: 10.2741/1159. [DOI] [PubMed] [Google Scholar]
- 33.McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 34.Meloni EG, Jackson A, Gerety LP, Cohen BM, Carlezon WA., Jr Role of the bed nucleus of the stria terminalis (BST) in the expression of conditioned fear. Ann N Y Acad Sci. 2006;1071:538–541. doi: 10.1196/annals.1364.059. [DOI] [PubMed] [Google Scholar]
- 35.Methippara MM, Alam MN, Szymusiak R, McGinty D. Effects of lateral preoptic area application of orexin-A on sleep-wakefulness. Neuroreport. 2000;11:3423–3426. doi: 10.1097/00001756-200011090-00004. [DOI] [PubMed] [Google Scholar]
- 36.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Modirrousta M, Mainville L, Jones BE. Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur J Neurosci. 2005;21:2807–2816. doi: 10.1111/j.1460-9568.2005.04104.x. [DOI] [PubMed] [Google Scholar]
- 38.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 39.Nitz D, Siegel JM. GABA release in posterior hypothalamus across sleep-wake cycle. Am J Physiol. 1996;271:R1707–R1712. doi: 10.1152/ajpregu.1996.271.6.R1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 41.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Portas CM, McCarley RW. Behavioral state-related changes of extracellular serotonin concentration in the dorsal raphe nucleus: a microdialysis study in the freely moving cat. Brain Res. 1994;648:306–312. doi: 10.1016/0006-8993(94)91132-0. [DOI] [PubMed] [Google Scholar]
- 43.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 44.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Salin-Pascual R, Gerashchenko D, Greco M, Blanco-Centurion C, Shiromani PJ. Hypothalamic regulation of sleep. Neuropsychopharmacology. 2001;25:S21–S27. doi: 10.1016/S0893-133X(01)00318-9. [DOI] [PubMed] [Google Scholar]
- 46.Saper CB, Cano G, Scammell TE. Homeostatic, circadian, and emotional regulation of sleep. J Comp Neurol. 2005;493:92–98. doi: 10.1002/cne.20770. [DOI] [PubMed] [Google Scholar]
- 47.Satoh S, Matsumura H, Nakajima T, Nakahama K, Kanbayashi T, Nishino S, Yoneda H, Shigeyoshi Y. Inhibition of rostral basal forebrain neurons promotes wakefulness and induces FOS in orexin neurons. Eur J Neurosci. 2003;17:1635–1645. doi: 10.1046/j.1460-9568.2003.02577.x. [DOI] [PubMed] [Google Scholar]
- 48.Siegel JM. Hypocretin (orexin): role in normal behavior and neuropathology. Ann Rev Psychol. 2004;55:125–148. doi: 10.1146/annurev.psych.55.090902.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suntsova N, Guzman-Marin R, Kumar S, Alam MN, Szymusiak R, McGinty D. The median preoptic nucleus reciprocally modulates activity of arousal-related and sleep-related neurons in the perifornical lateral hypothalamus. J Neurosci. 2007;27:1616–1630. doi: 10.1523/JNEUROSCI.3498-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol. 2002;543:665–677. doi: 10.1113/jphysiol.2002.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swanson LW. BRAIN MAPS: Structure of the Rat Brain. Second edn. New York: Elsevier; 1998. [Google Scholar]
- 52.Takahashi K, Wang QP, Guan JL, Kayama Y, Shioda S, Koyama Y. State-dependent effects of orexins on the serotonergic dorsal raphe neurons in the rat. Regul Pept. 2005;126:43–47. doi: 10.1016/j.regpep.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Thakkar MM, Ramesh V, Strecker RE, McCarley RW. Microdialysis perfusion of orexin-A in the basal forebrain increases wakefulness in freely behaving rats. Arch Ital Biol. 2001;139:313–328. [PubMed] [Google Scholar]
- 54.Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torrealba F, Yanagisawa M, Saper CB. Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience. 2003;119:1033–1044. doi: 10.1016/s0306-4522(03)00238-0. [DOI] [PubMed] [Google Scholar]
- 56.Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: lack of diurnal variation. Neurosci Lett. 1983;36:285–290. doi: 10.1016/0304-3940(83)90014-9. [DOI] [PubMed] [Google Scholar]
- 57.Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6:55–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- 58.Uschakov A, Gong H, McGinty D, Szymusiak R. Sleep-active neurons in the preoptic area project to the hypothalamic paraventricular nucleus and perifornical lateral hypothalamus. Eur J Neurosci. 2006;23:3284–3296. doi: 10.1111/j.1460-9568.2006.04860.x. [DOI] [PubMed] [Google Scholar]
- 59.Uschakov A, Gong H, McGinty D, Szymusiak R. Efferent projections from the median preoptic nucleus to sleep- and arousal-regulatory nuclei in the rat brain. Neuroscience. 2007;150:104–120. doi: 10.1016/j.neuroscience.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verret L, Goutagny R, Fort P, Cagnon L, Salvert D, Leger L, Boissard R, Salin P, Peyron C, Luppi PH. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]