Abstract
Background
Case reports have suggested that cardiomyopathy may be a complication of recessive dystrophic epidermolysis bullosa (RDEB).
Objective
To determine the risk of congestive heart failure (CHF) or cardiomyopathy in each major EB subtype.
Methods
These data represent systematic case findings and data collection performed throughout the continental United States from 1986 through 2002, by the National Epidermolysis Bullosa Registry. Study design is cross-sectional (n = 3280) with a nested randomly sampled longitudinal subcohort (n = 450). Frequencies of CHF and cardiomyopathy were determined by patient self-reporting, medical histories and review of medical records. In those who died, death certificates were reviewed and histories obtained from surviving family. Cumulative risks were stratified by cause and EB subtype.
Results
Cardiomyopathy was reported as early as within the first year of life. In patients having no other known risk factors for CHF or cardiomyopathy, the highest risk of cardiomyopathy was seen among patients with Hallopeau–Siemens RDEB (RDEB-HS), with a cumulative risk of 4·51% on or after age 20 years. The cumulative risk of cardiomyopathy was only 1·14% and 0·40% in non-Herlitz junctional EB (JEB) and non-Hallopeau–Siemens RDEB, respectively, and was not observed in any other EB subtype. When patients with coexistent chronic renal failure were included, the cumulative risk for RDEB-HS rose to 18·86% by age 35 years. About 30% of our patients affected with RDEB-HS died of CHF or cardiomyopathy, even those with no other known risk factors.
Conclusions
CHF and cardiomyopathy are uncommon complications in both major RDEB subtypes and non-Herlitz JEB, and may be fatal.
Keywords: cardiomyopathy, congestive heart failure, epidermolysis bullosa
Inherited epidermolysis bullosa (EB) encompasses four major groups of skin diseases [epidermolysis bullosa simplex (EBS), junctional epidermolysis bullosa (JEB), dominant dystrophic epidermolysis bullosa (DDEB) and recessive dystrophic epidermolysis bullosa (RDEB)], each characterized by marked mechanical fragility of epithelial tissues and the formation of blisters, erosions and poorly healing ulcers on the skin.1–4 Approximately 25 clinically distinctive phenotypes5 and hundreds of genotypes have now been described, encompassing mutations in at least 10 structural genes.4,6–8 More clinical variations and novel mutations will undoubtedly be reported in the future, given the remarkably protean nature of this group of diseases.
In 1996 two children with RDEB with dilated cardiomyopathy were reported, one of whom had a low selenium level.9 Echocardiographic examination of an additional 18 children failed to reveal additional cases, raising a question about the true frequency with which cardiomyopathy might occur in the setting of RDEB. Since that time, a few other children with RDEB have also been shown to have dilated cardiomyopathy.10
The present study was undertaken to ascertain the frequency of cardiomyopathy among the U.S. EB population, given the availability of the robust numbers of clinically well-characterized patients enrolled in the National EB Registry. On the basis of rigorous analysis of this registry's database, we present data on the cumulative risks for the development of congestive heart failure (CHF) or cardiomyopathy for each of the major subtypes of this disease.
Patients and methods
Data were obtained from 3280 consecutively enrolled patients with EB who were seen on behalf of the National EB Registry from September 1986 to April 2002, when federal funding for this National Institutes of Health (NIH)-supported project formally ended. During these 16 years, work was carried out by a number of co-investigators at several institutions (see below for list of collaborating institutions). The authorship of the present publication represents the research team at the project's Data Coordinating Center who took ultimate responsibility for data validation and the performance and interpretation of all biostatistical analyses, in addition to playing a pivotal role in data collection from the majority of the project's patients, their families and referring physicians.
Details of our data instrument and the methodologies used in case finding have been reported previously.11–13 Of note, the questionnaire included ‘congestive heart failure/cardiomyopathy’ as a response.
During the last 5 years of funding, all the work was confined to the University of North Carolina at Chapel Hill and Stanford University, and was conducted under the auspices and approval of the NIH-supported General Clinical Research Centers and the Institutional Review Boards at each of these two universities.
Whenever possible, each person enrolled was seen and physically examined at least once by one of the project's principal investigators. The diagnosis of EB was confirmed in every case by immunofluorescence antigenic mapping, EB-specific monoclonal antibody studies and transmission electron microscopy.5 Each patient was further subclassified to EB subtype using a widely used classification scheme that was reported in 199114 and updated in 2000 by an international panel of EB experts.5
In order to allow more rigorous analyses, we separated our patient population into several mutually exclusive EB subtypes, following the detailed criteria of the most recent classification scheme:5 EBS, Weber–Cockayne (EBS-WC); EBS herpetiformis, Dowling–Meara (EBS-DM); EBS, Koebner (EBS-K); EBS, all others (EBS-O); JEB, Herlitz (JEB-H); JEB, non-Herlitz subtypes (JEB-O); DDEB; generalized RDEB, Hallopeau–Siemens (RDEB-HS); and generalized RDEB, non-Hallopeau–Siemens (RDEB-nHS). Only 17 patients with RDEB inversa were enrolled in the project, and as none of them experienced CHF or cardiomyopathy, they were not included within the present set of analyses. Sufficient data existed on 2750 enrollees (83·8%) to permit subclassification into these major EB subtypes. This served as the final subpopulation from which cardiac data were extracted.
Approximately 450 well-classified subjects were further randomly selected for longitudinal follow-up on an every 2-year basis during the last 10 years of this project.11 This nested subpopulation was chosen so as to maximally sample those EB subtypes most at risk for significant extracutaneous complications, the latter based on previous publications.
All patients who reported having had CHF or cardiomyopathy were interviewed for further details of their signs and symptoms, as well as for the source of each diagnosis. Families of deceased patients were identically interviewed. The diagnosis of CHF was made by the presence of classic clinical findings and cardiomegaly on chest X-ray. For purposes of our analyses, the diagnosis of cardiomyopathy was reserved for those patients being given that diagnosis by their physicians. In those who had died, death certificates or autopsy reports served as another confirmation of the diagnosis. None of our patients underwent cardiac biopsies, however, to permit definitive tissue confirmation of cardiomyopathy.
Among the entire National EB Registry study population, 21 patients were reported to have had CHF or cardiomyopathy. Of these, six were eliminated from initial analysis following careful review of their medical records or interviews of the patients or their families. Reasons included lack of evidence of any cardiac disease including CHF (n = 1); presence of congenital heart disease (n = 1) or rheumatic heart disease (n = 1); onset at age 65 years of CHF as a consequence of atherosclerotic heart disease (n = 1); or lack of data on the time of onset of either CHF or cardiomyopathy (n = 2).
Three groups of patients were then separated for analysis: group 1 (n = 15), all patients reported to have CHF or CM, including five patients who had chronic renal failure [due to glomerulonephritis (n = 1), renal amyloidosis (n = 1), unknown aetiology (n = 3)]; group 2 (n = 10), excluding those having concurrent chronic renal failure; group 3 (n = 7), comprising only those patients who specifically carried the diagnosis of cardiomyopathy from a physician and lacked any other discernible potential confounding causes of CHF (including renal failure and other types of heart disease, acquired or congenital). In each of these patients, signs and symptoms of CHF, including cardiomegaly, were also present. This third group was believed to represent the most accurate estimate of possible cardiomyopathy among our entire EB study population.
Data were entered into specially designed templates originally based on Clinfo and later on EpiInfo software (Centers for Disease Control, Atlanta, GA, U.S.A.). SAS datasets (SAS Institute, Cary, NC, U.S.A.) were subsequently generated to facilitate the performance of lifetable analyses. All biostatistical analyses were conducted under the direct supervision of the project's designated biostatistician (C.S.). Computer-generated graphs were created using Excel.
Results
Frequencies of congestive heart failure or cardiomyopathy
CHF and cardiomyopathy were reported in only three EB subtypes – JEB-O, RDEB-HS and RDEB-nHS – among all of the participants in the National EB Registry. Table 1 summarizes their frequency of occurrence. The most common EB subtype affected was RDEB-HS, with about 7% reporting one of these complications. When cases were confined to those patients in whom the diagnosis of cardiomyopathy was specified in writing by a physician, the frequency dropped to about 3·6%. Only about 1% of patients with JEB-O or RDEB-nHS were noted to have had either CHF or cardiomyopathy, with frequencies dropping to about 0·5% and 0·4%, respectively, when only cardiomyopathy was considered.
Table 1.
Frequencies of congestive heart failure (CHF) and cardiomyopathy (CM) among National Epidermal Bullosa Registry participants
| Frequency (%) | |||
|---|---|---|---|
| EB subtype | CHF or CM (all reports) | CHF or CM, excluding CRF | CM (specified by physician) |
| JEB-O | 2/194 (1·04) | 2/194 (1·04) | 1/194 (0·52) |
| RDEB-HS | 10/140 (7·14) | 6/140 (4·29) | 5/140 (3·57) |
| RDEB-nHS | 3/267 (1·12) | 2/267 (0·75) | 1/267 (0·37) |
EB, epidermal bullosa; CRF, chronic renal failure; JEB-O, junctional EB, non-Herlitz subtypes; RDEB-HS, recessive dystrophic EB, Hallopeau–Siemens; RDEB-nHS, RDEB, non-Hallopeau–Siemens.
Cumulative and conditional risks of congestive heart failure or cardiomyopathy
Group 1 included all patients who were reported to have CHF or cardiomyopathy, and on whom age of onset was known, regardless of whether concurrent chronic renal failure was present. The highest risk was noted in those with RDEB-HS, with a cumulative risk of 0·72% by age 2, rising to 1·45%, 2·28%, 4·28%, 6·73%, 8·72%, and 18·86% by ages 3, 9, 15, 20, 25 and 35 (through age 65), respectively. The highest conditional risk, 11·11%, occurred between ages 30 and 35. Among those with RDEB-nHS, the cumulative risks were only 0·4%, 1·35%, and 2·75% at ages 1, 20 and 30 (through age 80), and in JEB-O, they were 1·14%, 1·14% and 4·94% by ages 3, 35 and 40 (through age 70), respectively.
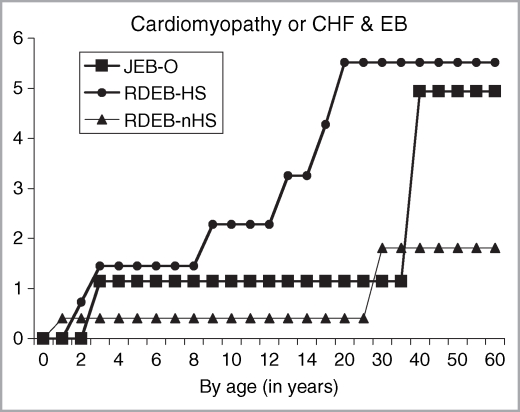
Figure 1 depicts the cumulative risk of CHF or cardiomyopathy within group 2 of our patient population, which excluded any patient who had a history of concurrent chronic renal failure. This complication was first reported by ages 3, 2 and 1 year of life in patients with JEB-O, RDEB-HS and RDEB-nHS, respectively. Among patients with JEB-O, the cumulative risk was 1·14% by age 3, rising to 4·94% on or after age 40 years, with the highest conditional risk, 3·85%, occurring between ages 35 and 40. In patients with RDEB-HS, the cumulative risks were 0·72%, 1·45%, 2·28% and 5·52% by ages 2, 3, 9 and 20 years. The highest conditional risk, 1·29%, occurred between ages 15 and 20. In our RDEB-nHS subpopulation, the cumulative risks were 0·40% and 1·81% by ages 1 and 30, respectively.
Fig 1.
Age-dependent cumulative risk of congestive heart failure (CHF) or cardiomyopathy in inherited epidermolysis bullosa (EB) expressed as percentage and stratified by EB subtype. JEB-O, junctional EB, non-Herlitz subtypes; RDEB-HS, recessive dystrophic EB, Hallopeau–Siemens; RDEB-nHS, RDEB, non-Hallopeau–Siemens.
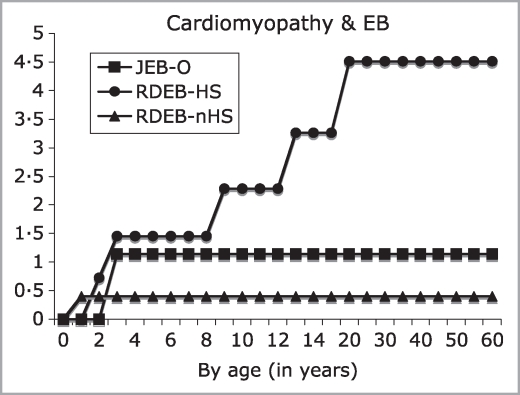
Figure 2 depicts the cumulative risk of cardiomyopathy among those patients lacking other discernible causes of CHF (group 3). Among patients with RDEB-HS, cumulative risks were 0·72%, 1·45%, 2·28%, 3·26%, and 4·51% by ages 2, 3, 9, 13 and 20 years, respectively, with the highest conditional risk, 1·29%, arising between ages 15 and 20 years. In contrast, the maximum cumulative risks in JEB-O and RDEB-nHS were 1·14% and 0·40% by ages 3 and 1, respectively.
Fig 2.
Age-dependent cumulative risk of cardiomyopathy in inherited epidermolysis bullosa (EB) expressed as percentage and stratified by EB subtype. JEB-O, junctional EB, non-Herlitz subtypes; RDEB-HS, recessive dystrophic EB, Hallopeau–Siemens; RDEB-nHS, RDEB, non-Hallopeau–Siemens.
Risk of death from congestive heart failure or cardiomyopathy
Among group 2, three out of 10 (30%) patients, all with RDEB-HS, died as a result of CHF or cardiomyopathy at ages 2·9, 8 and 21 years. Among those patients restricted to group 3, two deaths (out of seven, 28·6%) were directly attributed to cardiomyopathy. An additional patient with JEB-O who was also included within groups 2 and 3 died at age 3 of respiratory insufficiency following anaesthesia; dilated cardiomyopathy was noted at autopsy but was not listed as a cause of death. Two other patients with RDEB-HS included within group 1, who had concurrent chronic renal failure, reportedly died of CHF or cardiomegaly at ages 21 and 31 years. The older of these two also had numerous transfusions, which may have contributed to iron overload, whereas the younger patient with chronic renal failure had her renal disease for 5 years prior to the onset of CHF, and then died 3 months later, reportedly as a result of the latter complication.
Discussion
The term cardiomyopathy, first used in 1957, now encompasses three main subtypes – dilated, hypertrophic (idiopathic hypertrophic subaortic stenosis) and restrictive cardiomyopathies.15 A characteristic feature of dilated cardiomyopathy is CHF. There are many suggested causes of dilated cardiomyopathy, including micronutrient deficiencies (carnitine,16–18 selenium,19–21 possibly thiamine), iron overload (due to chronic transfusion therapy),22 chronic anaemia23 and viral infections (presenting as myocarditis).24–26 Of clinical pertinence to EB, the causal association of cardiomyopathy with selenium deficiency has recently been challenged.21,27
The first case of dilated cardiomyopathy in RDEB was reported in 1989 in a 17-year-old patient who had undergone repeated transfusions since age 9 years.28 At that time it was believed that this complication was caused by iron overload and secondary haemosiderosis. In 1996, Melville et al.9 reported the presence of fatal dilated cardiomyopathy in two unrelated children with RDEB. One child was found to have low selenium levels, suggesting that this micronutrient deficiency might be the possible cause. However, low selenium levels were also found in 14 of 25 other children with RDEB lacking evidence of cardiac disease, raising a question as to the clinical relevance of this finding in only one of the two children with cardiomyopathy. In a follow-up study with 7 years of observation, dilated cardiomyopathy was detected in six out of 61 (9·8%) children with RDEB who were evaluated within the same institution.10 The mean age of confirmation of the diagnosis by echocardiography was 8·7 years (range 5·8–12·5). Three of these six children died of their cardiac disease within 0·2 to 0·4 years. When data on each of these 61 patients were reviewed, statistically significant reductions in blood levels of free and total carnitine, but not selenium, were noted prior to nutritional supplementation, and none of these children was found to have any coexistent viral infection that might have played a causative role. These latter findings suggest the possibility that carnitine supplementation in recessive dystrophic EB children might be preventative against the development of dilated cardiomyopathy. In support of the latter, l-carnitine therapy has reversed cardiomyopathy in some patients having other underlying diseases associated with carnitine deficiency.16,29
Our findings are limited by the strength of the data collected, given the epidemiological study design of the National EB Registry. As noted elsewhere, these data may have been influenced by patient self-reporting, allowing for the possible introduction of reporting bias. Similarly, lack of cardiac muscle biopsy specimens and details of echocardiograms prevent definitive determination of the presence of cardiomyopathy, as opposed to some other cause of CHF. These data are therefore theoretically at risk of being influenced by misclassification bias, with under- or over-reporting of the frequency with which both conditions occur in patients with EB. However, every effort was made to confirm these diagnoses via systematic detailed interviews of patients and their immediate family (if deceased), and the review of their medical records, whenever available. As such, we are confident that all of these affected patients experienced at least CHF. Furthermore, since we excluded from our analyses any other known causes of CHF in children (i.e. congenital heart defects producing myocardial overload, or cardiac dysfunction resulting from their surgical repair or palliation),30 it is probable that most or all of our patients diagnosed with CHF did indeed have underlying dilated cardiomyopathy.
As noted previously, we grouped our patients into three increasingly restrictive strata, so as to ascertain the maximum (group 1) and minimum (group 3) cumulative risks for the development of cardiomyopathy among our large, well-characterized EB population. Group 1 represents the risk of CHF after all other identifiable confounders other than chronic renal failure were excluded. This would therefore capture all patients with cardiomyopathy, as well as those having other explanations for the presence of CHF, most notably chronic renal failure. Groups 2 and 3 were specifically considered separately, as a particular concern has been raised in the literature that cardiomyopathy might, in the setting of RDEB-HS, be caused by micronutrient deficiency, either carnitine or selenium. Among our patients in group 1, the cumulative risk rose as high as 18·86% for patients with RDEB-HS, 2·75% in RDEB-nHS and 4·94% in JEB-O. This makes the point that CHF is a clinically significant concern among patients with EB, most notably those with RDEB-HS, and, when compared with groups 2 and 3, that the chronic renal failure plays an important role in the development of CHF in these specific EB subtypes. While it is known that chronic renal failure may be a complication of RDEB-HS, typically the result of chronic glomerulonephritis or renal amyloidosis, and that chronic renal failure may be associated with CHF, it is not usually included among the causes of cardiomyopathy. When patients with chronic renal failure were excluded, as were those lacking a definitive diagnosis of cardiomyopathy, the cumulative risk of cardiomyopathy among EB patients was much lower, albeit still clinically significant.
Based on our data, in the absence of chronic renal failure, CHF and cardiomyopathy are uncommon occurrences among patients with EB, with the highest cumulative risks (4·51%) for cardiomyopathy or CHF/cardiomyopathy (5·52%), observed in patients with RDEB-HS. Lower cumulative risks also occurred in two other generalized EB subtypes, RDEB-nHS and JEB-O. These risks are much higher than those of the general population, where an annual incidence of cardiomyopathy of 1·1–1·2 per 100 000 children has been estimated for Australia31 and the U.S.A.32 It is also noteworthy that about 30% of our patients with RDEB-HS who had CHF or cardiomyopathy died as a result of these cardiac complications. Of clinical significance, none of our patients had evidence of ischaemic heart disease or any congenital cardiac anomaly that might alternatively explain the presence of CHF. Unfortunately, selenium and carnitine levels were not obtained for any of our affected patients, as much of our data collection preceded any suggestion within the literature that cardiac complications might arise in the setting of EB, or that selenium or carnitine deficiencies might play a pathological role in this disease.
It is quite likely that chronic anaemia, a well-recognized cause of dilated cardiomyopathy, plays a clinically relevant role in the development of CHF and cardiomyopathy in severe generalized EB, particularly RDEB-HS. Consistent with that, all of our patients with RDEB-HS suffered from severe multifactorial anaemia, although lack of data on haemoglobin levels at the time of onset or diagnosis of these cardiac findings prevents any quantitative comparison between those patients with and those without CHF or cardiomyopathy. Similarly, although it is known that patients with RDEB-nHS and JEB-O may also experience some degree of anaemia, insufficient data among our cohort prevent testing for any possible quantitative correlation with that risk factor in either of these two EB subtypes.
On the basis of our collective findings, surveillance for evidence of CHF and cardiomyopathy seems prudent in all infants and children with RDEB-HS, RDEB-nHS and JEB-O. Serum levels of carnitine and selenium should be monitored for possible deficiencies which could be reversed by aggressive nutritional supplementation. Similarly, haematological testing should be part of the routine management of patients with severe generalized EB, most notably RDEB-HS, and interventions should be undertaken to try to increase, at least partially, haemoglobin levels in those with severe anaemia. Based on our data, the presence of renal disease should also be sought, since it is clear that chronic renal failure also plays a role in the risk of CHF arising among patients with severe generalized EB.
Acknowledgments
The senior author (J.D.F.) gratefully acknowledges federal grant support for the National Epidermolysis Bullosa Registry throughout its first 16 years of existence. Such funding was awarded by the National Institute of Arthritis, Musculoskeletal and Skin Diseases (NO1 AM62271, NO1 AR22200, NO1 AR22201 and NO1 AR72233). This work was also supported (J.D.F.) by a NIAMS Midcareer Investigator Award for Patient-Oriented Research (K24 AR02098) and clinical fellowship awards from the Dystrophic Epidermolysis Bullosa Research Association of America and the Doris Duke Predoctoral Fellowship Program. Availability of the many resources of an NIH-supported General Clinical Research Center at the University of North Carolina at Chapel Hill was also critical to the success of this project during its last 12 funded years. We gratefully acknowledge the contributions of several other physicians to patient recruitment and data collection on behalf of the Registry throughout at least portions of its existence, most notably Drs Joseph McGuire and Eugene Bauer (Stanford University), D. Martin Carter (Rockefeller University), Virginia P. Sybert (University of Washington) and Amy Stein, Joy DeLeoz, Sarah Cash and David T. DeVries (University of North Carolina at Chapel Hill).
Conflicts of interest
None declared.
National Epidermolysis Bullosa Registry collaborating institutions
Data Coordinating Centers: Rockefeller University, 1986–1992; University of North Carolina at Chapel Hill, 1992–2002. Clinical Centers or subcontract sites for regional data collection: Rockefeller University (1986–1997); University of North Carolina at Chapel Hill (1990–2002), Stanford University (1989–2002); Washington University (1986–1989); University of Alabama at Birmingham (1986–1990); University of Washington (1986–1997); Children's Memorial Hospital, Chicago (1992–1995); University of Colorado (1992–1997).
References
- 1.Gedde-Dahl T., Jr . Epidermolysis Bullosa. A Clinical, Genetic and Epidemiologic Study. Baltimore: The Johns Hopkins Press; 1971. [Google Scholar]
- 2.Pearson RW. Clinicopathologic types of epidermolysis bullosa and their nondermatological complications. Arch Dermatol. 1988;124:718–25. [PubMed] [Google Scholar]
- 3.Fine JD, Bauer EA, McGuire J, Moshell A, editors. Epidermolysis Bullosa: Clinical, Epidemiologic, and Laboratory Advances, and the Findings of the National Epidermolysis Bullosa Registry. Baltimore: Johns Hopkins University Press; 1999. [Google Scholar]
- 4.Eady RAJ, Fine J-D, Burge SM. Genetic blistering diseases. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 7th edn. Oxford: Blackwell Publishing; 2004. pp. 40.1–32. [Google Scholar]
- 5.Fine J-D, Eady RAJ, Bauer EA, et al. Revised classification system for inherited epidermolysis bullosa: report of the Second International Consensus Meeting on diagnosis and classification of epidermolysis bullosa. J Am Acad Dermatol. 2000;42:1051–66. [PubMed] [Google Scholar]
- 6.Fuchs EV. The molecular biology of epidermolysis bullosa simplex. In: Fine JD, Bauer EA, McGuire J, Moshell A, editors. Epidermolysis Bullosa: Clinical, Epidemiologic, and Laboratory Advances, and the Findings of the National Epidermolysis Bullosa Registry. Baltimore: Johns Hopkins University Press; 1999. pp. 280–99. [Google Scholar]
- 7.Pulkkinen L, Uitto J, Christiano AM. The molecular basis of the junctional forms of epidermolysis bullosa. In: Fine JD, Bauer EA, McGuire J, Moshell A, editors. Epidermolysis Bullosa: Clinical, Epidemiologic, and Laboratory Advances, and the Findings of the National Epidermolysis Bullosa Registry. Baltimore: Johns Hopkins University Press; 1999. pp. 300–25. [Google Scholar]
- 8.Uitto J, Pulkkinen L, Christiano AM. The molecular basis of the dystrophic forms of epidermolysis bullosa. In: Fine JD, Bauer EA, McGuire J, Moshell A, editors. Epidermolysis Bullosa: Clinical, Epidemiologic, and Laboratory Advances, and the Findings of the National Epidermolysis Bullosa Registry. Baltimore: Johns Hopkins University Press; 1999. pp. 326–50. [Google Scholar]
- 9.Melville C, Atherton D, Burch M, et al. Fatal cardiomyopathy in dystrophic epidermolysis bullosa. Br J Dermatol. 1996;135:603–6. [PubMed] [Google Scholar]
- 10.Sidwell RU, Yates R, Atherton D. Dilated cardiomyopathy in dystrophic epidermolysis bullosa. Arch Dis Child. 2000;83:59–63. doi: 10.1136/adc.83.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fine JD, Johnson LB, Suchindran C, et al. The National Epidermolysis Bullosa Registry: organization, goals, methodologic approaches, basic demography, and accomplishments. In: Fine JD, Bauer EA, McGuire J, Moshell A, editors. Epidermolysis Bullosa: Clinical, Epidemiologic, and Laboratory Advances, and the Findings of the National Epidermolysis Bullosa Registry. Baltimore: Johns Hopkins University Press; 1999. pp. 79–100. [Google Scholar]
- 12.Fine J-D, Johnson LB, Weiner M, et al. Eye involvement in inherited epidermolysis bullosa (EB): experience of the National EB Registry. Am J Ophthalmol. 2004;138:254–62. doi: 10.1016/j.ajo.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 13.Fine J-D, Johnson LB, Weiner M, et al. Pseudosyndactyly and musculoskeletal deformities in inherited epidermolysis bullosa (EB): experience of the National Epidermal Bullosa Registry, 1986–2002. J Hand Surg (Br) 2005;30:14–22. doi: 10.1016/j.jhsb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Fine JD, Bauer EA, Briggaman RA, et al. Revised clinical and laboratory criteria for subtypes of inherited epidermolysis bullosa: a consensus report by the Subcommittee on Diagnosis and Classification of the National Epidermolysis Bullosa Registry. J Am Acad Dermatol. 1991;24:119–35. doi: 10.1016/0190-9622(91)70021-s. [DOI] [PubMed] [Google Scholar]
- 15.Towbin JA. Pediatric myocardial disease. Pediatr Clin North Am. 1999;46:289–312. doi: 10.1016/s0031-3955(05)70119-x. [DOI] [PubMed] [Google Scholar]
- 16.Waber LJ, Valle D, Neill C, et al. Carnitine deficiency presenting as familial cardiomyopathy: a treatable defect in carnitine transport. J Pediatr. 1982;101:700–5. doi: 10.1016/s0022-3476(82)80294-1. [DOI] [PubMed] [Google Scholar]
- 17.Winter SC, Szabo-Aczel S, Curry CJR, et al. Plasma carnitine deficiency: clinical observations in 51 pediatric patients. Am J Dis Child. 1987;141:660–5. doi: 10.1001/archpedi.1987.04460060076039. [DOI] [PubMed] [Google Scholar]
- 18.Paulson DJ. Carnitine deficiency-induced cardiomyopathy. Mol Cell Biochem. 1998;180:33–41. [PubMed] [Google Scholar]
- 19.Kanekura T, Yotsumoto S, Maeno N, et al. Selenium deficiency: report of a case. Clin Exp Dermatol. 2005;30:346–8. doi: 10.1111/j.1365-2230.2005.01746.x. [DOI] [PubMed] [Google Scholar]
- 20.Johnson RA, Baker SS, Fallon JT, et al. An occidental case of cardiomyopathy and selenium deficiency. N Engl J Med. 1981;304:1210–12. doi: 10.1056/NEJM198105143042005. [DOI] [PubMed] [Google Scholar]
- 21.Alissa EM, Bahijri SM, Ferns GA. The controversy surrounding selenium and cardiovascular disease: a review of the evidence. Med Sci Monit. 2003;9:RA9–18. [PubMed] [Google Scholar]
- 22.Rahko PS, Salerni R, Uretsky BF. Successful reversal by chelation therapy of congestive cardiomyopathy due to iron overload. J Am Coll Cardiol. 1986;8:436–40. doi: 10.1016/s0735-1097(86)80063-8. [DOI] [PubMed] [Google Scholar]
- 23.Lewis BS, Rachmilewitz EA, Amitai N, et al. Left ventricular function in thalassemia and effect of multiple transfusions. Am Heart J. 1978;96:636–45. doi: 10.1016/0002-8703(78)90201-6. [DOI] [PubMed] [Google Scholar]
- 24.Matsumori A. Molecular and immune mechanisms in the pathogenesis of cardiomyopathy – role of viruses, cytokines, and nitric oxide. Jpn Circ. 1997;61:275–91. doi: 10.1253/jcj.61.275. [DOI] [PubMed] [Google Scholar]
- 25.Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343:1388–98. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- 26.Morelli S, Dianzani C, Sgreccia A, et al. Reversible acute global left ventricular dysfunction in a patient with autosomal recessive dystrophic epidermolysis bullosa. Int J Cardiol. 2001;79:321–3. doi: 10.1016/s0167-5273(01)00433-8. [DOI] [PubMed] [Google Scholar]
- 27.Fett JD, Ansari AA, Sundstrom JB, et al. Peripartum cardiomyopathy: a selenium disconnection and an autoimmune connection. Int J Cardiol. 2002;86:311–16. doi: 10.1016/s0167-5273(02)00359-5. [DOI] [PubMed] [Google Scholar]
- 28.Brook MM, Weinhouse E, Jarenwattananon M, et al. Dilated cardiomyopathy complicating a case of epidermolysis bullosa dystrophica. Pediatr Dermatol. 1989;6:21–3. doi: 10.1111/j.1525-1470.1989.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 29.Zales VR, Benson DW Jr. Reversible cardiomyopathy due to carnitine deficiency from renal tubular wasting. Pediatr Cardiol. 1995;16:76–8. doi: 10.1007/BF00796822. [DOI] [PubMed] [Google Scholar]
- 30.Kay JD, Colan SD, Graham TP Jr. Congestive heart failure in pediatric patients. Am Heart J. 2001;142:923–8. doi: 10.1067/mhj.2001.119423. [DOI] [PubMed] [Google Scholar]
- 31.Nugent AW, Daubeney PEF, Chondros P, et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003;348:1639–46. doi: 10.1056/NEJMoa021737. [DOI] [PubMed] [Google Scholar]
- 32.Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647–55. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]