Abstract
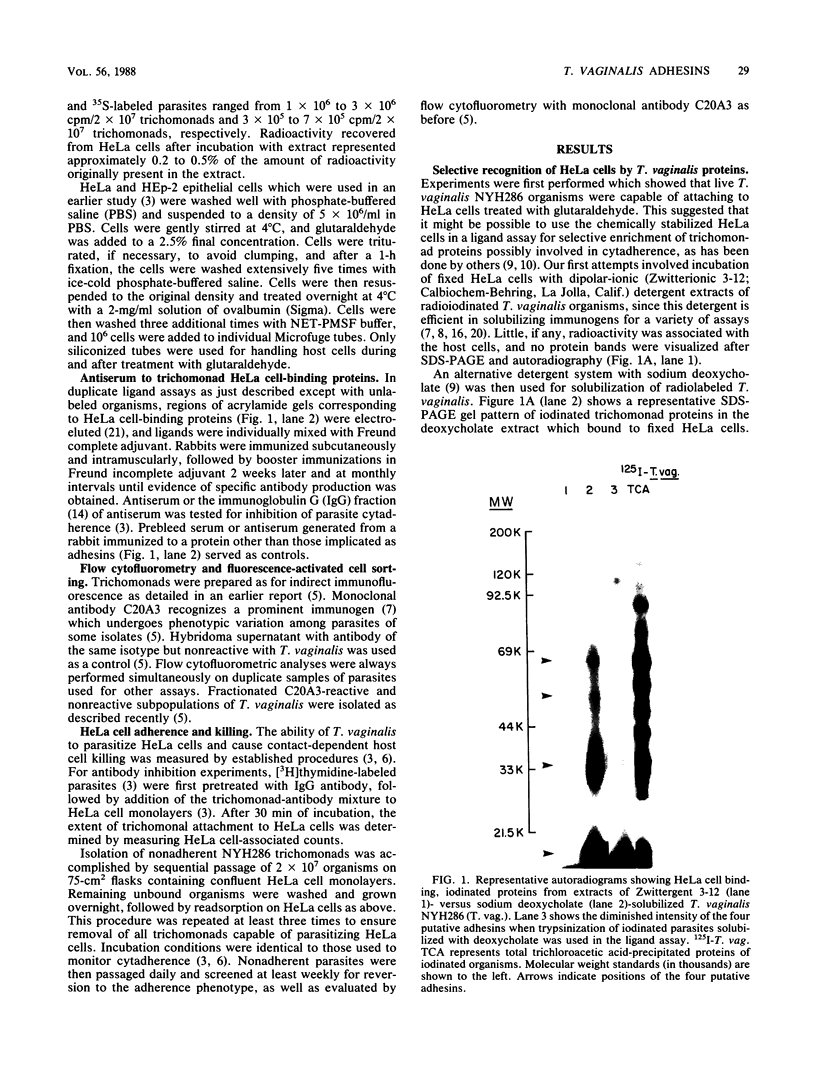
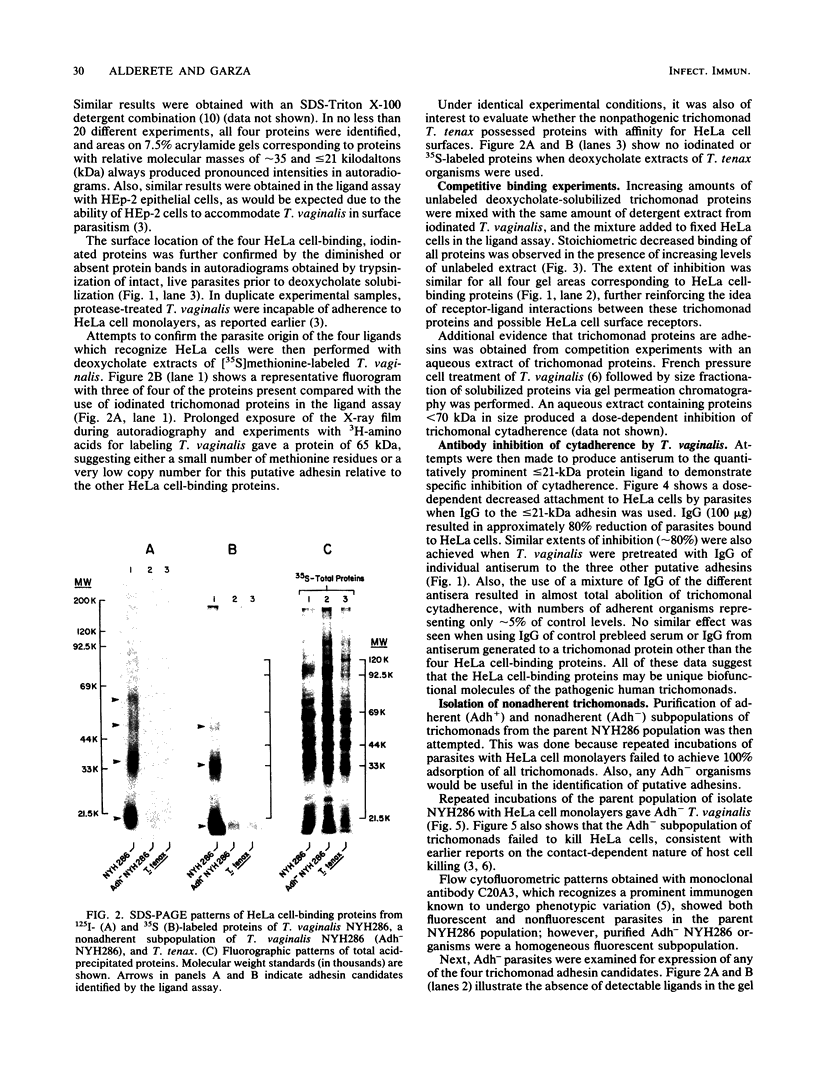
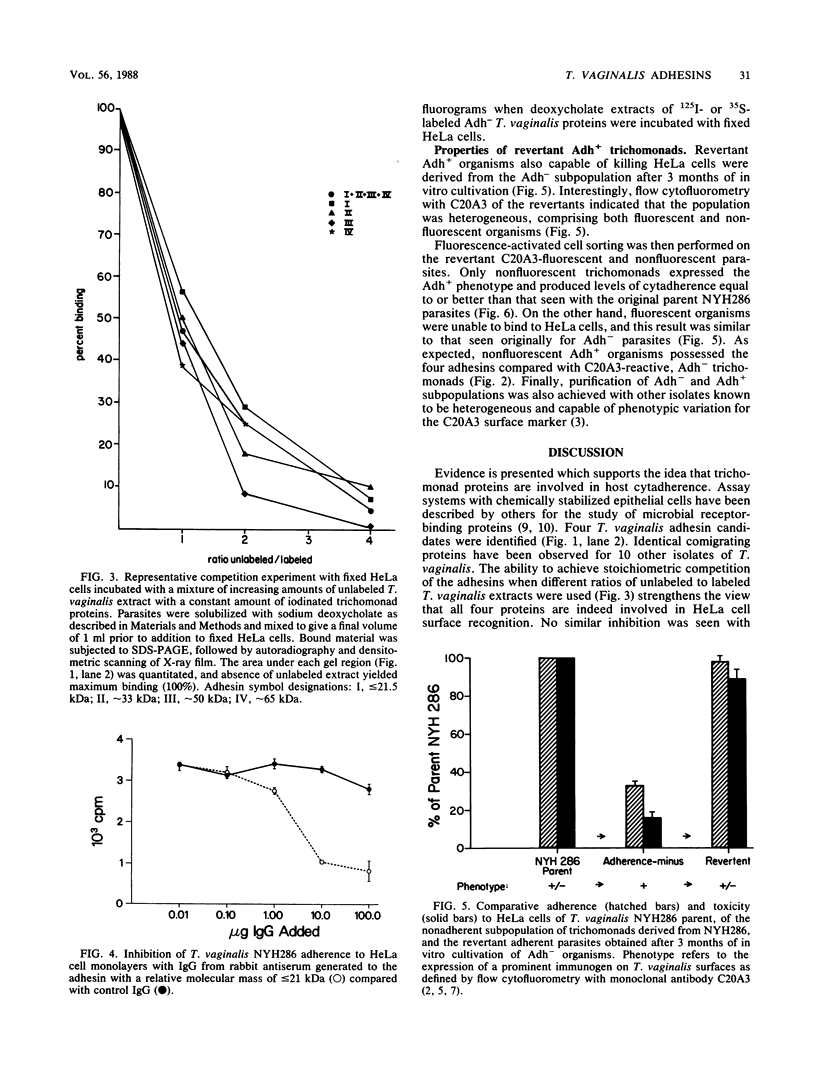
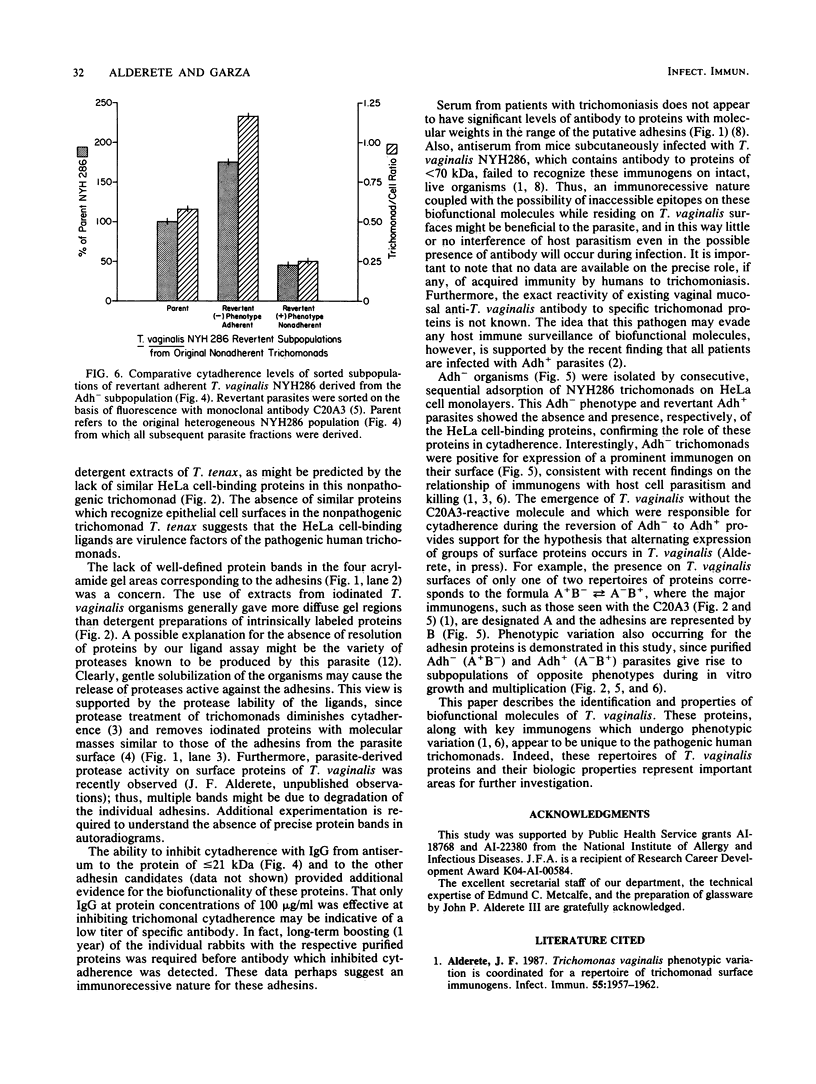
Trichomonas vaginalis NYH286 surface proteins which are candidates for mediating parasite cytadherence (adhesins) were identified. At least four trichomonad protein ligands ranging in relative molecular mass from 65 to less than or equal to 21 kilodaltons were found to selectively bind to chemically stabilized HeLa cells. The proteins were present on the surfaces of 10 different isolates of T. vaginalis examined; however, the nonpathogenic trichomonad T. tenax did not possess similar HeLa cell-binding proteins under identical experimental conditions, suggesting that these proteins are unique to the pathogenic human trichomonads. The surface nature of the candidate adhesins was confirmed by the ability of the proteins on intact, live organisms to be radioiodinated and to be removed with trypsin treatment. Rabbit antiserum (immunoglobulin G fraction) generated against adhesin proteins electroeluted from acrylamide preparations inhibited cytadherence compared with control immunoglobulin G. An adherence-negative subpopulation of T. vaginalis NYH286 organisms was also isolated. These nonadherent trichomonads did not synthesize the adhesin proteins. Interestingly, absence of adhesins from these parasites paralleled expression of a major immunogen known to undergo phenotypic variation. Revertant organisms derived from the adherence-minus subpopulation synthesized the adhesins and attached to HeLa cells. The emergence of revertant adherent T. vaginalis organisms also corresponded with the appearance of parasites which were without the major immunogen on their surface. Finally, it was determined that only those parasites lacking the major surface immunogen were capable of adherence and toxicity to HeLa cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Garza G. E. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect Immun. 1985 Dec;50(3):701–708. doi: 10.1128/iai.50.3.701-708.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Garza G., Smith J., Spence M. Trichomonas vaginalis: electrophoretic analysis and heterogeneity among isolates due to high-molecular-weight trichomonad proteins. Exp Parasitol. 1986 Apr;61(2):244–251. doi: 10.1016/0014-4894(86)90158-x. [DOI] [PubMed] [Google Scholar]
- Alderete J. F., Kasmala L., Metcalfe E., Garza G. E. Phenotypic variation and diversity among Trichomonas vaginalis isolates and correlation of phenotype with trichomonal virulence determinants. Infect Immun. 1986 Aug;53(2):285–293. doi: 10.1128/iai.53.2.285-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Pearlman E. Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br J Vener Dis. 1984 Apr;60(2):99–105. doi: 10.1136/sti.60.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L. Monoclonal antibody to a major surface glycoprotein immunogen differentiates isolates and subpopulations of Trichomonas vaginalis. Infect Immun. 1986 Apr;52(1):70–75. doi: 10.1128/iai.52.1.70-75.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L., Smith J., Spence M. Heterogeneity of Trichomonas vaginalis and discrimination among trichomonal isolates and subpopulations with sera of patients and experimentally infected mice. Infect Immun. 1985 Sep;49(3):463–468. doi: 10.1128/iai.49.3.463-468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F. Trichomonas vaginalis NYH286 phenotypic variation may be coordinated for a repertoire of trichomonad surface immunogens. Infect Immun. 1987 Sep;55(9):1957–1962. doi: 10.1128/iai.55.9.1957-1962.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Coombs G. H. Proteinases of Leishmania mexicana and other flagellate protozoa. Parasitology. 1982 Feb;84(1):149–155. doi: 10.1017/s003118200005174x. [DOI] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Gonenne A., Ernst R. Solubilization of membrane proteins by sulfobetaines, novel zwitterionic surfactants. Anal Biochem. 1978 Jun 15;87(1):28–38. doi: 10.1016/0003-2697(78)90565-1. [DOI] [PubMed] [Google Scholar]
- Krieger J. N., Ravdin J. I., Rein M. F. Contact-dependent cytopathogenic mechanisms of Trichomonas vaginalis. Infect Immun. 1985 Dec;50(3):778–786. doi: 10.1128/iai.50.3.778-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Host plasma proteins on the surface of pathogenic Trichomonas vaginalis. Infect Immun. 1982 Aug;37(2):755–762. doi: 10.1128/iai.37.2.755-762.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Baseman J. B., Alderete J. F. Treponema pallidum receptor binding proteins interact with fibronectin. J Exp Med. 1983 Jun 1;157(6):1958–1970. doi: 10.1084/jem.157.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Putative Treponema pallidum cytadhesins share a common functional domain. Infect Immun. 1985 Sep;49(3):833–835. doi: 10.1128/iai.49.3.833-835.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]





