Summary
Observations from nematodes to mammals indicate that insulin/insulin-like growth factor signaling (IIS) regulates lifespan. As in other organisms, IIS is conserved in mosquitoes and signaling occurs in multiple tissues. During bloodfeeding, mosquitoes ingest human insulin. This simple observation suggested that exogenous insulin could mimic the endogenous hormonal control of aging in mosquitoes, providing a new model to examine this phenomenon at the organismal and cellular levels. To this end, female Anopheles stephensi mosquitoes were maintained on diets containing human insulin provided daily in sucrose or three times weekly by artificial bloodmeal. Regardless of delivery route, mosquitoes provided with insulin at 1.7×10−4 and 1.7×10−3 μmol l−1, doses 0.3-fold and 3.0-fold higher than non-fasting blood levels, died at a faster rate than controls. In mammals, IIS induces the synthesis of reactive oxygen species and downregulates antioxidants, events that increase oxidative stress and that have been associated with reduced lifespan. Insulin treatment of mosquito cells in vitro induced hydrogen peroxide synthesis while dietary supplementation reduced total superoxide dismutase (SOD) activity and manganese SOD activity relative to controls. The effects of insulin on mortality were reversed when diets were supplemented with manganese (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP), a cell-permeable SOD mimetic agent, suggesting that insulin-induced mortality was due to oxidative stress. In addition, dietary insulin activated Akt/protein kinase B and extracellular signal-regulated kinase (ERK) in the mosquito midgut, suggesting that, as observed in Caenorhabditis elegans, the midgut may act as a ‘signaling center’ for mosquito aging.
Keywords: malaria, mosquito, Plasmodium, Anopheles, aging, insulin, oxidative stress, antioxidant
Introduction
Malaria is responsible for over 1 million deaths worldwide each year (Guinovart et al., 2006) and is caused by parasites of the genus Plasmodium that are transmitted by female Anopheles mosquitoes. Parasite development in the mosquito is initiated shortly after bloodfeeding and requires 10 days for completion, a time called the extrinsic incubation period (EIP). The midgut is a critical site for parasite development (Whitten et al., 2006) and is exposed, at bloodfeeding, to erythrocytes and parasites, and to parasite-derived factors and host-derived proteins such as insulin that can affect mosquito physiology and parasite development.
Insulin/insulin-like growth factor signaling (IIS) is highly conserved in mosquitoes and expressed in multiple tissues. Studies by Riehle and Brown (Riehle and Brown, 1999; Riehle and Brown, 2002; Riehle and Brown, 2003) of Aedes aegypti, the yellow fever mosquito, revealed that bovine insulin stimulated ovarian ecdysteroid synthesis and that this effect was transduced through a mosquito insulin receptor (INR) and the IIS-associated phosphatidylinositol 3-kinase (PI-3K) and Akt/protein kinase B (PKB). Studies by Lim et al. (Lim et al., 2005) indicated that the INR, Akt/PKB and the mitogen-activated protein kinase kinase DSOR1 were expressed in the midgut epithelium of the malaria vector A. stephensi and were activated by Plasmodium falciparum glycosylphosphatidylinositols, a parasite factor that can mimic insulin in mammalian hosts (Schofield and Hackett, 1993; Caro et al., 1996). Beier et al. (Beier et al., 1994) had previously shown that human insulin, albeit at levels vastly exceeding those in human blood, could alter the development of P. falciparum oocysts in the midguts of A. stephensi and Anopheles gambiae. In addition to the effects of exogenous insulin on different tissues, endogenous insulin-like peptides (ILPs) or their transcripts are detectable in numerous mosquito tissues, including the brains and midguts of A. aegypti (Riehle et al., 2006) and of A. gambiae (Krieger et al., 2004). Taken together, these observations suggested to us that insulin ingested with blood could function as a signal to multiple mosquito tissues, including the midgut, to have far-reaching effects on mosquito physiology.
In mammals, blood insulin levels are altered by malaria parasite infection, suggesting that, under natural conditions, feeding mosquitoes are subjected to a range of insulin concentrations. In mice (Elased and Playfair, 1994) and in humans (White et al., 1983; White et al., 1987) malaria parasite infection induces hypoglycemia, which is predictive of severe pathology and fatal outcome. In mice, hypoglycemia has been causally linked to hyperinsulinemia (Elased and Playfair, 1996). In humans, malaria parasite infection and quinine therapy of infection can also lead to hyperinsulinemia (White et al., 1983; Planche et al., 2005). Average insulin levels in hyperinsulinemic malaria patients were 1.6×10−4 μmol l−1, with the highest concentration at 4.7×10−4 μmol l−1 (White et al., 1983). These levels contrast with normal blood insulin levels, which range from 1.7×10−5 μmol l−1 (0.1 ng ml−1) at fasting to 5.9×10−4 μmol l−1 [3.4 ng ml−1 (Darby et al., 2001)] without fasting, indicating that blood levels of insulin can vary as much as 10- to 35-fold depending on nutrition and disease status.
One of the best-known effects of insulin signaling is the control of lifespan. Studies in C. elegans provide causal genetic evidence that lifespan is regulated by IIS (Baumeister et al., 2006). In C. elegans, signaling presumably is initiated by the binding of ILPs to the INR DAF-2, which activates AGE-1, a PI-3K, and Akt/PKB, which directly phosphorylates DAF-16, a forkhead/winged helix transcription factor, preventing its translocation to the nucleus (Lin et al., 2001). In C. elegans, daf-2 loss-of-function mutants not only live longer but also exhibit increased resistance to oxidative stress, whereas daf-16 loss-offunction mutants are short-lived and more susceptible to oxidative stress relative to wild-type (Ogg et al., 1997; Garsin et al., 2003). In C. elegans, the intestine and germ tissue play pivotal roles in signaling crosstalk. Indeed, the intestine has been defined as a ‘signaling center’ from which overexpressed daf-16 can completely restore the longevity of daf-16 germline-deficient nematodes and increase the lifespan of these mutants (Libina et al., 2003; Murphy et al., 2007). Key targets of DAF-16 regulation include mitochondrial manganese superoxide dismutase (MnSOD) and glutathione S-transferase (Murphy et al., 2003), two antioxidants that are closely linked to aging in C. elegans and other organisms (Sampayo et al., 2003; Ayyadevara et al., 2005).
The free radical theory of aging predicts that oxidative stress is a key determinant of lifespan (Humphries et al., 2006). Reactive oxygen species (ROS), such as superoxide and hydrogen peroxide (H2O2), are generated during cellular metabolism, especially during mitochondrial energy production. Unregulated production of ROS damages DNA and proteins and the accumulation of this damage is believed to be an underlying cause of aging. Among invertebrate model organisms, the importance of oxidative stress in aging has been demonstrated in studies of SOD-deficient Drosophila melanogaster and in C. elegans. In D. melanogaster, exogenous provision of antioxidants increased the lifespan of SOD-deficient flies and improved their tolerance to oxidative stress (Magwere et al., 2006). Similarly, studies in C. elegans have demonstrated that provision of antioxidants extended lifespan in oxidatively stressed but not in unstressed nematodes (Keaney et al., 2004), while others showed a direct effect of SOD/catalase mimetics in the growth medium on the lifespan of normal nematodes (Melov et al., 2000).
In this study, we show that dietary provision of human insulin at levels found in circulating blood to A. stephensi significantly decreased the lifespan of treated mosquitoes relative to controls. Reversal of these effects by dietary provision of MnTBAP, a cell-permeable SOD mimetic agent (Faulkner et al., 1994), suggests that reduced SOD activity and damaging ROS, which may include H2O2, account for a measurable component of mortality in insulin-fed mosquitoes. Human insulin activates proteins associated with insulin signaling in the mosquito midgut, suggesting that the control of aging by IIS is evolutionarily conserved in the mosquito and that, as has been observed in C. elegans (Libina et al., 2003), the midgut may function as a signaling center for mosquito lifespan. In the light of the fact that hyperinsulinemia can be associated with human malaria infection (Planche et al., 2005), our data also suggest that the lifespan of mosquitoes in nature is directly regulated by this blood-derived hormone, perhaps to an even greater degree when human parasite infection is prevalent.
Materials and Methods
Cell culture and reagents
Immortalized, embryo-derived A. stephensi cell lines, ASE (Kurtti and Munderloh, 1989) and MSQ43 (provided by the Department of Entomology, Water Reed Army Institute of Research, Washington, DC, USA), were cultured in modified minimal essential medium (MEM; Gibco, Invitrogen, Carlsbad, CA, USA) containing 5% heat-inactivated fetal bovine serum at 28°C under 5% CO2. Chemicals, antisera and other reagents were purchased from the following companies: human serum and erythrocytes from Continental Services Group (Miami, FL, USA); human erythrocyte catalase, PD98059, human insulin, protease inhibitor cocktail, adenosine 5′-triphosphate (ATP), monoclonal mouse anti-phospho-ERK antisera and horseradish peroxidase (HRP)-conjugated anti-mouse IgG from Sigma-Aldrich (St Louis, MO, USA); bovine serum albumin from Fisher Scientific (Waltham, MA, USA); antibodies to phosphorylated Akt/PKB (Ser473), total Akt/PKB and total ERK from Cell Signaling (Charlottesville, VA, USA); HRP-conjugated anti-rabbit IgG from Biosource International (Invitrogen); SuperSignal West Pico chemiluminescent detection kit from Pierce (Rockford, IL, USA); 5,6-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA) from Molecular Probes (Invitrogen); and SOD assay kit from Cayman Chemical (Ann Arbor, MI, USA). All other chemicals and reagents were obtained from Sigma-Aldrich or Fisher Scientific.
Mosquito rearing and dietary provision of human insulin
Anopheles stephensi Liston (Indian wild-type strain) were reared and maintained at 27°C and 75% relative humidity. The use of mice or hamsters for a blood source in the rearing of A. stephensi is in compliance with all federal guidelines and institutional policies.
Insulin and MnTBAP were provided to A. stephensi via 10% sucrose or via an artificial bloodmeal. For feeding studies with 10% sucrose solution, 100–250 female A. stephensi aged 3–5 days from a single cohort were transferred into 1 gallon (∼3.79 l per US gallon) cartons. Treatment cartons were provided with cotton pads soaked with 15 ml of 10% sucrose supplemented with 1.7×10−4 μmol l−1 human insulin or an equivalent volume of insulin buffer (25 mmol l−1 Hepes buffer, pH 8.2) as a control. Other treatment cartons received sucrose solution supplemented with insulin and 0.05 mmol l−1 MnTBAP (in buffer) or with MnTBAP alone. Cotton pads were changed twice daily and dead insects were counted and removed from all cartons daily.
For feeding studies with artificial bloodmeals, mosquitoes in 1 gallon (N=70–100) or 5 gallon cartons (N=300–500) were fed via a Hemotek circulation system (Discovery Workshops, Accrington, UK) every Monday, Wednesday and Friday until all insects were dead. Artificial bloodmeals provided via the Hemotek were composed of washed human erythrocytes and saline (15 mmol l−1 NaCl, 10 mmol l−1 NaHCO3, 1 mmol l−1 ATP, pH 7.0) supplemented with 1.7×10−3 or 1.7×10−5 μmol l−1 insulin or an equivalent volume of insulin buffer as a control. Additional treatment cartons received artificial bloodmeals supplemented with insulin and 0.05 mmol l−1 MnTBAP or with MnTBAP alone. The use of anonymously collected human blood products for these procedures is in compliance with all federal guidelines and institutional policies. Mosquitoes were allowed to feed for approximately 1 h to ensure that the majority of insects engorged. All treatment groups were provided with 10% sucrose-soaked cotton pads between bloodfeedings and with oviposition cups for egg laying that were changed after each bloodmeal. Dead mosquitoes were counted daily and removed from each container.
Western blot analyses of mosquito midgut proteins
For Western blot analyses, 3- to 5-day-old female A. stephensi were allowed to feed on artificial bloodmeals containing 1.7×10−5, 1.7×10−3 or 1.7 μmol l−1 insulin (Lim et al., 2005), or on a buffer-supplemented artificial bloodmeal as a control. After feeding, midguts of blood-fed mosquitoes were dissected in phosphate-buffered saline (PBS, pH 7.4; Gibco) with protease inhibitor cocktail. For analyses of protein phosphorylation, 30 midguts from each treatment group were dissected at 30 min post-bloodmeal. Blood was removed by puncturing the midguts with minuten probes and washing the tissue twice on ice with PBS plus protease inhibitor cocktail. Dissected, washed midguts were triturated in 80 μl of lysis buffer [10 mmol l−1 Tris-HCl pH 7.4, 100 mmol l−1 NaCl, 1 mmol l−1 EDTA, 1 mmol l−1 EGTA, 1 mmol l−1 NaF, 20 mmol l−1 Na4P2O7, 2 mmol l−1 Na3VO4, 0.1% sodium dodecyl sulfate (SDS), 0.5% sodium deoxycholate, 1% Triton X-100, 10% glycerol, 1 mmol l−1 phenylmethylsulfonyl fluoride, 60 μg ml−1 aprotinin, 10 μg ml−1 leupeptin and 1 μg ml−1 pepstatin]. Cell debris was removed by centrifugation for 30 min at 4°C. Protein concentration was measured by Bradford assay (BioRad, Hercules, CA, USA) relative to a standard curve of bovine serum albumin.
Midgut protein lysates were diluted with sample buffer (125 mmol l−1 Tris-HCl pH 6.8, 10% glycerol, 10% SDS, 0.006% Bromophenol blue, 130 mmol l−1 dithiothreitol) and heated to 95°C for 5 min. Equivalent amounts of midgut proteins from each treatment group were electrophoretically separated through 12% polyacrylamide, then transferred to nitrocellulose membrane using a semi-dry blotter (BioRad). Membranes were blocked with Tris-buffered saline (TBS, pH 7.4) containing 0.1% Tween 20 (TBS-T) and 5% (w/v) dry skimmed milk powder. After washing with TBS-T, the membranes were incubated overnight with a 1:1000 dilution of anti-phospho-Akt/PKB (Ser473) antibody or a 1:10 000 dilution of monoclonal anti-phospho-ERK antisera. The sequences of peptides used to generate the phospho-specific antisera are 100% conserved with predicted amino acid sequences from A. gambiae (not shown) and, as such, were expected to recognize relevant A. stephensi proteins. Membranes were washed and incubated with a 1:1000 dilution of horseradish peroxidase (HRP)-conjugated anti-rabbit IgG or a 1:60 000 dilution of HRP-conjugated anti-mouse IgG. Peroxidase activity was detected with the SuperSignal West Pico chemiluminescent detection kit. To assess loading, identical paired membranes were probed with 1:1000 anti-total ERK or 1:1000 anti-total Akt/PKB antibody and processed as above. Signal intensities of cross-reacting proteins were measured using a GS-800 calibrated densitometer (BioRad) and normalized against values obtained for control midguts.
Measurement of SOD activity
For these studies, treatment groups of 100 female A. stephensi aged 3 days from a single cohort were transferred to 1 gallon cartons. Mosquitoes were provided with cotton pads soaked with 10% sucrose supplemented with 1.7×10−4 μmol l−1 human insulin or an equivalent volume of insulin buffer as a control. Every third day, 10 mosquitoes from each treatment group were collected and homogenized by trituration on ice for 20 min in 400 μl of cold lysis buffer (20 mmol l−1 Hepes, pH 7.2 with 1 mmol l−1 EGTA, 210 mmol l−1 mannitol, 70 mmol l−1 sucrose). Samples were briefly sonicated (five 5 s pulses) and then centrifuged at 300 g for 10 min. Lysate supernatants were diluted 1:5 for determination of total SOD and MnSOD activity according to the manufacturer's instructions. Measurement of SOD activity is based on the detection of superoxide generated by xanthine oxidase and hypoxanthine. Activity in each lysate was determined based on a standard curve. MnSOD activity was measured in the same lysate samples following sample treatment with 3–9 mmol l−1 KCN, which inhibits Cu/Zn SOD and FeSOD.
Measurement of intracellular reactive oxygen species
For these assays, 1×105 A. stephensi MSQ43 or ASE cells were plated in 96-well plates in medium without Phenol red, grown overnight, then made quiescent in serum-free media for 3 h. Serum-deprived cells were stimulated with several concentrations of human insulin or with insulin buffer or 500 μmol l−1 H2O2 as controls. For some assays, cells were pre-treated with catalase for 1 h before treatment. A 10 mmol l−1 stock solution of DCF-DA was freshly prepared in ethanol for each assay. Cells were incubated for 10 min with 10 μmol l−1 DCF-DA at room temperature in the dark. Cells were then immediately monitored using 488 nm excitation/530 nm emission settings on a microplate fluorometer (Fluorocount™; Packard, Ramsey, MN, USA).
Statistical analyses
Survival analyses were performed using the Kaplan Meier method (Kaplan and Meier, 1958) and the significance of differences between survival curves was calculated using the Wilcoxon test. Wilcoxon statistics were used in our analyses because this test gives more weight to mortality at early times (Allison, 1995), which is important considering the biology of our system (see Discussion). Curves were considered significantly different at P=0.05. The statistical software used was SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). Mortality rate (Fig. 1B and Fig. 2B) was estimated as the negative natural log of (1−qx), where qx is age-specific mortality (Tatar and Carey, 1995).
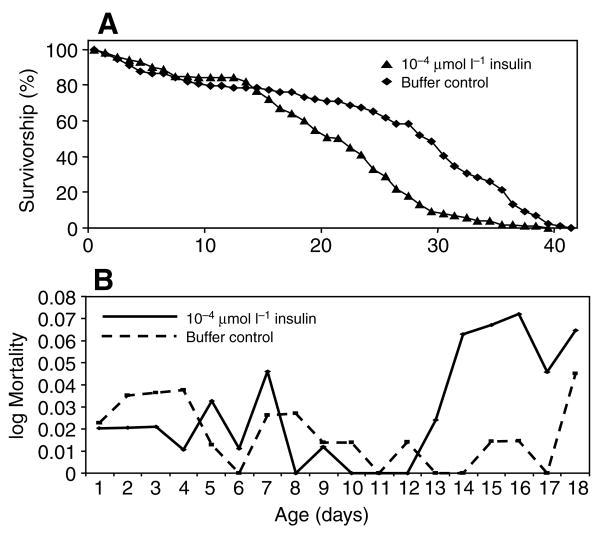
Fig. 1.
Example survivorship curve (A) and mortality rate (B; see Materials and methods) showing that human insulin provided in sucrose increased the mortality of A. stephensi relative to controls. These plots correspond to the data for experiment 2, Table 1. Female mosquitoes were provided with 10% sucrose-soaked cotton pads with 1.7×10−4 μmol l−1 human insulin or with an equivalent volume of insulin buffer as a control in sucrose. Cotton pads were changed twice daily. Dead insects were counted and removed daily from treatment and control cartons.
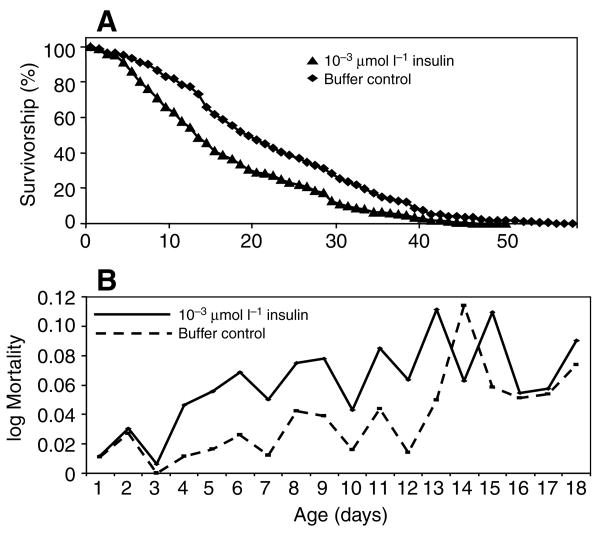
Fig. 2.
Example survivorship curve (A) and mortality rate (B) showing that human insulin provided by artificial bloodmeal increased the mortality of A. stephensi relative to controls. These plots correspond to the data for experiment 3, Table 2. Female mosquitoes were provided with 1.7×10−3 μmol l−1 human insulin or with an equivalent volume of insulin buffer by artificial bloodmeal every Monday, Wednesday and Friday until all insects were dead. Oviposition cups and 10% sucrose pads were provided between bloodmeals. Dead insects were counted and removed daily from treatment and control cartons.
Data from SOD assays were analyzed by 3-way factorial ANOVA, where the main effects were age, replicate and treatment. Because the main effect of age was not significant, nor were any interactions with other effects and age, it was not considered for analyses. Variation among replicates was expected due to the nature of the experiments; thus we compared means for treatments with each other using the Student–Neuman–Keuls test with α=0.05. Data from DCF-DA assays were analyzed by ANOVA and the Bonferroni post-test with α=0.05.
Results
Insulin-induced mortality in A. stephensi and the effects of MnTBAP
To test whether insulin delivered via sucrose could reduce mosquito lifespan, A. stephensi females in 1 gallon cartons (N≈ 100–250 per carton) were provided with 10% sucrose supplemented with 1.7×10−4 μmol l−1 human insulin or with 10% sucrose with an equivalent volume of insulin buffer (25 mmol l−1 Hepes, pH 8.2) as a control. A total of six such experiments were conducted. An example survivorship curve is presented in Fig. 1A. A depiction of the mortality rate, or instantaneous probability of death, is presented in Fig. 1B to the age of 18 days (see Discussion for the relevance of this time period). The data for these figures correspond to Experiment 2 in Table 1. Comparisons using the Kaplan Meier method followed by the Wilcoxon test showed that in three out of the six experiments, regardless of mosquito density, the curves differed, with the insulin-treated mosquitoes having a higher mortality rate according to at least one of the tests (Table 1). From day 12 to day 18, the daily risk of mortality for mosquitoes was higher for insulin-fed mosquitoes than for the control mosquitoes (Fig. 1B). Across the six experiments, the median lifespan of buffer-fed mosquitoes was 8.5% longer than that of insulin-fed mosquitoes.
Table 1.
Effect of human insulin via sucrose on lifespan
| Buffer | Insulin | Wilcoxon statistic | ||||
|---|---|---|---|---|---|---|
| Experiment | N | Days | N | Days | ||
| 1 | 206 | 29 | 257 | 25 | 0.0003 | |
| 2 | 89 | 30 | 100 | 23 | <0.0001 | |
| 3 | 107 | 25 | 124 | 24 | NS | |
| 4 | 95 | 24 | 102 | 25 | NS | |
| 5 | 99 | 24 | 100 | 24.5 | NS | |
| 6 | 102 | 33 | 94 | 31 | <0.0001 | |
Median lifespans (days) and statistical results from six independent experiments comparing A. stephensi provided with 1.7×10−4 μmol l−1 human insulin (or buffer as a control) in 10% sucrose.
The above results led us to test the effects of human insulin ingested in an artificial bloodmeal, which simulates a more natural route of delivery. To eliminate confounding effects of insulin in serum, we fed mosquitoes an artificial meal containing washed erythrocytes in saline (150 mmol l−1 NaCl, 10 mmol l−1 NaHCO3, 1 mmol l−1 ATP, pH 7.0). Human insulin was added at 1.7×10−3 or 1.7×10−5 μmol l−1. Control mosquitoes were given an artificial bloodmeal supplemented with an equivalent volume of insulin buffer. Assays were performed using 1 gallon cartons (N≈70–100 A. stephensi per carton) or 5 gallon cartons (N≈300–500 A. stephensi per carton).
Insulin at 1.7×10−5 μmol l−1 had no effect on lifespan relative to the control treatment (data not shown). However, 1.7×10−3 μmol l−1 insulin had a negative effect on survivorship. An example survivorship curve is presented in Fig. 2A, which corresponds to experiment 3 in Table 2. Fig. 2B represents the mortality rate during this experiment; the daily risk of mortality for mosquitoes fed blood supplemented with insulin was higher than those fed blood supplemented with buffer nearly every day during the first 18 days of the experiment. Experiments 1 and 2 were conducted in small cartons with sample sizes of approximately 70–100 mosquitoes per treatment and showed no significant differences between the treatment and control groups, despite the numerical differences between median lifespans (Table 2). However, experiments 3–5 were conducted with sample sizes of approximately 300–500 mosquitoes per treatment and all three experiments had curves that differed, with the insulin-treated mosquitoes having a higher mortality rate (Table 2). Across the five experiments, the median lifespan of buffer-fed mosquitoes was 20.07% longer than that of insulin-fed mosquitoes.
Table 2.
Effect of human insulin via bloodmeal on lifespan
| Buffer | Insulin | Wilcoxon statistic | ||||
|---|---|---|---|---|---|---|
| Experiment | N | Days | N | Days | ||
| 1 | 102 | 23 | 93 | 21 | NS | |
| 2 | 79 | 29 | 66 | 25 | NS | |
| 3 | 455 | 19 | 508 | 13 | <0.0001 | |
| 4 | 304 | 21 | 327 | 18 | 0.001 | |
| 5 | 456 | 28 | 460 | 25 | 0.001 | |
Median lifespans (days) and statistical results from five independent experiments comparing A. stephensi provided with 1.7×10−3 μmol l−1 human insulin (or buffer as a control) via artificial bloodmeal. In experiment 5, two additional treatment groups were included (0.05 mmol l−1 MnTBAP and MnTBAP + insulin); the results from these groups and associated statistics are discussed in the text.
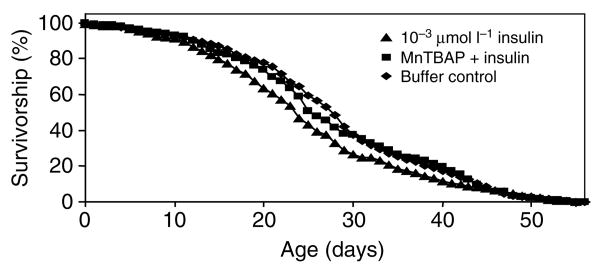
To test the hypothesis that insulin-induced mortality was due to oxidative stress, we provided 1.7×10−3 μmol l−1 insulin by artificial bloodmeal to approximately 450 female A. stephensi in 5 gallon cartons in the presence or absence of 0.05 mmol l−1 MnTBAP (experiment 5 in Table 2 and Fig. 3). Controls included mosquitoes provided with insulin buffer alone or with MnTBAP alone in the artificial meal. As noted in Table 2, insulin treatment in experiment 5 increased mosquito mortality compared with the buffer control. When MnTBAP was provided with insulin, the mortality rate of the mosquitoes was no longer different from those fed on the buffer treatment (median lifespan for the MnTBAP + insulin was 26 days versus 28 days for the control). Treatment with MnTBAP alone also produced a survivorship curve (not shown) that was not different from the control according to the Wilcoxon statistic (P=0.064). The median lifespan for mosquitoes fed MnTBAP alone was slightly shortened (4.7%) compared with the control.
Fig. 3.
Example survivorship curve showing that human insulin provided by artificial bloodmeal increased the mortality of A. stephensi relative to controls and that this effect was reversed by the provision of the cell-permeable SOD mimetic agent MnTBAP in the presence of insulin. This example corresponds to experiment 5, Table 2. Female mosquitoes were provided with 1.7×10−3 μmol l−1 human insulin, 0.05 mmol l−1 MnTBAP alone (not shown), human insulin + MnTBAP, or with an equivalent volume of insulin buffer by artificial bloodmeal every Monday, Wednesday and Friday until all insects were dead. Oviposition cups and 10% sucrose pads were provided between bloodmeals. Dead insects were counted and removed daily from treatment and control cartons.
Insulin-induced H2O2 synthesis in A. stephensi cells
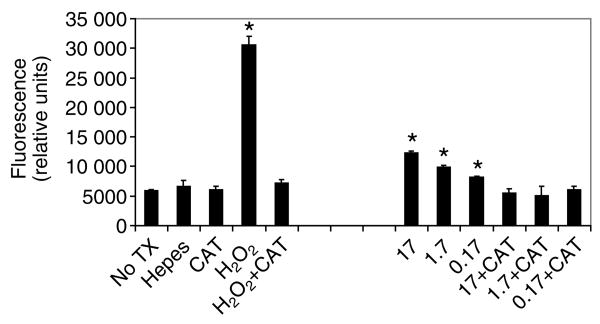
Based on our in vivo assays that suggested the insulin-dependent increase in mortality was due to increased oxidative stress, we examined the effects of insulin on H2O2 synthesis in A. stephensi MSQ43 cells in vitro. H2O2 synthesis has been studied in human HepG2 cells (Carnesecchi et al., 2006) and human fibroblasts (Ceolotto et al., 2004) treated with 100 nmol l−1 and 1 μmol l−1 insulin, concentrations that are 6(×103)-fold and 6(×104)-fold higher than normal fasting levels (Darby et al., 2001). In our assays, cells were stimulated with insulin at 0.17, 1.7 or 17 μmol l−1 [104- to 106-fold higher than fasting levels (Darby et al., 2001)]. MSQ43 cells showed dose-dependent increases in H2O2 production in response to human insulin (Fig. 4); these increases were consistent with 30–75% increases in H2O2 synthesis reported for human cells (Carnesecchi et al., 2006; Ceolotto et al., 2004). Heat inactivation of insulin eliminated these effects (not shown) and pre-treatment of cells with catalase prevented insulin-stimulated production of H2O2 (Fig. 4). Identical results were obtained with a second A. stephensi cell line, ASE (data not shown).
Fig. 4.
Human insulin dose-dependently induced hydrogen peroxide synthesis in A. stephensi MSQ43 cells. MSQ43 cells were stimulated with human insulin (Ins, 0.17–17 μmol l−1) and assayed for H2O2 using DCF-DA. Controls included no treatment (No TX), treatment with an equivalent volume of insulin buffer (Hepes), treatment with 100 units ml−1 catalase (CAT), treatment with 500 μmol l−1 H2O2, and pre-treatment with catalase (+CAT) followed by treatment with H2O2 or insulin. Data are represented as mean relative fluorescence units ± s.e.m. (N=3). An asterisk denotes a significant difference (α=0.05) between treatment and no treatment (No TX).
Reduction in SOD activity in mosquito tissues following dietary provision of insulin
The induction of H2O2 synthesis by insulin stimulation of A. stephensi cells suggested that increased mortality following insulin feeding could result from enhanced synthesis of ROS in vivo. However, the effects of insulin were observed in vitro. Further, oxidative stress is influenced not only by ROS levels but also by antioxidant activity. In C. elegans and in mammals, MnSOD is a mitochondrial antioxidant that is negatively regulated by the IIS (Murphy et al., 2003; Brown-Borg et al., 2002). Further, enhanced MnSOD activity via inhibition of IIS has been associated with increased lifespan in C. elegans and in D. melanogaster (Murphy et al., 2003; Clancy et al., 2001). These observations suggested that insulin-induced mortality in A. stephensi could result from decreased MnSOD activity in insulin-fed mosquitoes.
To test this hypothesis, we repeated our feeding studies with 100 mosquitoes each in 1 gallon cartons provided with a physiological concentration (1.7×10−4 μmol l−1) of insulin or insulin buffer in 10% sucrose-soaked cotton pads. Every 3 days, samples of insects from each group were assayed for SOD activity. Treatments were compared using a 3-way factorial design with the main effects being day (the age of the mosquitoes at the time of sampling), replicate and treatment. There was no significant effect of day (F=0.87, d.f.=10, P=0.56), but the main effects of replicate (F=116.65, d.f.=2, P<0.001) and treatment (F=30.38, d.f.=3, P<0.001) were both significant. There was a significant interaction between day and treatment (F=2.90, d.f.=18, P=0.001). When treatments were compared using the Student–Neuman–Keuls test (α=0.05), both total SOD and MnSOD activities in insulin-fed mosquitoes were reduced relative to the controls (Fig. 5).
Fig. 5.
Human insulin provided in sucrose decreased total superoxide dismutase (SOD) and MnSOD activities in A. stephensi. Female mosquitoes were provided with 10% sucrose-soaked cotton pads with 1.7×10−4 μmol l−1 human insulin or with an equivalent volume of insulin buffer in sucrose. Cotton pads were changed twice daily. Every third day, insulin-fed and control mosquitoes were sampled for total SOD activity; the same samples were treated with KCN and re-analyzed to quantify MnSOD activity. Data are represented as mean enzyme activities for N=3 replicates.
Human insulin activates ERK and Akt/PKB in the A. stephensi midgut epithelium
Based on our observations, we reasoned that human insulin was functioning, in part, by activating IIS proteins in the midgut, the tissue most proximal to ingested blood. To address this question, four groups of female A. stephensi were provided with artificial bloodmeals supplemented with 1.7, 1.7×10−3 or 1.7×10−5 μmol l−1 human insulin or with artificial bloodmeals supplemented with equivalent volumes of insulin buffer as controls. Relative to controls, dose-dependent increases in phosphorylated (activated) ERK and Akt/PKB were observed at 30 min after feeding in groups provided with 1.7, 1.7×10−3 and 1.7×10−5 μmol l−1 insulin (Fig. 6).
Fig. 6.
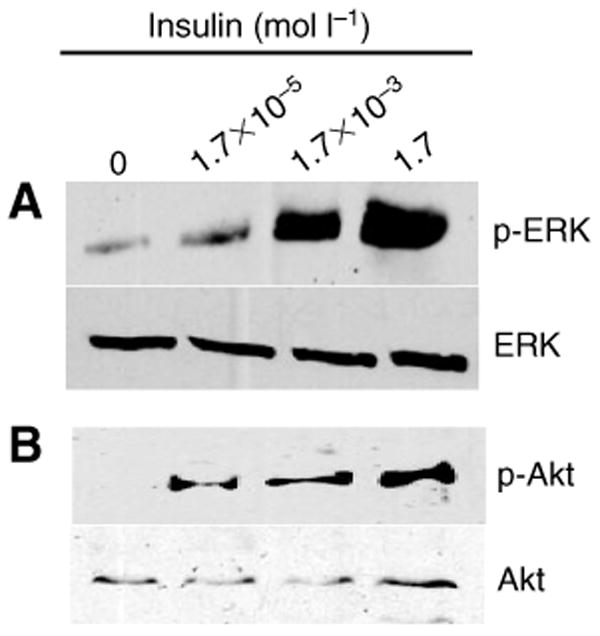
Human insulin stimulated extracellular signal-regulated kinase (ERK, A) and Akt/protein kinase B (PKB, B) phosphorylation in the A. stephensi midgut. Midgut proteins prepared from A. stephensi at 30 min after feeding on artificial bloodmeal supplemented with human insulin were probed with anti-phospho-ERK antisera (p-Erk, 1:10 000; A) or with anti-phospho-Akt antibody (p-Akt, 1:1000; B). Anti-total ERK (ERK) or anti-total Akt/PKB (Akt) antibody was used to assess loading.
Discussion
In our studies, we demonstrated that human insulin, provided as a dietary supplement to mosquitoes at levels found in circulating human blood, decreased the lifespan of these insects relative to controls. Interestingly, insulin had a greater effect on median lifespan when provided via artificial bloodmeal than in sucrose. In related work, we have observed that multiple blood-derived components can activate intersecting signaling pathways to regulate mosquito physiology (Akman-Anderson et al., 2007), suggesting that cross-talking factors from blood are required for maximal insulin action. In addition to blood-specific factors, the consumption of blood may initiate endogenous responses in the mosquito that are critical to the action of ingested insulin. We discuss one of these possibilities below.
Our results with insulin are consistent with the prediction from numerous studies on lifespan extension in model organisms that enhanced insulin signaling would lead to a shortened lifespan (reviewed in Giannakou and Partridge, 2007; Luckhart and Riehle, 2007). For example, mutations in daf-16, which mimic exclusion of this transcription factor from the nucleus during insulin signaling, shorten lifespan in C. elegans (Ogg et al., 1997). However, our studies differ from previous studies of genetic mutants of C. elegans and D. melanogaster in that insulin is a normal constituent of the mosquito bloodmeal, suggesting that ingested insulin regulates the normal physiology of A. stephensi under natural conditions.
Analyses of H2O2 synthesis by A. stephensi cells in vitro suggest that the reduction in mosquito lifespan by dietary insulin may result directly from enhanced oxidative stress. Indirect effects of insulin-induced H2O2, however, have also been described. Specifically, Mahadev et al. (Mahadev et al., 2001a; Mahadev et al., 2001b) demonstrated that insulin-induced H2O2 not only inhibits redox-sensitive protein-tyrosine phosphatases that regulate phosphorylation at early points in signaling, but also regulates the activation of PI-3K and Akt/PKB in the downstream signaling pathway, indicating that insulin-induced H2O2 can function as a feed-forward signal to enhance the downstream effects of signaling.
Although insulin-induced H2O2 may only indirectly affect survivorship via an impact on signaling, our data also showed that dietary human insulin down-regulated total SOD and MnSOD activity in vivo over the lifespan of the mosquito, effects that would be predicted to directly enhance oxidative stress in affected tissues. Our results are consistent with previous microarray data from C. elegans that linked insulin signaling with antioxidant gene expression (Murphy et al., 2003) and also a recent study of the effects of IIS on MnSOD expression and activity in rat cells (Li et al., 2006). Specifically, Li et al. (Li et al., 2006) demonstrated that insulin-like growth factor-1 induced Akt-dependent phosphorylation of forkhead transcription factor FOXO3a in rat cells. Phosphorylation excluded FOXO3a from a binding site on the MnSOD promoter, resulting in reduced expression and levels of MnSOD (Li et al., 2006).
Total SOD activity is composed of multiple enzymes including MnSOD, which is located in the mitochondria, two forms of Cu/ZnSOD and FeSOD. Among the cellular antioxidants, MnSOD has perhaps been the most closely associated with aging or senescence due to its localization in the mitochondria, the source of metabolic ROS. As such, a large body of data (Sanz et al., 2006) supports the ‘mitochondrial free radical theory of aging’ which posits that free radical damage to mitochondrial DNA is directly linked to aging. We hypothesized from our data, therefore, that a deficiency in MnSOD activity could enhance free radical damage of mosquito mitochondrial proteins and decrease survivorship in insulin-fed mosquitoes. We tested this hypothesis by provision of MnTBAP in the presence and absence of insulin via artificial bloodmeal. We had expected that the antioxidant activity of MnTBAP would result in a longer lifespan in insects fed only the antioxidant relative to the buffer controls, so were surprised to see a decrease in median lifespan of 4.7%. Although this was somewhat unexpected, Magwere et al. (Magwere et al., 2006) also observed that exogenous antioxidants did not extend the lifespan of normal, wild-type D. melanogaster. Further, Bayne et al. (Bayne et al., 2005) reported that while overexpression of MnSOD and catalase in D. melanogaster protected the insects from experimental oxidative stress, normal lifespan and physical fitness were adversely affected, suggesting that some level of ROS is essential for normal physiological processes. In contrast to the effects of MnTBAP alone, reversal of the effects of insulin by provision of MnTBAP, which has been used to complement a lack of mitochondrial SOD in mutant mice (Melov et al., 1998), supports a hypothesis of insulin-induced mitochondrial damage.
While the direct effects of exogenous insulin are important, these cannot be studied to the exclusion of possible indirect effects on the endogenously produced mosquito ILPs. The existence of seven A. gambiae ILP genes and eight A. aegypti ILP genes with complex expression patterns in multiple tissues (Riehle et al., 2006; Krieger et al., 2004) suggests that the same is true for A. stephensi. The biological roles of these peptides are not yet known, but we suggest that cross-talk is likely between ingested insulin and the endogenous ILPs. Specifically, a number of studies indicate that mammalian insulin action is mediated by positive feedback that enhances insulin secretion and perhaps also insulin biosynthesis (Leibiger et al., 2002). In this light, it is possible that ingested insulin not only activates the A. stephensi IIS for direct downstream effects but also activates the secretion and perhaps biosynthesis of A. stephensi ILPs that enhance or extend these effects to other tissues. For example, signal propagation from the midgut could include downstream effectors of the IIS (e.g. H2O2) and mosquito ILPs that are synthesized and released to propagate these effects in other tissues.
In the field, only approximately 6% of A. gambiae survive to 14 days (Gillies and Wilkes, 1965), while mark–release–recapture studies suggest that the percentage of A. stephensi surviving to an age of 12 days is less than 1% (Quraishi et al., 1966; Reisen and Aslamkhan, 1979). These observations suggest that these important malaria parasite vectors in sub-Saharan Africa (A. gambiae) and southeast Asia, India and parts of the Middle East (A. stephensi) live only a few days longer than the 10 day EIP required for the completion of parasite development. As such, a 15–20% decrease in lifespan, as we have observed for A. stephensi fed insulin in blood, could have significant epidemiological implications. Further, the mosquitoes that are most likely to transmit malaria parasites are those that ingest an infective bloodmeal within 2 days of emergence as adults. Significance by the Wilcoxon test (Table 2) suggests that mortality at early times contributes to the major effects of insulin on survivorship. In addition, our measurements of mortality rate showed that mosquitoes that fed on blood supplemented with insulin suffered a higher probability of death nearly every day during this critical 2 week period. Young female mosquitoes that ingest malaria parasite-infected meals from hosts with elevated insulin levels in the first few days after emergence, therefore, will experience higher mortality than would mosquitoes consuming blood from uninfected hosts, an effect that could also impact on parasite transmission.
A significant proportion of countries in sub-Saharan Africa report human malaria prevalence in excess of 50% during 4–6 month transmission seasons (Omumbo et al., 2005; Gemperli et al., 2006). These observations would suggest that our experimental design of provision of effective concentrations of insulin at each bloodmeal for the duration of mosquito lifespan could faithfully represent feeding conditions in highly endemic areas, with the prediction that natural mortality patterns of feeding mosquitoes could be impacted. In this light, we suggest that IIS, driven by natural feeding behavior, has a significant impact on the capacity of anopheline mosquitoes to successfully transmit malaria parasites. As such, intervention strategies that are cognizant of these effects can for the first time turn the evolutionarily conserved physiologies of invertebrate and human hosts into gain for malaria transmission control.
Acknowledgments
We thank Dr M. Riehle and Dr M. Brown for help with the manuscript and N. Soonthornpong and K. W. Cheung for technical support. This work was supported by NIH grants R01AI60664 and R01AI50663 to S.L. in a facility constructed with support from NIH NCRR C06 RR-12088-01.
List of Symbols and Abbreviations
- ATP
adenosine 5′-triphosphate
- DCF-DA
5,6-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
- DSOR1
mitogen-activated protein kinase kinase
- EIP
extrinsic incubation period
- ERK
extracellular signal-regulated kinase
- H2O2
hydrogen peroxide
- HRP
horseradish peroxidase
- IIS
insulin/insulin-like growth factor signaling
- ILPs
insulin-like peptides
- INR
insulin receptor
- MnTBAP
manganese (III) tetrakis (4-benzoic acid) porphyrin
- PI-3K
phosphatidylinositol 3-kinase
- PKB
protein kinase B
- ROS
reactive oxygen species
- SOD
superoxide dismutase
References
- Akman-Anderson L, Vodovotz Y, Zamora R, Luckhart S. Bloodfeeding as an interface of mammalian and arthropod immunity. In: Beckage NA, editor. Insect Immunology. San Diego: Academic Press; 2007. pp. 151–180. [Google Scholar]
- Allison PD. Survival Analysis Using the SAS System: A Practical Guide. Cary, NC: SAS Publishers; 1995. [Google Scholar]
- Ayyadevara S, Dandapat A, Singh SP, Benes H, Zimniak L, Reis RJ, Zimniak P. Lifespan extension in hypomorphic daf-2 mutants of Caenorhabditis elegans is partially mediated by glutathione transferase CeGSTP2-2. Aging Cell. 2005;4:299–307. doi: 10.1111/j.1474-9726.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- Baumeister R, Schaffitzel E, Hertweck M. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J Endocrinol. 2006;190:191–202. doi: 10.1677/joe.1.06856. [DOI] [PubMed] [Google Scholar]
- Bayne AC, Mockett RJ, Orr WC, Sohal RS. Enhanced catabolism of mitochondrial superoxide/hydrogen peroxide and aging in transgenic Drosophila. Biochem J. 2005;391:277–284. doi: 10.1042/BJ20041872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier MS, Pumpuni CB, Beier JC, Davis JR. Effects of para-aminobenzoic acid, insulin, and gentamicin on Plasmodium falciparum development in anopheline mosquitoes (Diptera: Culicidae) J Med Entomol. 1994;31:561–565. doi: 10.1093/jmedent/31.4.561. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Romanick MA, Kennedy MA. Effects of growth hormone and insulin-like growth factor-1 on hepatocyte antioxidative enzymes. Exp Biol Med. 2002;227:94–104. doi: 10.1177/153537020222700203. [DOI] [PubMed] [Google Scholar]
- Carnesecchi S, Carpentier JL, Foti M, Szanto I. Insulin-induced vascular endothelial growth factor expression is mediated by the NADPH oxidase NOX3. Exp Cell Res. 2006;312:3413–3424. doi: 10.1016/j.yexcr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Caro HN, Sheikh NA, Taverne J, Playfair JH, Rademacher TW. Structural similarities among malaria toxins insulin second messengers, and bacterial endotoxin. Infect Immun. 1996;64:3438–3441. doi: 10.1128/iai.64.8.3438-3441.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceolotto G, Bevilacqua M, Papparella I, Baritono E, Franco L, Corvaja C, Mazzoni M, Semplicini A, Avogaro A. Insulin generates free radicals by an NAD(P)H, phosphatidylinositol 3′-kinase-dependent mechanism in human skin fibroblasts ex vivo. Diabetes. 2004;53:1344–1351. doi: 10.2337/diabetes.53.5.1344. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Darby SM, Miller ML, Allen RO, LeBeau M. A mass spectrometric method for quantitation of intact insulin in blood samples. J Anal Toxicol. 2001;25:8–14. doi: 10.1093/jat/25.1.8. [DOI] [PubMed] [Google Scholar]
- Elased K, Playfair JH. Hypoglycemia and hyperinsulinemia in rodent models of severe malaria infection. Infect Immun. 1994;62:5157–5160. doi: 10.1128/iai.62.11.5157-5160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elased KM, Playfair JH. Reversal of hypoglycaemia in murine malaria by drugs that inhibit insulin secretion. Parasitology. 1996;112:515–521. doi: 10.1017/s0031182000066087. [DOI] [PubMed] [Google Scholar]
- Faulkner KM, Liochev SI, Fridovich I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J Biol Chem. 1994;269:23471–23476. [PubMed] [Google Scholar]
- Garsin DA, Villanueva JM, Begun J, Kim DH, Sifri CD, Calderwood SB, Ruvkun G, Ausubel FM. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science. 2003;300:1921. doi: 10.1126/science.1080147. [DOI] [PubMed] [Google Scholar]
- Gemperli A, Sogoba N, Fondjo E, Mabaso M, Bagayoko M, Briet OJ, Anderegg D, Liebe J, Smith T, Vounatsou P. Mapping malaria transmission in West and Central Africa. Trop Med Int Health. 2006;11:1032–1046. doi: 10.1111/j.1365-3156.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci. 2007;32:180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Gillies MT, Wilkes TJ. A study of the age-composition of populations of Anopheles gambiae Giles and A. funestus Giles in North-Eastern Tanzania. Bull Entomol Res. 1965;56:237–262. doi: 10.1017/s0007485300056339. [DOI] [PubMed] [Google Scholar]
- Guinovart C, Navia MM, Tanner M, Alonso PL. Malaria: burden of disease. Curr Mol Med. 2006;6:137–140. doi: 10.2174/156652406776055131. [DOI] [PubMed] [Google Scholar]
- Humphries KM, Szweda PA, Szweda LI. Aging: a shift from redox regulation to oxidative damage. Free Radic Res. 2006;40:1239–1243. doi: 10.1080/10715760600913184. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Keaney M, Matthijssens F, Sharpe M, Vanfleteren J, Gems D. Superoxide dismutase mimetics elevate superoxide dismutase activity in vivo but do not retard aging in the nematode Caenorhabditis elegans. Free Radic Biol Med. 2004;37:239–250. doi: 10.1016/j.freeradbiomed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Krieger MJ, Jahan N, Riehle MA, Cao C, Brown MR. Molecular characterization of insulin-like peptide genes and their expression in the African malaria mosquito, Anopheles gambiae. Insect Mol Biol. 2004;13:305–315. doi: 10.1111/j.0962-1075.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- Kurtti TJ, Munderloh UG. Advances in the Definition of Culture Media for Mosquito Cells. Boca Raton: CRC Press; 1989. [Google Scholar]
- Leibiger IB, Leibiger B, Berggren PO. Insulin feedback action on pancreatic beta-cell function. FEBS Lett. 2002;532:1–6. doi: 10.1016/s0014-5793(02)03627-x. [DOI] [PubMed] [Google Scholar]
- Li M, Chiu JF, Mossman BT, Fukagawa NK. Down-regulation of manganese-superoxide dismutase through phosphorylation of FOXO3a by Akt in explanted vascular smooth muscle cells from old rats. J Biol Chem. 2006;281:40429–40439. doi: 10.1074/jbc.M606596200. [DOI] [PubMed] [Google Scholar]
- Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- Lim J, Gowda DC, Krishnegowda G, Luckhart S. Induction of nitric oxide synthase in Anopheles stephensi by Plasmodium falciparum: mechanism of signaling and the role of parasite glycosylphosphatidylinositols. Infect Immun. 2005;73:2778–2789. doi: 10.1128/IAI.73.5.2778-2789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- Luckhart S, Riehle MA. The insulin signaling cascade from nematodes to mammals: insights into innate immunity of Anopheles mosquitoes to malaria parasite infection. Dev Comp Immunol. 2007;31:647–656. doi: 10.1016/j.dci.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magwere T, West M, Riyahi K, Murphy MP, Smith RA, Partridge L. The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech Ageing Dev. 2006;127:356–370. doi: 10.1016/j.mad.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Mahadev K, Wu X, Zilbering A, Zhu L, Lawrence JT, Goldstein BJ. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J Biol Chem. 2001a;276:48662–48669. doi: 10.1074/jbc.M105061200. [DOI] [PubMed] [Google Scholar]
- Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001b;276:21938–219342. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- Melov S, Schneider JA, Day BJ, Hinerfeld D, Coskun P, Mirra SS, Crapo JD, Wallace DC. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat Genet. 1998;18:159–163. doi: 10.1038/ng0298-159. [DOI] [PubMed] [Google Scholar]
- Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Murphy CT, Lee SJ, Kenyon C. Tissue entrainment by feedback regulation of insulin gene expression in the endoderm of Caenorhabditis elegans. Proc Natl Acad Sci USA. 2007 doi: 10.1073/pnas.0709613104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Omumbo JA, Hay SI, Snow RW, Tatem AJ, Rogers DJ. Modelling malaria risk in East Africa at high-spatial resolution. Trop Med Int Health. 2005;10:557–566. doi: 10.1111/j.1365-3156.2005.01424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planche T, Dzeing A, Ngou-Milama E, Kombila M, Stacpoole PW. Metabolic complications of severe malaria. Curr Top Microbiol Immunol. 2005;295:105–136. doi: 10.1007/3-540-29088-5_5. [DOI] [PubMed] [Google Scholar]
- Quraishi MS, Esghi N, Faghih MA. Flight range, lengths of gonotrophic cycles, and longevity of P-32-labeled Anopheles stephensi mysorensis. J Econ Entomol. 1966;59:50–55. doi: 10.1093/jee/59.1.50. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Aslamkhan M. A release-recapture experiment with the malaria vector, Anopheles stephensi Liston, with observations on dispersal, survivorship, population size, gonotrophic rhythm and mating behaviour. Ann Trop Med Parasitol. 1979;73:251–269. doi: 10.1080/00034983.1979.11687255. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Brown MR. Insulin stimulates ecdysteroid production through a conserved signaling cascade in the mosquito Aedes aegypti. Insect Biochem Mol Biol. 1999;29:855–860. doi: 10.1016/s0965-1748(99)00084-3. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Brown MR. Insulin receptor expression during development and a reproductive cycle in the ovary of the mosquito Aedes aegypti. Cell Tissue Res. 2002;308:409–420. doi: 10.1007/s00441-002-0561-8. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Brown MR. Molecular analysis of the serine/threonine kinase Akt and its expression in the mosquito Aedes aegypti. Insect Mol Biol. 2003;12:225–232. doi: 10.1046/j.1365-2583.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Fan Y, Cao C, Brown MR. Molecular characterization of insulin-like peptides in the yellow fever mosquito, Aedes aegypti: expression, cellular localization, and phylogeny. Peptides. 2006;27:2547–2560. doi: 10.1016/j.peptides.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Sampayo JN, Olsen A, Lithgow GJ. Oxidative stress in Caenorhabditis elegans: protective effects of superoxide dismutase/catalase mimetics. Aging Cell. 2003;2:319–326. doi: 10.1046/j.1474-9728.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- Sanz A, Pamplona R, Barja G. Is the mitochondrial free radical theory of aging intact? Antioxid Redox Signal. 2006;8:582–599. doi: 10.1089/ars.2006.8.582. [DOI] [PubMed] [Google Scholar]
- Schofield L, Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993;177:145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Carey J. Nutrition mediates reproductive trade-offs with age-specific mortality in the beetle Callosobruchus maculatus. Ecology. 1995;76:2066–2073. [Google Scholar]
- White NJ, Warrell DA, Chanthavanich P, Looareesuwan S, Warrell MJ, Krishna S, Williamson DH, Turner R. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983;309:61–66. doi: 10.1056/NEJM198307143090201. [DOI] [PubMed] [Google Scholar]
- White NJ, Miller KD, Marsh K, Berry CD, Turner RC, Williamson DH, Brown J. Hypoglycaemia in African children with severe malaria. Lancet. 1987;1:708–711. doi: 10.1016/s0140-6736(87)90354-0. [DOI] [PubMed] [Google Scholar]
- Whitten MM, Shiao SH, Levashina EA. Mosquito midguts and malaria: cell biology, compartmentalization and immunology. Parasite Immunol. 2006;28:121–130. doi: 10.1111/j.1365-3024.2006.00804.x. [DOI] [PubMed] [Google Scholar]