Abstract
The posterodorsal portion of the medial amygdala (MePD) is sexually dimorphic in several rodent species. In several other brain nuclei, astrocytes change morphology in response to steroid hormones. We visualized MePD astrocytes using glial-fibrillary acidic protein (GFAP) immunocytochemistry. We compared the number and process complexity of MePD astrocytes in adult wildtype male and female rats and testicular feminized mutant (TFM) male rats that lack functional androgen receptors (ARs) to determine whether MePD astrocytes are sexually differentiated and whether ARs have a role. Unbiased stereological methods revealed laterality and sex differences in MePD astrocyte number and complexity. The right MePD contained more astrocytes than the left in all three genotypes, and the number of astrocytes was also sexually differentiated in the right MePD, with males having more astrocytes than females. In contrast, the left MePD contained more complex astrocytes than did the right MePD in all three genotypes, and males had more complex astrocytes than females in this hemisphere. TFM males were comparable to wildtype females, having fewer astrocytes on the right and simpler astrocytes on the left than do wildtype males. Taken together, these results demonstrate that astrocytes are sexually dimorphic in the adult MePD and that the nature of the sex difference is hemisphere-dependent: a sex difference in astrocyte number in the right MePD and a sex difference in astrocyte complexity in the left MePD. Moreover, functional ARs appear to be critical in establishing these sex differences in MePD astrocyte morphology.
Keywords: androgen, glia, adult plasticity, stereology, testicular feminization mutation, astrocytes
The amygdala is implicated in the control of many social behaviors, including fear, anxiety, and social interactions (LeDoux et al., 1998; Bishop et al., 2004; Adolphs et al., 2005; Williams et al., 2005; Mah et al., 2007; Spezio et al., 2007; Truit et al., 2007). Interestingly, many behavioral disorders in which the amygdala has been implicated have clear sex biases in the human population. For example, schizophrenia, autism, and drug addiction are all more common in males, while anxiety disorders and depression are more common in females (American Psychiatric Association, 2000). Many aspects of the amygdala are sexually dimorphic in a variety of mammalian species, lending support to the idea that structural differences in the brain may underlie biases in behavior and disease susceptibility, and that the amygdala may be a key player in the control of such behaviors.
In rats the medial amygdala is composed of several subnuclei that are extensively interconnected (Grove, 1988a,b; Alheid et al., 1995; Shammah-Lagnado et al., 2000). The posterodorsal medial amygdala (MePD) in particular receives input from chemosensory and hypothalamic regions and projects to the preoptic area and ventral medial hypothalamic nucleus, both of which play important roles in reproductive behaviors. The MePD normally contains a high concentration of both androgen receptors (ARs) and estrogen receptors (ERs; Simerly et al., 1990), and is highly sexually dimorphic in adults. The MePD in male rats has a larger overall volume (Hines et al., 1992), contains larger neuronal somata, more neurons (Morris et al., 2008), and greater dendritic spine density than the MePD in female rats (Rasia-Filho et al., 2004). The adult MePD is also highly plastic. Castration of adult male rats eliminates the sex difference in regional volume and neuronal soma size (Cooke et al., 1999) and causes deficits in noncontact erections and ultrasonic vocalizations, while treatment with androgens and/or estrogens prevents these changes following castration (Cooke et al., 2003).
Typically, estrogen acting on ERs is regarded as the pathway responsible for hormone-induced masculinization in the rodent brain. However, recent results indicate that AR also contributes to sexual differentiation of many brain regions, including the MePD. Testicular feminized mutant (TFM) male rats lack functional AR and are insensitive to androgens (Yarbrough et al., 1990), and thus represent an important tool for assessing the role of androgens in the origin of neural sex differences. We have found that several aspects of MePD morphology are only partially masculinized in TFM males, indicating that ARs are necessary for complete masculinization of this nucleus (Morris et al., 2005), but where androgens act to masculinize the MePD has not been established.
Gonadal hormones not only influence neuronal morphology, but also modulate the morphology of nonneuronal cells such as astrocytes, which are thought to contribute critically to some forms of neural plasticity. For example, estrogens increase the complexity of astrocyte arbors in the arcuate nucleus and preoptic area of the hypothalamus (reviewed in Garcia-Segura et al., 1994; Garcia-Segura and McCarthy, 2006). Moreover, androgens increase the number of astrocytes in hippocampal cultures (Gatson and Singh, 2007). Astrocytes are also known to express steroid hormone receptors, including both ARs and ERs (Doncarlos et al., 2005; Lorenz et al., 2005). Thus, steroid hormones may act directly on astrocytes to regulate their morphology and function. Given the implicated role of astrocytes in adult neural plasticity, their steroid responsiveness in some brain regions, and the highly plastic nature of the adult MePD, we speculated that astrocytes might exhibit sexually dimorphic morphologies in the MePD. Indeed, reports of sex differences in the MePD in both the density of glial fibrillary acidic protein (GFAP) immunoreactivity and in the number of Nissl-stained glial cells (Martinez et al., 2006; Morris et al., 2008) suggest that MePD astrocytes may be sexually dimorphic in both number and process complexity. We now report sex differences in both measures, and that TFM males are like females in having fewer and less complex MePD astrocytes than wildtype males, indicating that both the number and complexity of MePD astrocytes in rats are normally masculinized through the activation of ARs.
MATERIALS AND METHODS
Animals
Ninety to 120-day-old wildtype male, wildtype female, and TFM male rats were obtained from our TFM colony at Michigan State University. Female carriers of this spontaneous mutation of the AR gene have been bred with Long Evans male rats from Charles Rivers (Portage, MI) for over 10 generations. Estrous cycle was not monitored for the females used in this study, thus differences in ovarian hormones were not accounted for. Animals were housed three per cage in a single colony room in standard rat cages with food and water available ad libitum. Lights were on at 0700 and off at 1900 hours. Animals were cared for in accordance with the guidelines set forth by the National Institutes of Health and all procedures were approved by the Institutional Animal Care and Use Committee at Michigan State University.
Animals were given an overdose of sodium pentobarbital (120 mg/kg, ip). Once the animals were deeply anesthetized (showing no reflex response to either tail or foot pinch) they were intracardially perfused with 0.9% saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4, ≈250 mL/animal). The brains were then removed and postfixed for 5 hours at 4°C in the same fixative solution and stored in 30% phosphate-buffered sucrose at 4°C for 48 hours. The left cortex of each brain was scored with a shallow cut to mark that hemisphere. Brains were sectioned coronally on a freezing microtome at 40 μm through the region of interest. Sections were collected into cryoprotectant (de Olmos et al., 1978) and stored at -20°C. Every third section through the rostrocaudal extent of the MePD was processed for immunocytochemistry.
Histology
For immunocytochemistry, 0.1 M phosphate-buffered saline with 0.3% Triton X-100 (PBS-T; 140 mM NaCl, 10.7 mM KCl, 1 mM KH2PO4, 10 mM Na2HPO4, pH 7.4) served as a rinsing agent between all incubations and as a dilutant. Upon removal from cryoprotectant, tissue was placed into Netwell plates (Corning Life Sciences) and thoroughly rinsed. Sections were then incubated for 20 minutes in 1% sodium borohydride followed by a 25-minute incubation in 1% H2O2, 1-hour incubation in 10% normal horse serum (Chemicon, Temecula, CA), and a 24-hour incubation at 4°C in mouse anti-GFAP monoclonal antiserum (1:50,000, Chemicon, MAB360). The antiserum was prepared against purified GFAP from porcine spinal cord. In Western blots the antiserum recognizes a single band at ≈51 kDA (manufacturer’s technical information). Staining of sections through the amygdala and hypothalamus produced a pattern of GFAP immunoreactivity that was identical to other descriptions (Mong et al., 1999; Rasia-Filho et al., 1999; Martinez et al., 2006).
Following incubation in primary antiserum, tissue was incubated in biotinylated rat-absorbed horse antimouse secondary antiserum (1:500, Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. This was followed by a 1-hour incubation in peroxidase avidinbiotin complex solution at half the recommended strength (Elite ABC kit, Vector). Horseradish peroxidase (HRP) was visualized using NiCl-enhanced diaminobenzidine (DAB, Sigma, St. Louis, MO) in a 0.05 M in Tris Buffer, pH 7.2. After rinsing to quench the peroxidase reaction, tissue was mounted on gel-subbed slides, dried, dehydrated in alcohol rinses, and counterstained using Harris hematoxylin solution (Sigma), with 10% lithium carbonate used as the bluing agent. Slides were then coverslipped with Permount.
Stereological analysis
A Zeiss Axioplan II microscope with the visual field captured by an Optronics MicroFire digital video camera and displayed on a monitor was used to quantify the number and complexity of astrocytes within the region of interest. Using StereoInvestigator software (v. 7.0, MBF Bioscience, Williston, VT), the perimeter of the MePD was traced in serial sections at low magnification. MePD boundaries were identified using the standard rat atlas (Paxinos and Watson, 2005), in conjunction with previously established standards within our laboratory (Morris et al., 2005, 2007) and others (Hines et al., 1992; Canteras et al., 1995). Several consistent morphological features were used to accurately identify and trace the MePD, including the angle and length of the optic tracts, the overlap of the optic tract with the cerebral peduncle, the descent of the stria terminalis into adjacent regions, and the dense collection of neurons forming the posteroventral medial amygdala. After tracing the boundaries of the MePD at low magnification, astrocytes were counted and traced using a 100× Plan-NeoFluar, 1.3 N.A. oilimmersion objective. Slides were coded to ensure that all measures were performed by an observer who was “blind” to the group membership of each animal. Images of astrocytes were captured using the image capture option in StereoInvestigator. Images were resized and adjusted using Adobe Photoshop (v. 7.0, San Jose, CA). All images received a +5 increase in brightness through a brightness/contrast adjustment layer available in the software.
Astrocyte number
An optical fractionator probe (West et al., 1991) was used to generate an unbiased estimate of astrocyte numbers within the boundaries of the MePD. The optical fractionator estimates the total number of objects within a space based on a sample representing a known fraction of that space. The method utilizes a 3D probe with a fixed, specified volume inserted into the tissue. Initial probe insertion is random within the region of interest but then proceeds at fixed intervals allowing for an unbiased and accurate estimate of the number of objects within the entire region of interest. Probe dimensions were 45 × 45 μm with a height of 6–9 μm. The coefficient of error (CE; Gunderson m = 1) for each hemisphere was at or below 0.10.
Criteria for identifying an astrocyte included a distinct and recognizable nucleus in a plane of focus within the sampling probe’s Z-value, and from which at least two GFAP-labeled fibers extended. Such nuclei were often, although not always, smaller than surrounding (presumably neuronal) nuclei, often elliptical in shape, and lacking a clear nucleolus. At times, especially around vascular pathways and at cortical boundaries, GFAP immunoreactivity was dense enough to obscure the nucleus of origin, and in these cases no cells were marked or traced. These methods produced estimates of overall MePD volume, number of astrocytes, and average area of astrocyte nuclei per hemisphere for each subject. The number of cells counted per hemisphere ranged from ≈250 – 400 depending on the sex and hemisphere of the subject.
Astrocyte process complexity
Neurolucida software (v. 7.0, MBF Bioscience) was used to trace and measure the entire visible arbor of 24 randomly selected astrocytes per subject. The MePD was traced via the criteria and methodology described above and a fractionator probe was used to define random tracing sites throughout the MePD, with 12 sites per hemisphere. At each of these sites the astrocyte nearest the randomly placed marker, but at least 30 μm from the MePD boundary, was traced in its entirety. The average number of primary processes, average number of branch points, average number of branch endings, and average branch length were calculated for each animal.
Additionally, each astrocyte was classified into one of four groups based on overall morphological complexity following a classification scheme developed by Mong and McCarthy (1999). In brief, Group I astrocytes exhibit few and short primary processes (Fig. 1A). Group II astrocytes possess more and longer primary processes but few secondary processes (Fig. 1B). Group III possess more secondary processes and finally Group IV astrocytes have large and complex arbors with numerous secondary processes (Mong et al., 1996; Mong et al., 1999) (Fig. 1C,D). The percentage of astrocytes within the MePD that fall into each of the categories was calculated for each of the three experimental groups (male, female, and TFM male).
Fig. 1.
Photomicrographs depicting astrocytes of varying complexity. A: A Group 1 astrocyte, which has few and short processes. B: A Group 2 astrocyte, which has several processes but branches remain unelaborated. C: A Group 3 astrocyte, which has multiple elaborated processes. D: A Group 4 astrocyte, which is highly elaborated. Because of the single plane of focus depicted, only a portion of the full extent of arbor complexity is visible here. Complex astrocytes frequently demonstrated multiple branches that emerged as the plane of focus was moved through the tissue. Scale bar = 50 μm.
Statistical analysis
Separate two-way mixed-design analyses of variance (ANOVA) were conducted for each dependent variable (MePD volume, astrocyte number, number of primary processes, number of branch points, number of branch endings, and branch length). The left and right hemispheres served as a repeated measure and genotype (male, female, TFM) as a between-group measure. This was followed by one-way ANOVAs for each individual hemisphere and LSD post-hoc analysis to determine differences between genotypes on each side. For all analyses, results are expressed as mean ± SEM (standard error of the mean), and a P-value of 0.05 was considered statistically significant, with N = number of animals per group reported in figures.
RESULTS
Regional volume
Mixed design 2-way ANOVA (left and right hemisphere as repeated measures) revealed a main effect of sex on MePD volume (P < 0.01) in addition to a main effect of hemisphere (P < 0.01) and a sex by hemisphere interaction (P < 0.001). In all three groups the right hemisphere had greater regional volume than the left (males: P < 0.01; TFMs: P < 0.05; females: P < 0.05, Fig. 2A). Post-hoc analysis revealed that the volume of the MePD varied significantly across genotypes only in the right hemisphere (P < 0.05). Thus, the overall main effect of genotype is due to differences in the right but not the left hemisphere. Additional post-hoc analyses revealed that the right MePD of males has a greater volume than that of either TFM males or females (Ps <0.04, Fig. 2A) and that volume of the right MePD is not different in TFM males and females (P = 0.323). These data agree well with estimates of MePD volume based on Nissl-stained material (Morris et al., 2005, 2008), suggesting that the MePD boundaries revealed by GFAP and hematoxylin is comparable to that shown by Nissl stain.
Fig. 2.
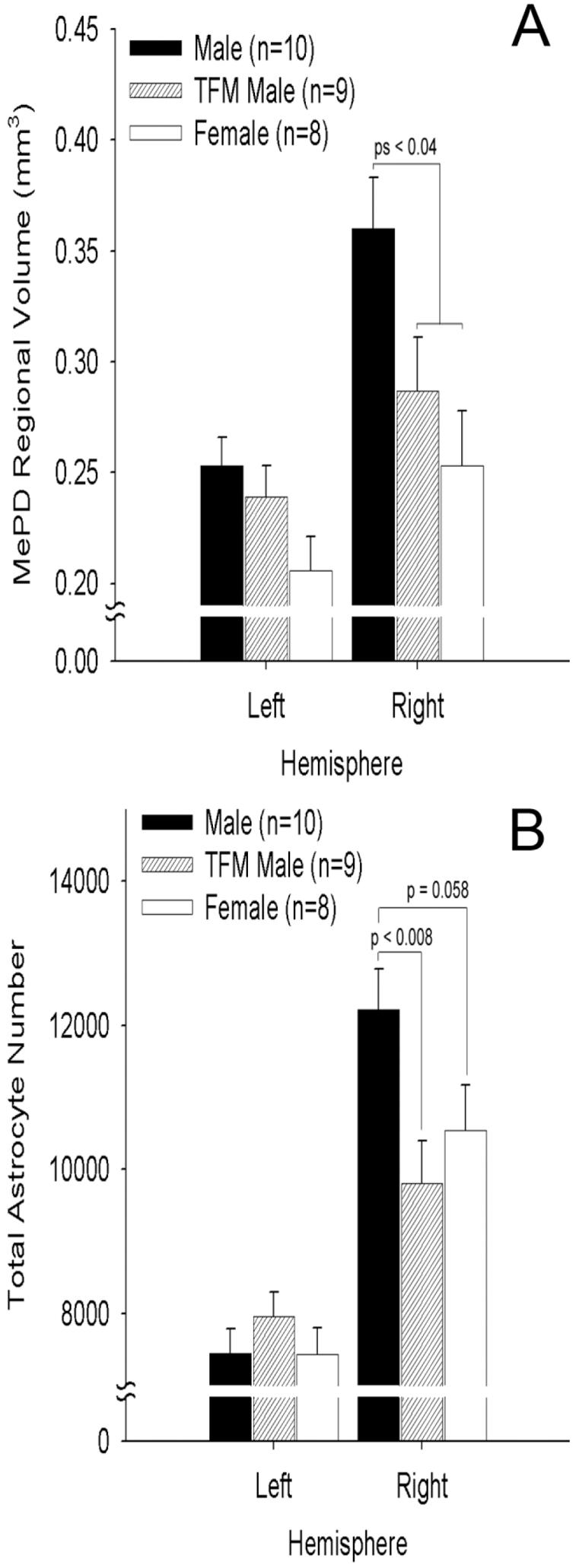
Estimated regional volume (A) and total astrocyte number (B) in the posterodorsal medial amygdala (MePD) of adult wildtype male and female rats and testicular feminized mutant (TFM) male rats, which lack functional AR. The MePD volume is significantly larger on the right than the left in all three groups, but this laterality was most dramatic in males. The right hemisphere is also significantly larger in males than in females and TFM males. The right MePD contained significantly more astrocytes than the left in all three groups (B). This laterality was most prominent in males, leading to a sex difference in astrocyte number only on the right side. Note that TFM males have fewer astrocytes than wildtype males but no more than do females in the right MePD, indicating that the sex difference in astrocyte number depends on AR. In contrast, the left hemisphere showed no effect of genotype. Values are means of n = 8–10 rats/group (±SEM).
Astrocyte numbers
There was a main effect of hemisphere (P < 0.001) on the number of MePD astrocytes indicating that astrocytes are more numerous on the right than on the left side in all three groups (males: P < 0.001; TFMs: P < 0.01; females: P < 0.001, Fig. 1B). There was no significant main effect of genotype on the number of astrocytes but there was a significant hemisphere by sex interaction (P = 0.01, Fig. 2B), which led to separate one-way ANOVAs for each hemisphere. Although astrocyte number does not differ significantly by genotype in the left hemisphere (P = 0.49), it does in the right hemisphere (P < 0.05). Additional post-hoc analyses revealed that wildtype males have more astrocytes on the right than do TFM males (P < 0.01) and marginally significantly more do females (P = 0.058). Thus, in the left hemisphere, astrocyte numbers are not sexually dimorphic, whereas in the right hemisphere astrocyte numbers are sexually dimorphic, with wildtype males having more than wildtype females and TFM males. Since TFM male rats lack functional ARs but have slightly higher than normal male levels of androgen (Roselli et al., 1987), the difference between wildtype and TFM males in astrocyte number suggests that functional ARs are necessary to reach normal masculine levels.
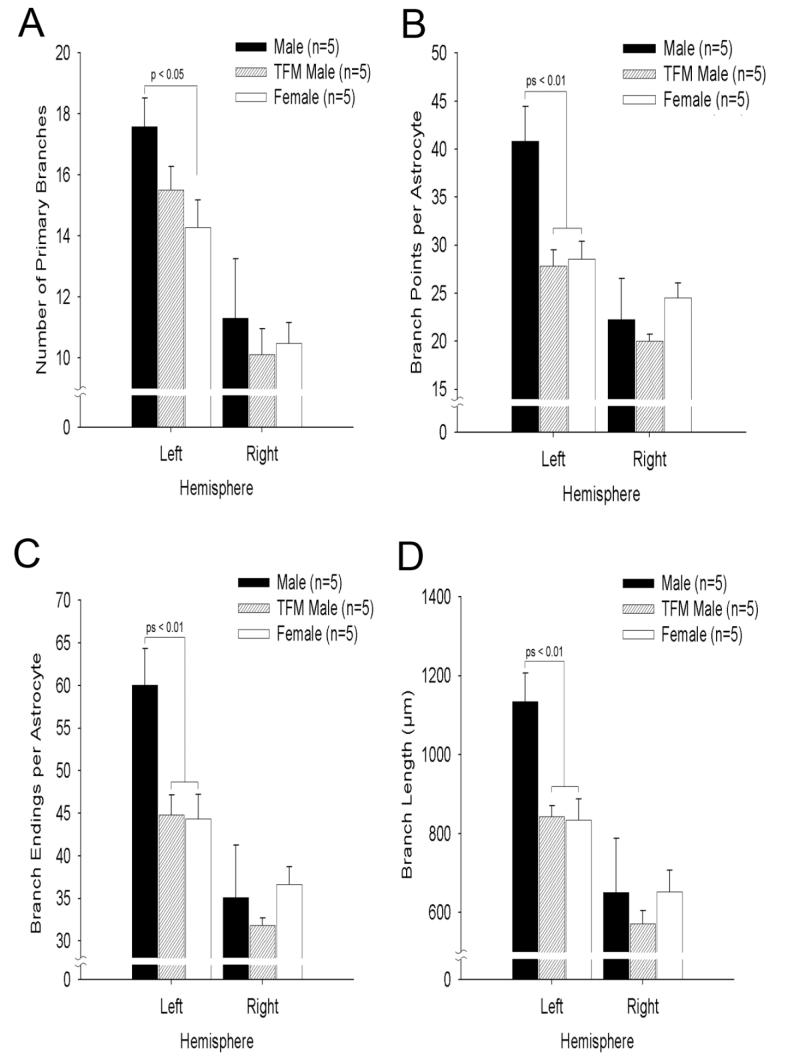
Astrocyte complexity
Astrocyte complexity was assessed using several measures: the number of primary branches, the number of branch points, the number of branch endings, and the average length of branches. In all complexity measures we found a significant main effect of laterality (Ps < 0.005) with the left hemisphere consistently exhibiting more complex astrocytes than the right (Fig. 3). We also found significant main effects of genotype on each measure of astrocyte complexity (Ps < 0.05) except for number of primary branches, where there was no main effect of genotype. Despite finding no significant interactions of genotype with hemisphere on any measure of astrocyte complexity, post-hoc analyses revealed that astrocyte complexity varied significantly by genotype on three of the four measures (number of branch points, number of branch endings, and average branch length), but only in the left hemisphere (Ps < 0.01). Further analyses of astrocyte measures indicated that left MePD astrocytes of wildtype males are more complex than those of females on all four measures, having on average more primary branches as well as total number of branches, more branch points, and longer branches than do astrocytes in the left MePD of females (Ps < 0.5, Fig. 3). Except for average number of primary branches, males also showed more complex astrocytes than did TFM males on the left MePD (Ps < 0.01, Fig. 3). Thus, although the number of astrocytes in the left hemisphere was not sexually differentiated, astrocyte complexity was. This is in contrast to the right hemisphere, which exhibits an AR-dependent sexual dimorphism in total astrocyte numbers but exhibits no sex differences in astrocyte complexity. Furthermore, the sex difference in astrocyte complexity in the left MePD also appears to be AR-dependent, as TFM males did not significantly differ from females in any measure of astrocyte complexity.
Fig. 3.
Estimated number of primary branches (A), branch points (B), branch endings (C), and average branch length (D) in the MePD of adult wildtype male, TFM male, and female rats. In all three groups, astrocytes in the left MePD had more primary branches, more branch points, more branch endings, and a larger average branch length than those on the right, indicating that astrocytes in the left MePD have more complex morphologies than astrocytes in the right. Moreover, there was a significant sex difference in astrocyte complexity in the left MePD, with all measures indicating that males have more complex astrocytes than females on the left side. TFM males are not significantly different from females for any measure of astrocyte complexity. Data from TFM males demonstrates that masculinization of astrocyte complexity in the left MePD depends on AR. Values are means of n = 5 rats/group (±SEM).
A principle components analysis for each hemisphere revealed that all four measures of astrocytes arbor complexity loaded very strongly onto a single factor, suggesting that the four measures used are closely related and could be viewed as a single measure of overall arbor complexity in that hemisphere. One-way ANOVAs examining this single factor in each hemisphere confirmed a significant effect of genotype on overall arbor complexity in the left MePD (P < 0.01) but no effect in the right MePD. Post-hoc analysis of the left MePD revealed that males have greater overall arbor complexity than either TFM males or females (Ps < 0.01).
Morphology classification
Astrocyte arbor complexity was measured and analyzed using methodology established by Mong and McCarthy (1999). Chi-square analysis indicates that distribution of the four classes of astrocytes is different in males compared to females or TFM males (Ps < 0.01), in the left MePD, but not the right, with males having more class IV astrocytes in the left hemisphere than do females or TFMs. Furthermore, TFM males did not differ from females (P = 0.611). Thus, this nominal method of assessing astrocytes arbor complexity agrees well with the quantitative measures of complexity. Both indicate that males exhibit more complex astrocytes than females or TFMs, but only in the left hemisphere, and that TFM males are feminine in their astrocyte complexity. GFAP labeling may not reveal ultrafine astrocyte processes, so these data should not be taken as absolute measures of astrocyte complexity, but as relative measures across the three groups.
Astrocyte nuclear size
Analysis of the average area of individual astrocyte nuclei revealed no main effects of sex or hemisphere. However, there were several correlations; in the left MePD, average astrocytic nuclear size across all genotypes correlated positively with the number of primary branches and the average branch length (Pearson correlations: 0.72 and 0.53, respectively). No measure of astrocyte complexity correlated with astrocyte nuclear size in the right MePD. These correlations suggest that nuclear size may become critical when astrocytic branching becomes extensive as in the left MePD.
DISCUSSION
Prior investigations demonstrated that the adult MePD is highly plastic and responds to steroid hormones (Cooke et al., 1999, 2003; Morris 2005, 2008). Because astrocytes are implicated in regulating neuronal plasticity and are known to be steroid-responsive in some brain regions (Garcia-Segura et al., 1994; Mong et al., 1999; Mong and McCarthy, 2002), we asked whether astrocytes in the adult MePD are sexually dimorphic in number and/or complexity and, if so, whether such differences are sensitive to hormone action via ARs. We found sex differences in, and dramatic laterality of, astrocyte number and complexity in this nucleus, and by utilizing TFM males that lack functioning AR we also determined that masculinization of astrocyte number and complexity in the MePD requires functional AR.
MePD laterality
Laterality in adult MePD anatomy is not unexpected and has been reported previously in the literature. Briefly, the adult right MePD is larger in volume than the left based on Nissl-stained material (Morris et al., 2005). Surprisingly, although the left MePD is smaller in volume, it contains more neurons and is sometimes reported to contain neurons that have larger cell bodies than those in the right MePD (Morris et al., 2008). Although reports have detailed MePD connectivity in the rat (Canteras et al., 1992; Coolen et al., 1998), hemisphere differences in these parameters are either absent or unreported.
The current results report a new and dramatic lateralization in the anatomy of the adult rat MePD, with the right having more astrocytes than the left (Fig. 2), while the left has more complex astrocytes than the right (Fig. 3). The increased number of astrocytes in the right MePD may not be particularly surprising, given its larger volume compared to the left. However, data on neuronal number makes it clear that larger regional volume need not predict greater cell number (Morris et al., 2008). The increased complexity of astrocytes in the left MePD, as revealed in several morphological measurements, was unexpected. The use of a previously established astrocyte complexity classification scheme (Mong and McCarthy, 1999) also suggests that the left hemisphere has more complex astrocytes than the right (Table 1).
TABLE 1.
Percent of Astrocytes within Each of the Four Categories of Complexity,1 by Genotype, and Hemisphere
| Astrocyte group |
Left hemisphere |
Right hemisphere |
||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Male | 0.0 | 3.3 | 23.3 | 73.3 | 23.3 | 20.0 | 25.0 | 31.6 |
| TFM | 5.0 | 13.3 | 31.6 | 50.0 | 8.3 | 21.6 | 36.6 | 33.3 |
| Female | 1.6 | 11.7 | 36.7 | 50.0 | 15.0 | 18.3 | 28.3 | 38.3 |
Group 1 astrocytes are the least complex; group 4 are the most complex.
Thus, it appears that astrocytes follow different strategies to populate each side of the MePD: fewer but more complex astrocytes on the left versus more but simpler astrocytes on the right. Understanding the significance of this dramatic laterality is a challenge. The left MePD contains more neurons than the right (Morris et al., 2008) and prior to puberty contains more dendritic endings and more excitatory synapses than the right MePD (Cooke and Wooley 2005; Cooke et al., 2007). Given that astrocytes ensheath neurons and synapses, the increased number of neurons and synapses on the left may explain why astrocytic processes are more extensive on this side. On the other hand, astrocytes in the right MePD are more numerous but significantly less complex. The simpler morphology of right MePD astrocytes is reminiscent of less mature glia (Sancho-Tello et al., 1995), suggesting that there may be a higher turnover of astrocytes in the right MePD than the left. This would lead to a greater proportion of young astrocytes with less complex arbors. Because adult treatment with testosterone can increase the number of apparent glia (based on counts in Nissl) in the right but not the left MePD of adult females (Morris et al., 2008), androgens may influence ongoing turnover of astrocytes in the right MePD by regulating their genesis and/or survival. Indeed, finding more newly generated glia in the medial amygdala of male than female rats supports this view (Ahmed et al., 2008). In contrast, the left MePD may contain a more stable, and hence older and more complex, population of astrocytes. Combining cell birth and/or cell death markers with astrocytic markers such as GFAP could help to address this possibility in future experiments.
How this laterality in astrocyte morphology relates to behavior will be more challenging to understand. Unfortunately, reports describing the functional consequences of unilateral medial amygdala lesions in rodent models are rare. The few reports available suggest that the right medial amygdala may exert a stronger influence on the hypothalamic-pituitary-gonadal axis than the left in both sexes (Sanchez and Dominguez 1995; Gerendai et al., 1995). It is also possible that hemispheric and sex differences exist in the communication between the MePD and other brain regions.
Reports of functional laterality in the amygdala of humans are more common and offer a consistent portrait (LaBar et al., 1998; Canli et al., 2002; Hamann et al., 2004; Cahill et al., 2004). For example, Cahill et al. (2001) found that in males the activity of the right amygdala, but not the left, was related to recall of emotional memory. In females, activity of the left amygdala, but not the right, was related to such processing. Furthermore, by examining patients in a resting state using functional MRI (fMRI) Kilpatrick et al. (2006) found that the right amygdala exhibited a more widespread distribution of functional connectivity at rest in men than in women, and that the left amygdala exhibited a more widespread distribution of functional connectivity at rest in women than in men. Interestingly, the connectivity pattern was also different for males and females. Kilpatrick et al. (2006) concluded that during resting states there are dramatic differences in the functional networks formed by the amygdala in men and women, and that there is evidence for a female-left, male-right pattern of lateralization.
Perhaps, as is suggested in the human literature, sex differences and hemisphere differences in the rodent MePD reflect differences in functional connectivity with other regions. It is clear that several behaviors are influenced by the medial amygdala and it is likely that astrocytes in this region are involved in restructuring of neural connections in response to gonadal hormones. Recent evidence that astrocytes play a critical role in regulating neuronal responses and cerebral blood flow (Schummers et al., 2008) raises the possibility that sex differences in asymmetrical activity of the human amygdala may be related to sex differences in the asymmetry of astrocyte number or morphology. However, the importance of greater astrocyte numbers in the right hemisphere and greater astrocyte complexity in the left hemisphere for brain function and behavior requires further study.
Astrocytes and MePD anatomy
Dissociations between neuron number and sexually dimorphic regional volume are not common in the literature. Because the MePD is one such rare example (Cooke et al., 2003; Morris et al., 2008), we expected that astrocytes might be a large determinant of regional volume. Although significant correlations existed between regional volume and astrocyte number in the right hemisphere, astrocyte density (regional volume/astrocyte number) was not equivalent across sexes or hemispheres (data not shown), which one would expect if astrocyte number and regional volume were closely linked. In fact, significant differences were found in astrocyte density, with females having greater density compared to males, a result consistent with previous reports of sex differences in GFAP immunoreactivity in the MePD based on optical density (Rasia-Filho et al., 2002). Accordingly, in our multiple regression analysis examining which measures significantly contribute to right regional volume, total astrocyte number emerged as a significant positive predictor (P < 0.05).
In the left hemisphere, astrocytic primary branches, branch endings, and branch length all demonstrated significant positive correlations with left hemisphere volume. Interestingly, left hemisphere volume also significantly correlated with average astrocyte nuclear size. Thus, right MePD volume appears loosely linked to astrocyte numbers and left MePD volume may be linked to overall astrocyte complexity and average astrocyte nuclear size. Future experiments that manipulate androgens and measure astrocyte morphology, may clarify which components change with androgen-induced changes in regional volume and neuronal soma size.
Since astrocytes appear to regulate the formation and stabilization of dendritic spines (Ullian et al., 2001; Nishida and Okabe 2007), one might expect a relationship between astrocytes and dendritic complexity in the MePD. In prepubescent rats, the volume occupied by glia and the number of dendritic branch endings are greater in males than in females in the left hemisphere (Cooke et al., 2007). In adults, males have greater dendritic spine density than females in the MePD, but hemispheric information is not available (Rasia-Filho et al., 2004). Depending on which hemisphere is considered, adult sex differences in spine density would depict two very different pictures of astrocyte-neuron interactions. Increased branch complexity in astrocytes has been associated with an increase in functional synapses in other brain regions (Pfrieger and Barres 1997; Elmariah et al., 2005) but has also been associated with decreases in dendritic spines (Mong et al., 2001). Currently, it is not clear how various aspects of astrocyte morphology (number vs. complexity) relate to neurons, or whether this relationship varies in a region-specific manner.
However, it is also possible that the male bias in astrocyte number in the right MePD is related to synaptic connectivity. MePD volume occupied by glia has been reported to be sexually monomorphic in the right hemisphere prior to puberty in mice (Cooke et al., 2007), suggesting that sex differences in glial number emerge during puberty. Given that neuron-astrocyte communication is important in normal pubertal development in the hypothalamus (Dziedzic et al., 2003; Prevot et al., 2003), perhaps part of the rewiring of the MePD during puberty is accomplished through gliogenesis, with glia leading the way in establishing sex differences in connectivity, similar to the role glia may play in establishing sex differences in neuron number in the bird song system (Nordeen and Nordeen, 1996). These new astrocytes may aid in the formation and maintenance of crucial neuronal connections that ultimately contribute to the expression of sexspecific behaviors. Future studies examining the interaction between MePD astrocytes and local dendrites in each hemisphere are needed to understand the role of astrocytes in modulating MePD structure and function.
Influence of AR
We found striking sex differences in astrocyte morphology in the MePD, with adult wildtype males having more astrocytes than females on the right and more complex astrocytes than females on the left. Moreover, MePD astrocytes in TFM males were like those in females and not like those in wildtype males. The number of astrocytes in the right MePD of TFM males was equivalent to that of females and significantly less than that of males. All measures of astrocyte complexity except the number of primary branches were also reduced in TFM males compared to wildtype males. Also based on the classification method, TFM males exhibited a distribution of astrocytes complexities that matched that of females, but differed from males (Table 1). Finally, TFM males did not significantly differ from females for any astrocyte measure, in either hemisphere. These results clearly indicate that the sex differences in the number of astrocytes in the right MePD and astrocyte complexity in the left MePD of adult rats are dependent on functional AR. ARs may act to promote the extension and elaboration of branches of already established astrocytes on the left side, whereas ARs may promote gliogenesis on the right. Either of these actions may be due to organizational effects of androgens during development or activational effects during adulthood. How ARs exert these different actions on the two sides is not clear but perhaps lateralization of expression of ARs or critical cofactors are involved.
AR may influence astrocyte morphology through direct or indirect pathways. If AR is found in MePD astrocytes, then androgens could act directly on these cells to produce changes. Testosterone treatment increases the number of astrocytes in the female rodent hippocampus (Conejo et al., 2003), and cultured astrocytes taken from the optic nerve, which contain AR, respond to androgens with increases in GFAP and/or proliferation (Aqapova et al., 2006). Furthermore, in cultured cortical astrocytes, activation of membrane-bound AR leads to proliferation and an increase in GFAP (Gatson and Singh, 2007) further suggesting that direct androgen-induced modulation of astrocytes might occur in the MePD. However, the majority of AR-containing cells in the adult MePD are neurons (Greco et al., 1998; Lorenz et al., 2005), despite reports of astrocytic AR in other brain regions (DonCarlos et al., 2005).
Alternatively, androgens may be acting on neuronal AR in the MePD or elsewhere to affect MePD astrocytes indirectly. Androgens may stimulate neurons to communicate with astrocytes via a chemical messenger or may induce morphological changes in neurons, which astrocytes respond to. In the arcuate nucleus, evidence suggests that estrogens induce GABA synthesis and release in local neurons, which then act on GABA receptors in surrounding astrocytes to induce growth of astrocyte processes (Mong et al., 2002). A similar, but AR-dependent, mechanism may occur in the MePD. Because AR protein is defective throughout life in TFM males, our data do not address the stage at which AR promotes masculinization of MePD astrocytes. Nor do they address whether adult androgen manipulations affect astrocyte morphology in the MePD of either hemisphere. Such information will add greatly to our understanding of how gonadal hormones influence the MePD to regulate behavior.
ACKNOWLEDGMENTS
The authors thank Dr. Michelle Johnson for assistance with principle components analysis and Cindy Knaff, Heather Malinowski, and Diane Redenius for technical assistance.
Grant sponsor: National Institutes of Health (NIH); Grant numbers: NS28421 and NS0450195.
LITERATURE CITED
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;6433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Ahmed EI, Schultz KM, Zehr JL, Lorenz BH, DonCarlos LL, Sisk CL.Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions Nat Neurosci 2008. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, De Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system. Academic Press; Orlando, FL: 1995. pp. 495–578. [Google Scholar]
- American Psychiatric Association Diagnostic and statistical manual of mental disorders 2000APA; Washington, DC: 4th text revision ed.). [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci. 2004;24:10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MT. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol Learn Mem. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an fMRI investigation. Learn Mem. 2004;11:261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci U S A. 2002;99:10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Connections of the posterior nucleus of the amygdala. J Comp Neurol. 1992;324:143–179. doi: 10.1002/cne.903240203. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala — a Phal study in the rat. J Comp Neurol. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Conejo NM, González-Pardo H, Pedraza C, Navarro FF, Vallejo G, Arias JL. Evidence for sexual difference in astrocytes of adult rat hippocampus. Neurosci Lett. 2003;339:119–122. doi: 10.1016/s0304-3940(02)01484-2. [DOI] [PubMed] [Google Scholar]
- Cooke BM. Steroid-dependent plasticity in the medial amygdala. Neuroscience. 2006;138:997–1005. doi: 10.1016/j.neuroscience.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Sexually dimorphic synaptic organization of the medial amygdala. J Neurosci. 2005;25:10759–10767. doi: 10.1523/JNEUROSCI.2919-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci U S A. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Breedlove SM, Jordan CL. Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Horm Behav. 2003;43:336–346. doi: 10.1016/s0018-506x(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Stokas MR, Woolley CS. Morphological sex differences and laterality in the prepubertal medial amygdala. J Comp Neurol. 2007;20(501):904–915. doi: 10.1002/cne.21281. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Cunningham RL, Claiborne BJ, McGinnis MY. Pubertal exposure to anabolic androgenic steroids increases spine densities on neurons in the limbic system of male rats. Neuroscience. 2007;150:609–615. doi: 10.1016/j.neuroscience.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Olmos J, Haryd H, Heimer L. the aferent connections of the main and accessory olfactory bulb formations in the rat: an experimental HRP-study. J Comp Neurol. 1987;181:213–244. doi: 10.1002/cne.901810202. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Sarkey S, Lorenz B, Azcoitia I, Garcia-Ovejero D, Huppenbauer C, Garcia-Segura LM. Novel cellular phenotypes and subcellular sits for androgen in the forebrain. Neuroscience. 2005;138:801–807. doi: 10.1016/j.neuroscience.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Dziedzic B, Prevot V, Lomniczi A, Jung H, Cornea A, Ojeda SR. Neuron-to-glia signaling mediated by excitatory amino acid receptors regulates ErbB receptor function in astroglial cells of the neuroendocrine brain. J Neurosci. 2003;23:915–926. doi: 10.1523/JNEUROSCI.23-03-00915.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmariah SB, Oh EJ, Hughes EG, Balice-Gordon RJ. Astrocytes regulate inhibitory synapse formation via Trk-mediated modulation of postsynaptic GABAA receptors. J Neurosci. 2005;25:3638–3650. doi: 10.1523/JNEUROSCI.3980-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Chowen JA, Duenas M, Torres-Aleman I, Naftolin F. Gonadal steroids as promoters of neuro-glial plasticity. Psychoneuroendocrinology. 1994;19:445–453. doi: 10.1016/0306-4530(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, McCarthy MM. Minireview: role of glia in neuroendocrine function. Endocrinology. 2006;145:1082–1086. doi: 10.1210/en.2003-1383. [DOI] [PubMed] [Google Scholar]
- Gatson JW, Singh M. Activation of a membrane-associated androgen receptor promotes cell death in primary cortical astrocytes. Endocrinology. 2007;148:2458–2464. doi: 10.1210/en.2006-1443. [DOI] [PubMed] [Google Scholar]
- Gerendai I, Csaba Z, Vokó Z, Csernus V. Involvement of a direct neural mechanism in the control of gonadal functions. J Steroid Biochem Mol Biol. 1995;53:299–305. doi: 10.1016/0960-0760(95)00067-a. [DOI] [PubMed] [Google Scholar]
- Greco B, Edwards DA, Michael RP, Clancy AN. Androgen receptors and estrogen receptors are colocalized in male rat hypothalamic and limbic neurons that express Fos immunoreactivity induced by mating. Neuroendocrinology. 1998;67:18–28. doi: 10.1159/000054294. [DOI] [PubMed] [Google Scholar]
- Grove EA. Neural associations of the substantia innominata in the rat: afferent connections. J Comp Neurol. 1988a;277:315–346. doi: 10.1002/cne.902770302. [DOI] [PubMed] [Google Scholar]
- Grove EA. Efferent connections of the substantia innominata in the rat. J Comp Neurol. 1988b;277:347–364. doi: 10.1002/cne.902770303. [DOI] [PubMed] [Google Scholar]
- Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci. 2004;7:411–416. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579:321–326. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Sachs BD. Disparate effects of small medial amygdala lesions on noncontact erection, copulation, and partner preference. Physiol Behav. 2002;76:443–447. doi: 10.1016/s0031-9384(02)00682-0. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Sachs BD, Sakuma Y. Importance of the medial amygdala in rat penile erection evoked by remote stimuli from estrous females. Behav Brain Res. 1998;91:215–222. [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1998;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz B, Garcia-Segura LM, DonCarlos LL. Cellular phenotype pf androgen receptor-immunoreactive nuclei in the developing and adult rat brain. J Comp Neurol. 2005;492:456–468. doi: 10.1002/cne.20763. [DOI] [PubMed] [Google Scholar]
- Mah L, Zarate CA, Jr, Singh J, Duan YF, Luckenbaugh DA, Manji HK, Drevets WC. Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biol Psychiatry. 2007;61:765–775. doi: 10.1016/j.biopsych.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Martinez FG, Hermel EES, Xavier LL, Viola GG, Riboldi J, Rasia-Filho AA, Achaval M. Gonadal hormone regulation of glial fibrillary acidic protein immunoreactivity in the medial amygdala subnuclei across the estrous cycle and in castrated and treated female rats. Brain Res. 2006;1108:117–126. doi: 10.1016/j.brainres.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Mong JA, McCarthy MM. Steroid-induced developmental plasticity in hypothalamic astrocytes: implications for synaptic patterning. J Neurobiol. 1999;40:602–619. doi: 10.1002/(sici)1097-4695(19990915)40:4<602::aid-neu14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Mong JA, McCarthy MM. Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Brain Res Dev Brain Res. 2002;139:151–158. doi: 10.1016/s0165-3806(02)00541-2. [DOI] [PubMed] [Google Scholar]
- Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, Roberts RC, Kelly JJ, McCarthy MM. Gonadal steroids reduce the density of axospinous synapses in the developing rat arcuate nucleus: an electron microscopy analysis. J Comp Neurol. 2001;432:259–267. doi: 10.1002/cne.1101. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Dugger BN, Breedlove SM. Partial demasculinization of several brain regions in adult male (XY) rats with a dysfunctional androgen receptor gene. J Comp Neurol. 2005;487:217–226. doi: 10.1002/cne.20558. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in rats. J Comp Neurol. 2008;506:851–859. doi: 10.1002/cne.21536. [DOI] [PubMed] [Google Scholar]
- Nishida H, Okabe S. Direct astrocytic contacts regulate local maturation of dendritic spines. J Neurosci. 2007;27:331–340. doi: 10.1523/JNEUROSCI.4466-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW. Sex difference among nonneuronal cells precedes sexually dimorphic neuron growth and survival in an avian song control nucleus. J Neurobiol. 1996;30:531–542. doi: 10.1002/(SICI)1097-4695(199608)30:4<531::AID-NEU8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson W. Amsterdam. Elsevier; 2005. The rat brain in stereotaxic coordinates. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- Prevot V, Rio C, Cho GJ, Lomniczi A, Heger S, Neville CM, Rosenthal NA, Ojeda SR, Corfas G. Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J Neurosci. 2003;23:230–239. doi: 10.1523/JNEUROSCI.23-01-00230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasia-Filho AA, Xavier LL, dos Santos P, Gehlen G, Achaval M. Glial fibrillary protein immunodetection and immunoreactivity in the anterior and posterior medial amygdala of male and female rats. Brain Res Bull. 2002;58:67–75. doi: 10.1016/s0361-9230(02)00758-x. [DOI] [PubMed] [Google Scholar]
- Rasia-Filho AA, Fabian C, Rigoti KM, Achaval M. Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience. 2004;126:839–847. doi: 10.1016/j.neuroscience.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Diedrich SL, Sisk CL. Effects of gonadal steroids during pubertal development on androgen and estrogen receptor-alpha immunoreactivity in the hypothalamus and amygdala. J Neurobiol. 2000;44:361–368. [PubMed] [Google Scholar]
- Roselli CE, Salisbury RL, Resko JA. Genetic evidence for androgen-dependent and independent control of aromatase activity in the rat brain. Endocrinology. 1987;121:2205–2210. doi: 10.1210/endo-121-6-2205. [DOI] [PubMed] [Google Scholar]
- Sanchez MA, Dominguez R. Differential effects of unilateral lesions in the medial amygdala on spontaneous and induced ovulation. Brain Res Bull. 1995;38:313–317. doi: 10.1016/0361-9230(95)00094-u. [DOI] [PubMed] [Google Scholar]
- Sancho-Tello M, Valles S, Montoliu C, Renau-Piqueras J, Guerri C. Developmental pattern of GFAP and vimentin expression in the rat brain and radial glial cultures. Glia. 1995;15:157–166. doi: 10.1002/glia.440150208. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1628–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Beltramino CA, McDonald AJ, Miselis RR, Yang M, de Olmos J, Heimer L, Alheid GF. Supracapsular bed nucleus of the stria terminalis contains central and medial extended amygdala elements: evidence from anterograde and retrograde tracing experiments in the rat. J Comp Neurol. 2000;422:533–555. doi: 10.1002/1096-9861(20000710)422:4<533::aid-cne5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Huang PY, Castelli F, Adolphs R. Amygdala damage impairs eye contact during conversations with real people. J Neurosci. 2007;11(27):3994–3997. doi: 10.1523/JNEUROSCI.3789-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Williams LM, Barton MJ, Kemp AH, Bryant RA, Meares RA, Peduto AS, Gordon E. Distinct amygdala-autonomic arousal profilesin response to fear signals in healthy males and females. Neuroimage. 2005;28:618–626. doi: 10.1016/j.neuroimage.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Yarbrough WG, Quarmby VE, Simental JA, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson BM. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem. 1990;265:8893–8900. [PubMed] [Google Scholar]