Abstract
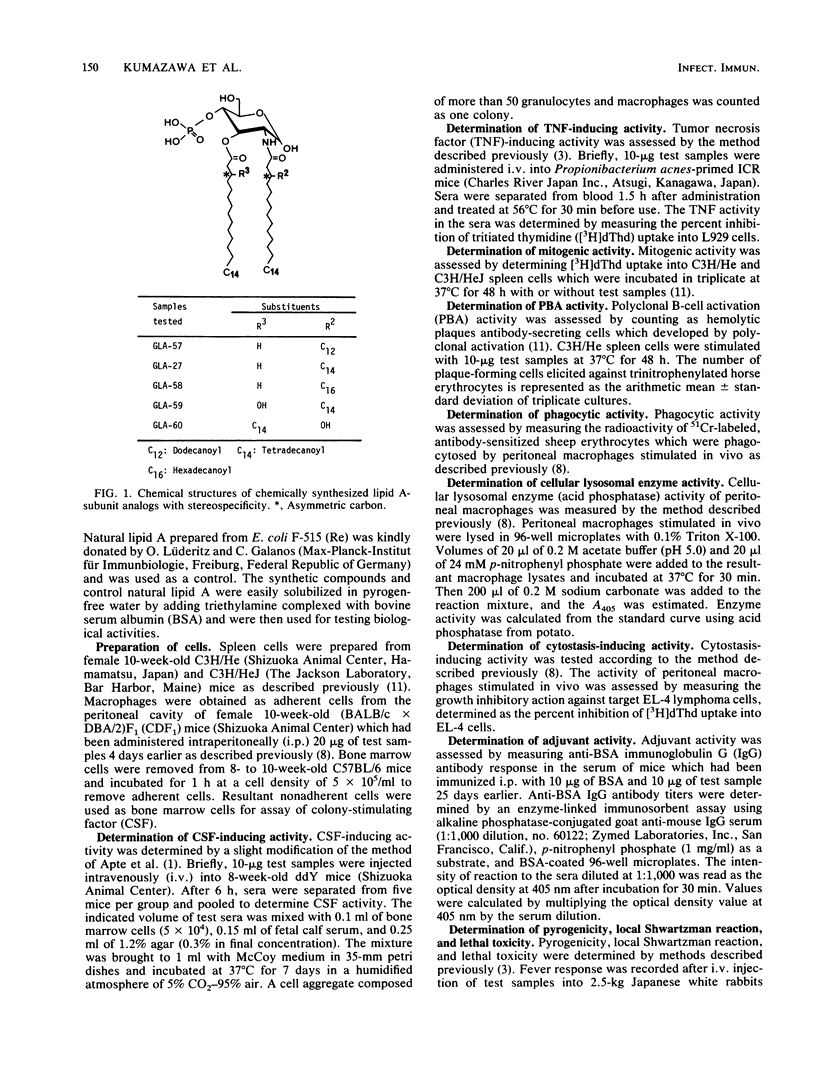
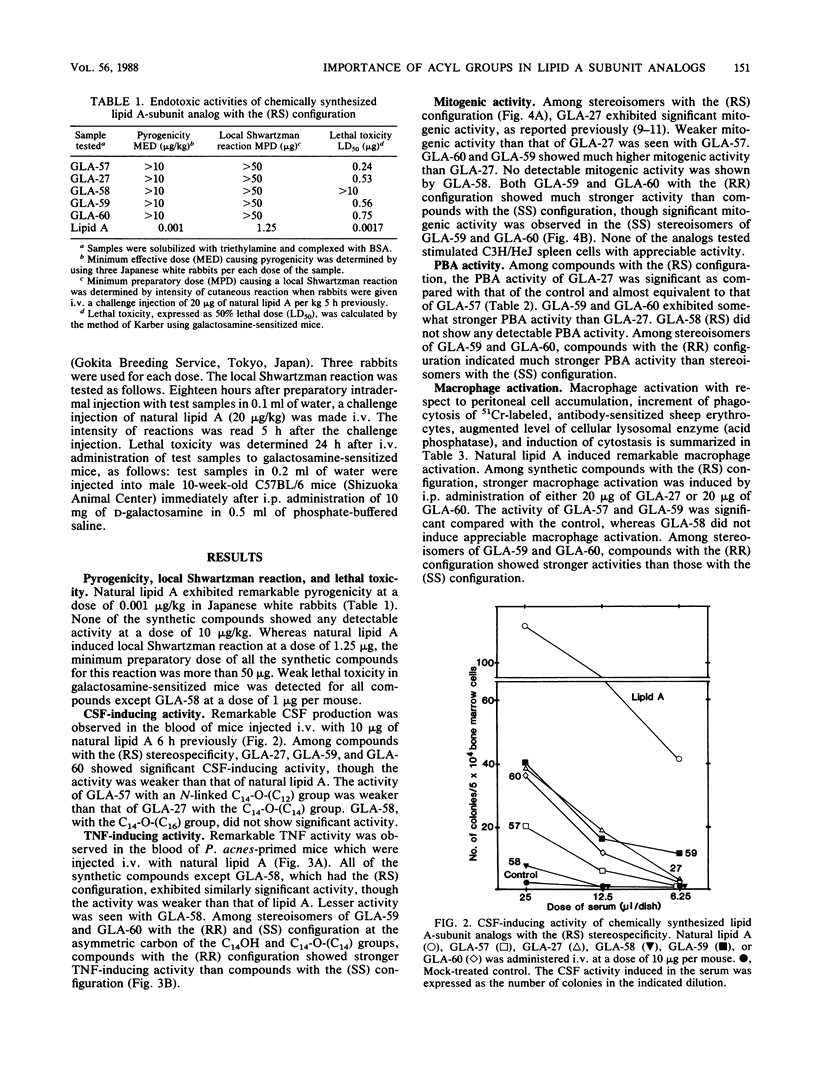
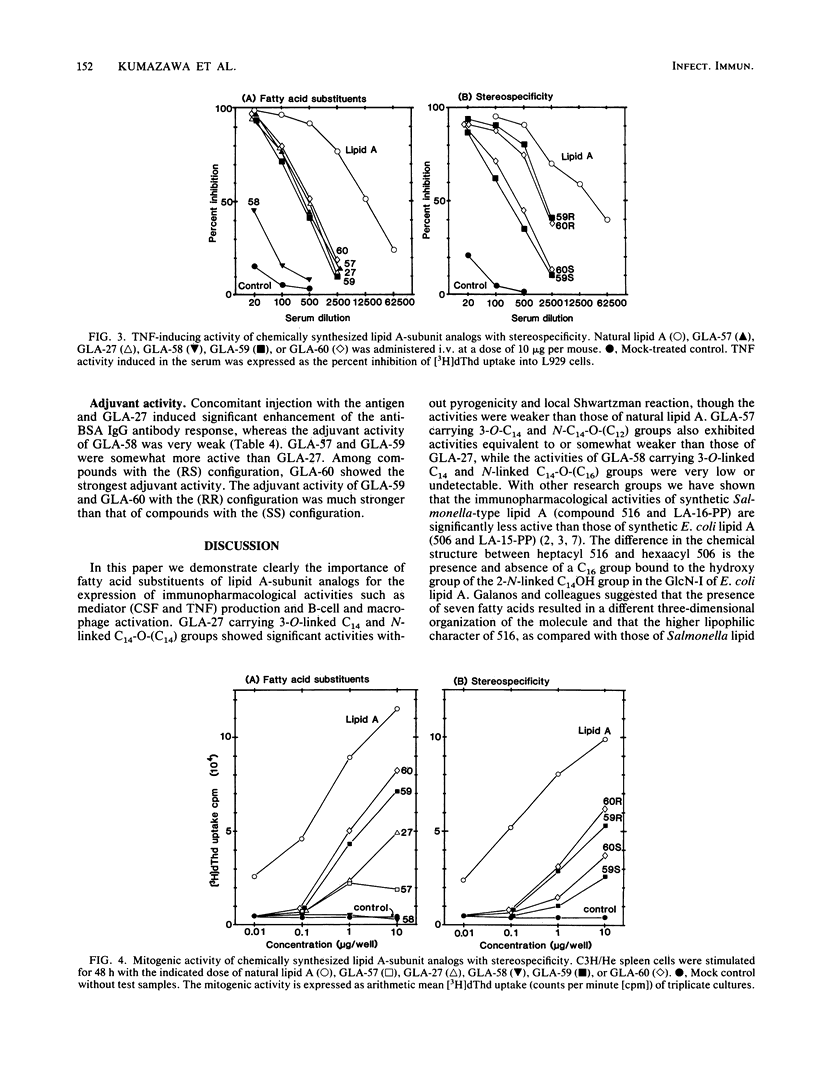
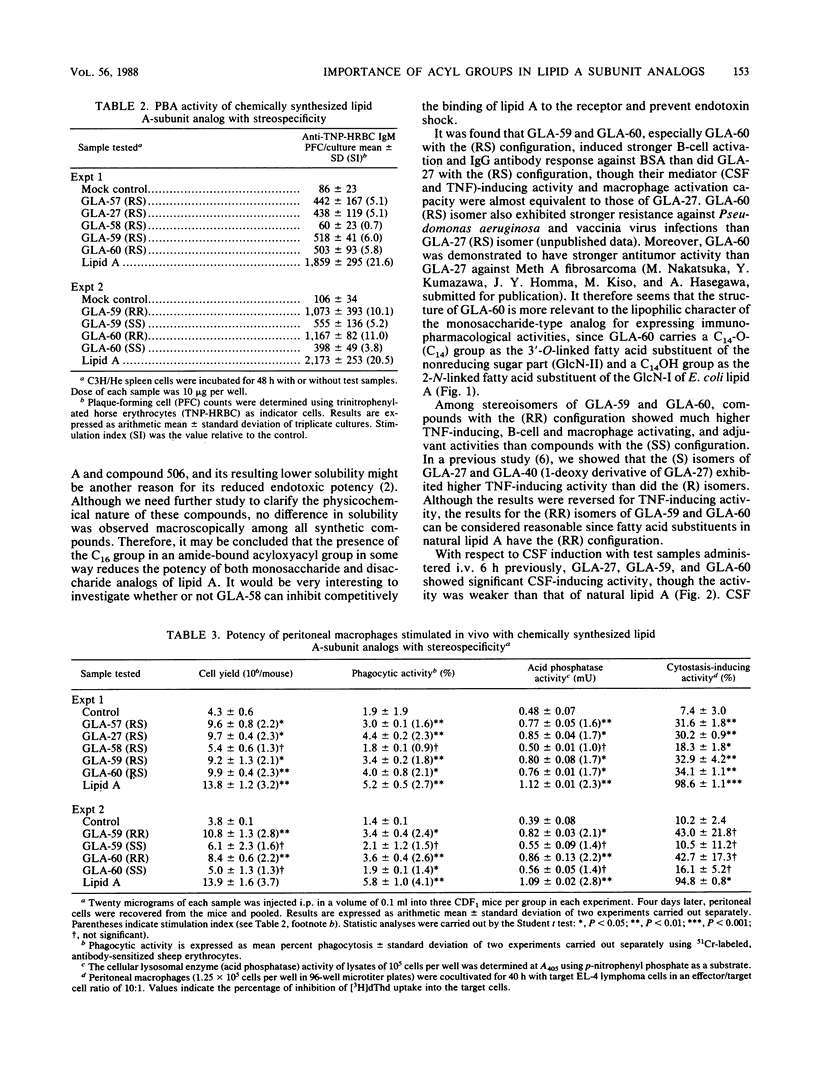
The immunopharmacological activities of chemically synthesized lipid A-subunit analogs, 4-O-phosphono-D-glucosamine derivatives carrying different N- and 3-O-linked acyl groups, were investigated. None of the synthetic compounds tested exhibited any detectable pyrogenicity at a dose of 10 micrograms/kg. Weaker lethal toxicity in galactosamine-sensitized mice was detected at 1 microgram per mouse for all the synthetic compounds except GLA-58. Among (RS) stereoisomers of 4-O-phosphono-D-glucosamine derivatives carrying a 3-O-tetradecanoyl (C14) group with different N-linked acyloxyacyl groups, i.e., 3-dodecanoyloxytetradecanoyl [C14-O-(C12)], 3-tetradecanoyloxytetradecanoyl [C14-O-(C14)], and 3-hexadecanoyloxytetradecanoyl [C14-O-(C16)] groups (termed GLA-57, GLA-27, and GLA-58, respectively), GLA-27 exhibited significant colony-stimulating factor-inducing and tumor necrosis factor-inducing activities, mitogenicity, polyclonal B-cell activation activity, macrophage activation, and adjuvanticity. The activities of GLA-57, which had an N-linked C14-O-(C12) group, were equivalent to or somewhat weaker than those of GLA-27 with a C14-O-(C14) group. Significant immunopharmacological activities were not observed for GLA-58, carrying a C14-O-(C16) group bound to the amino group. GLA-59, carrying 3-O-linked 3-hydroxytetradecanoyl (C14OH) and N-linked C14-O-(C14) groups, showed much higher activities than GLA-27, GLA-60, a compound which possesses the same fatty acid substituents as GLA-59 but with reversed binding sites, showed the strongest B-cell activation and adjuvant activities among the synthetic compounds. Among stereoisomers of GLA-59 and GLA-60 composed of fatty acid substituents with the (RR) and (SS) configuration, compounds with the (RR) configuration elicited stronger activities than the (SS) stereoisomers. The importance of fatty acid substituents, including stereospecificity for the expression of immunopharmacological activities of 4-O-phosphono-D-glucosamine derivatives, was demonstrated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte R. N., Galanos C., Pluznik D. H. Lipid A, the active part of bacterial endotoxins in inducing serum colony stimulating activity and proliferation of splenic granulocyte/macrophage progenitor cells. J Cell Physiol. 1976 Jan;87(1):71–78. doi: 10.1002/jcp.1040870110. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Freudenberg M., Brade L., Schade U., Rietschel E. T., Kusumoto S., Shiba T. Biological activity of synthetic heptaacyl lipid A representing a component of Salmonella minnesota R595 lipid A. Eur J Biochem. 1986 Oct 1;160(1):55–59. doi: 10.1111/j.1432-1033.1986.tb09939.x. [DOI] [PubMed] [Google Scholar]
- Kanegasaki S., Tanamoto K., Yasuda T., Homma J. Y., Matsuura M., Nakatsuka M., Kumazawa Y., Yamamoto A., Shiba T., Kusumoto S. Structure-activity relationship of lipid A: comparison of biological activities of natural and synthetic lipid A's with different fatty acid compositions. J Biochem. 1986 Apr;99(4):1203–1210. doi: 10.1093/oxfordjournals.jbchem.a135583. [DOI] [PubMed] [Google Scholar]
- Kiso M., Tanaka S., Fujita M., Fujishima Y., Ogawa Y., Ishida H., Hasegawa A. Synthesis of the optically active 4-O-phosphono-D-glucosamine derivatives related to the nonreducing-sugar subunit of bacterial lipid A. Carbohydr Res. 1987 Apr 15;162(1):127–140. doi: 10.1016/0008-6215(87)80207-0. [DOI] [PubMed] [Google Scholar]
- Kotani S., Takada H., Takahashi I., Tsujimoto M., Ogawa T., Ikeda T., Harada K., Okamura H., Tamura T., Tanaka S. Low endotoxic activities of synthetic Salmonella-type lipid A with an additional acyloxyacyl group on the 2-amino group of beta (1-6) glucosamine disaccharide 1,4'-bisphosphate. Infect Immun. 1986 Jun;52(3):872–884. doi: 10.1128/iai.52.3.872-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa Y., Ikeda S., Takimoto H., Nishimura C., Nakatsuka M., Homma J. Y., Yamamoto A., Kiso M., Hasegawa A. Effect of stereospecificity of chemically synthesized lipid A-subunit analogues GLA-27 and GLA-40 on the expression of immunopharmacological activities. Eur J Immunol. 1987 May;17(5):663–667. doi: 10.1002/eji.1830170513. [DOI] [PubMed] [Google Scholar]
- Kumazawa Y., Matsuura M., Homma J. Y., Nakatsuru Y., Kiso M., Hasegawa A. B cell activation and adjuvant activities of chemically synthesized analogues of the nonreducing sugar moiety of lipid A. Eur J Immunol. 1985 Feb;15(2):199–201. doi: 10.1002/eji.1830150218. [DOI] [PubMed] [Google Scholar]
- Kumazawa Y., Matsuura M., Maruyama T., Homma J. Y., Kiso M., Hasegawa A. Structural requirements for inducing in vitro B lymphocyte activation by chemically synthesized derivatives related to the nonreducing D-glucosamine subunit of lipid A. Eur J Immunol. 1986 Sep;16(9):1099–1103. doi: 10.1002/eji.1830160912. [DOI] [PubMed] [Google Scholar]
- Kumazawa Y., Matsuura M., Nakatsuru-Watanabe Y., Fukumoto M., Nishimura C., Homma J. Y., Inage M., Kusumoto S., Shiba T. Mitogenic and polyclonal B cell activation activities of synthetic lipid A analogues. Eur J Immunol. 1984 Feb;14(2):109–114. doi: 10.1002/eji.1830140202. [DOI] [PubMed] [Google Scholar]
- Lasfargues A., Charon D., Trigalo F., Ledur A., Szabo L., Chaby R. Analysis of the lipopolysaccharide-induced cytostatic activity of macrophages, by the use of synthetic models. Cell Immunol. 1986 Mar;98(1):8–17. doi: 10.1016/0008-8749(86)90262-5. [DOI] [PubMed] [Google Scholar]
- Lehmann V., Freudenberg M. A., Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med. 1987 Mar 1;165(3):657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura M., Kojima Y., Homma J. Y., Kubota Y., Yamamoto A., Kiso M., Hasegawa A. Biological activities of chemically synthesized analogues of the nonreducing sugar moiety of lipid A. FEBS Lett. 1984 Feb 27;167(2):226–230. doi: 10.1016/0014-5793(84)80131-3. [DOI] [PubMed] [Google Scholar]
- Matsuura M., Kojima Y., Homma J. Y., Kumazawa Y., Yamamoto A., Kiso M., Hasegawa A. Effects of backbone structures and stereospecificities of lipid A-subunit analogues on their biological activities. J Biochem. 1986 May;99(5):1377–1384. doi: 10.1093/oxfordjournals.jbchem.a135606. [DOI] [PubMed] [Google Scholar]
- Matsuura M., Yamamoto A., Kojima Y., Homma J. Y., Kiso M., Hasegawa A. Biological activities of chemically synthesized partial structure analogues of lipid A. J Biochem. 1985 Nov;98(5):1229–1237. doi: 10.1093/oxfordjournals.jbchem.a135389. [DOI] [PubMed] [Google Scholar]
- Nishijima M., Amano F., Akamatsu Y., Akagawa K., Tokunaga T., Raetz C. R. Macrophage activation by monosaccharide precursors of Escherichia coli lipid A. Proc Natl Acad Sci U S A. 1985 Jan;82(2):282–286. doi: 10.1073/pnas.82.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima M., Raetz C. R. Characterization of two membrane-associated glycolipids from an Escherichia coli mutant deficient in phosphatidylglycerol. J Biol Chem. 1981 Oct 25;256(20):10690–10696. [PubMed] [Google Scholar]
- Raetz C. R., Purcell S., Takayama K. Molecular requirements for B-lymphocyte activation by Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4624–4628. doi: 10.1073/pnas.80.15.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Akiyama S., Masuzawa T., Yanagihara Y., Nakamoto S., Takahashi T., Ikeda K., Achiwa K. Antitumor activity and biological effects of chemically synthesized monosaccharide analogues of lipid A in mice. Chem Pharm Bull (Tokyo) 1985 Oct;33(10):4621–4624. doi: 10.1248/cpb.33.4621. [DOI] [PubMed] [Google Scholar]
- Takahashi I., Kotani S., Takada H., Tsujimoto M., Ogawa T., Shiba T., Kusumoto S., Yamamoto M., Hasegawa A., Kiso M. Requirement of a properly acylated beta(1-6)-D-glucosamine disaccharide bisphosphate structure for efficient manifestation of full endotoxic and associated bioactivities of lipid A. Infect Immun. 1987 Jan;55(1):57–68. doi: 10.1128/iai.55.1.57-68.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Mascagni P., Nashed M. A., Anderson L., Raetz C. R. Fatty acyl derivatives of glucosamine 1-phosphate in Escherichia coli and their relation to lipid A. Complete structure of A diacyl GlcN-1-P found in a phosphatidylglycerol-deficient mutant. J Biol Chem. 1983 Jun 25;258(12):7379–7385. [PubMed] [Google Scholar]



