The medial entorhinal cortex (MEC), home of the “grid cells” (Hafting et al., 2005), is a component of a large and complex system that connects widespread areas of the cerebral cortex with the hippocampus, known as the medial temporal lobe (MTL) system. It is generally agreed that this system supports declarative memory (Eichenbaum & Cohen, 2001; Squire et al., 2007). Notably, several investigators pursue the notion that components of this system support spatial information processing that underlies navigation, path integration, and cognitive mapping (e.g. McNaughton et al., 2006), and it is not clear whether these spatial processing functions are considered the same or distinct from the role of this system in memory (e.g., Leutgeb et al., 2005; Bird & Burgess, 2008). Many of the papers in this special issue focus on the role of the MEC and its grid cells in spatial processing. In contrast, here we consider the anatomical and functional organization of the entire MTL memory system, with particular attention paid to the MEC and neighboring parts of the parahippocampal region as components of that system. We first provide an overview of the anatomy of the system, wherein the inputs and outputs of the MEC and neighboring cortical areas will be highlighted. We then briefly review the literature on the functional roles of major components of the MTL system, contrasting functions of the hippocampus and the adjacent cortical areas including the MEC. Next we expand on the role of the MEC and its closest cortical associate, the parahippocampal cortex, in humans and animals. At the conclusion of this review we suggest a role for the MEC that is quite different than the navigational function espoused by other papers in this special issue.
The anatomy of the MTL system
The organization of the MTL system involves bidirectional pathways between the cerebral cortex and the hippocampus, and these pathways are largely conserved across mammalian species (Manns & Eichenbaum, 2006). In each species these pathways are composed of three main levels: association areas of the cerebral cortex, the parahippocampal region, and the hippocampus. With regard to the cerebral cortex, there are considerable differences among species in the functions of the cortical areas that are connected with the medial temporal areas and in the proportions of connections from different areas. However, across species, the cortical areas that send inputs into, and receive outputs from the medial temporal areas include all higher order “association” areas and no primary sensory or motor cortices. Furthermore, across species, the cortical association areas do not connect directly with the hippocampus, but instead connect to a collection of interconnected cortical areas that lie outside the hippocampus called the parahippocampal region. Areas of the parahippocampal region then connect with the hippocampus. The flow of information through the cerebral cortex, parahippocampal region, and hippocampus can be described as a hierarchy of connectivity in which widespread areas of the cerebral cortex funnel information onto multiple areas within the parahippocampal region, whose outputs converge on the hippocampus (Kerr, 2007; Lavenex and Amaral, 2000; Witter et al., 2000). Importantly, the outputs of hippocampal processing are directed back down the hierarchy to areas of the parahippocampal region, which in turn send their outputs back to the areas of the cerebral cortex from which the inputs originated.
Considerable attention has focused on the organization and inputs and outputs of the parahippocampal region, which lies in the middle of this hierarchical, bidirectional pathway (Burwell, 2000, Witter et al., 1989; Figure 1). The major subdivisions of the parahippocampal region are the perirhinal cortex, the parahippocampal cortex (called postrhinal cortex in rodents), and the entorhinal cortex (Burwell and Amaral, 1998; Suzuki and Amaral, 1994). Furthermore a major anatomical and functional distinction is made between the lateral entorhinal cortex (LEC) and medial entorhinal cortex (MEC). Information flows between areas of the cerebral cortex and components of the parahippocampal region and between the parahippocampal region and areas of the hippocampus through two partially distinct channels. The perirhinal cortex receives inputs from areas that identify the nonspatial identity of stimuli. In contrast, parahippocampal/postrhinal cortex receives inputs from many areas that appear to be involved in processing the spatial content of sensory information. Thus, in the monkey, whereas the perirhinal cortex receives more inputs from areas along the ventral visual pathway considered important for object recognition, the parahippocampal cortex receives more inputs from areas along the dorsal visual stream considered important for spatial attention and visuospatially guided actions. In the rat, there is no clear cut segregation of the visual system into dorsal and ventral visual streams, yet the perirhinal and postrhinal cortices receive disproportionate spatial and nonspatial information, respectively (Burwell and Amaral, 1998). Thus the rodent perirhinal cortex receives prominent inputs from the polymodal ventral temporal association area (TEv). By contrast, the postrhinal cortex instead receives prominent spatial inputs from areas such as posterior parietal cortex.
Figure 1.
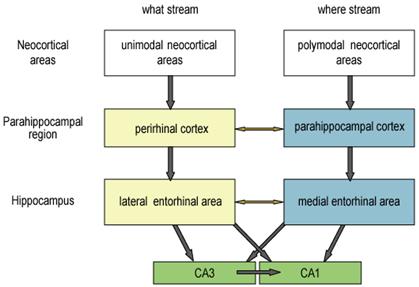
Schematic representation of anatomical connections between neocortex, parahippocampal region, and hippocampus.
Furthermore, the separation of spatial and nonspatial information is at least partially maintained until it is combined in the hippocampus. In both the rat and the monkey, the perirhinal cortex tends to project more to the lateral entorhinal area (LEC) whereas the parahippocampal/postrhinal cortex tends to project more to the medial entorhinal area (MEC; Witter et al., 2000). Interestingly, this system parallels the pattern of interconnectivity of the dorsal and ventral streams such that, despite direct connections between perirhinal and parahippocampal cortices and direct connections between LEC and MEC (Burwell, 2000), the most prominent pathways are those that separate nonspatial and spatial information. In addition, the LEC and MEC send separate projections to the hippocampus in both the rat and the macaque. Both pathways project to all hippocampal subregions, but the pattern in which the fibers from both pathways terminate in hippocampal targets differs between CA3 and dentate gyrus on one hand and CA1 and subiculum on the other (Witter et al., 2000). The projections from LEC and MEC target the same subsets of dentate and CA3 cells. In contrast, the projections from LEC target portions of CA1 and subiculum that differ from the portions targeted by the projections arising in MEC. More specifically, projections arising in LEC target CA1 and subiculum cells near the subiculum/CA1 border, whereas projections arising in MEC target CA1 and subiculum cells farthest from the subiculum/CA1 border. Thus, information passing through LEC and MEC appears to be combined in the dentate gyrus and CA3 but kept partially separate in the subiculum and CA1.
In sum, most of the neocortical input to the perirhinal cortex comes from association areas that process unimodal sensory information about qualities of objects (i.e., “what” information), whereas most of the neocortical input to the parahippocampal/postrhinal cortex comes from areas that process polymodal spatial (“where”) information. There are connections between the perirhinal cortex and parahippocampal cortex, but “what” and “where” streams of processing remain largely segregated as the perirhinal cortex projects primarily to the lateral entorhinal cortex whereas the parahippocampal/postrhinal cortex projects mainly to the medial entorhinal cortex. Modest direct connections also link medial and lateral entorhinal areas, however, the convergence of “what” and “where” information largely occurs within the hippocampus, where even at that point there exist distinct patterns of convergent and separated representations.
Distinct functions of major components of the MTL
Growing evidence from a variety of experimental approaches strongly supports the attribution of distinct functions to specific areas of MTL that parallels the anatomical organization introduced above. Evidence from human amnesic patients and functional neuroimaging in normal humans, neuropsychological evaluations on the effects of selective lesions of MTL areas in animals, and single neuron recordings and other methods of monitoring neural activity in animals, together largely support a separation of non-spatial object processing in the components of the parahippocampal region that receive “what” stream inputs versus spatial processing in components of the parahippocampal region that receive inputs from the “where” steam. Furthermore, these findings also are consistent with the notion that “what” and “where” information are combined within the hippocampus.
Perirhinal cortex and LEC
Considerable data indicate that the perirhinal and lateral entorhinal cortex process information about specific objects, and signal the familiarity of those objects. Thus a prominent characteristic of neurons in the perirhinal and lateral entorhinal areas is that they respond at high firing rates to specific sensory stimuli (Suzuki & Eichenbaum, 2000). For example, in monkeys, a large proportion of neurons are visually responsive, and in rats a large fraction of neurons are responsive to visual and olfactory stimuli (Miller et al., 1993; Suzuki et al., 1997; Zhu et al., 1995, Young et al., 1997). Furthermore, nearly half of all visually responsive neurons in LEC and nearly all visually responsive neurons in perirhinal cortex are stimulus selective in monkeys (Miller et al., 1993; Suzuki et al., 1997). In rats, 35% of odor responsive neurons in LEC and 11% of odor responsive neurons in perirhinal cortex are stimulus selective (Young et al., 1997). Furthermore, these neurons show remarkable memory properties for their optimal driving stimulus. In both species, substantial proportions of stimulus specific neurons can sustain firing during a memory delay period in a delayed matching or non-matching to sample task when the optimal stimulus is no longer present. And the stimulus-driven responses of a large proportion of these neurons are either enhanced or suppressed when a matching test stimulus is presented; repetition suppression is the more prominent of the stimulus specific memory signals. Some of these neurons can show repetition suppression for short periods, whereas in others this stimulus specific memory signal is lasting (Brown & Xiang, 1998; Brown and Aggleton, 2001).
Results from functional imaging studies in humans also indicate that the perirhinal cortex plays a special role in the identification of familiar stimuli (Stern et al., 1996). In a meta-analysis of studies using verbal and non-verbal pictoral stimuli, Henson et al. (2003) concluded that the perirhinal cortex is strongly differentially activated by novel compared to familiar stimuli across modalities. A recent review of studies by Eichenbaum et al. (2007) reported that 13 of 16 studies revealed activation of the perirhinal and/or lateral entorhinal cortex associated with the ability to recognize specific stimuli based on their familiarity without recollection of stimulus associations. In general, activation was greater during encoding of items subsequently rated as highly familiar than for encoding of items that were subsequently forgotten. Conversely, during retrieval, activation in the same areas was lower for items that were highly familiar than for items that were forgotten. These findings are entirely consistent with the results from single neuron recordings in rats and monkeys, revealing substantial commonality among species in identifying a specific role for the perirhinal cortex and LEC in the encoding of specific sensory stimuli and subsequent familiarity with these stimuli.
Parahippocampal/postrhinal cortex and MEC
There is considerable recent evidence that the parahippocampal/postrhinal cortex and MEC are specialized for processing spatial information. Studies in humans have indentified a “parahippocampal place area” within the parahippocampal cortex, which is activated by presentation of spatial scenes but not single or multiple objects (Epstein & Kanwisher, 1998; Epstein, 2005). In rats, whereas perirhinal and LEC neurons have poor spatial coding properties, MEC neurons show strong spatial coding (Quirk et al., 1992; Fyhn et al., 2004; Hargreaves et al., 2005), and there is some evidence of weak spatial firing in the postrhinal cortex (Burwell et al., 1998; Burwell and Hafeman, 2003). In particular, the MEC is the location of the grid cells, boundary cells, and head direction cells that reflect different aspects of spatial processing (Moser and Moser, 2008; Sargolini et al., 2006; Solstad et al., unpublished). Notably, the patterns of spatial firing by grid cells in MEC distinguish changes in spatial contexts (Barry et al., 2007; Fyhn et al., 2007).
Furthermore, in contrast to the perirhinal cortex and LEC, substantial data indicate that the parahippocampal/postrhinal cortex and MEC do not process the novelty or familiarity of distinct sensory stimuli, but instead respond to changes in spatial information. Results of functional imaging studies show striking dissociations within some of these regions. For example, Pihlajamki et al., (2004) reported that changes in the identity of presented objects activated the perirhinal cortex but not the parahippocampal cortex, whereas changes in the spatial arrangement of familiar objects activated the parahippocampal cortex and not the perirhinal cortex. Buffalo et al. (2006) presented subjects with visual objects in different places on a computer screen with instructions to remember the object or place independent of the alternative parameter, and observed activation of the perirhinal cortex during object and spatial encoding versus anterior parahippocampal cortex only during spatial encoding; they interpreted the perirhinal responses to spatial cues as a consequence of parahippocampal inputs for that source of information.
In a similar experiment on rats, Wan et al. (1999) observed activation of the immediate early gene fos in perirhinal cortex in response to a change in visual object stimuli, but no fos response to a change in object locations. The converse results were observed in the postrhinal cortex, that is, a response to spatial rearrangement but not to changing objects. In addition, whereas object recognition is impaired following perirhinal damage, object-location recognition is deficient following postrhinal damage in rats (Gaffan et al., 2004) and monkeys (Malkova and Mishin, 2003; Alvarado and Bachevalier, 2005). Similarly, Norman and Eacott (2005) reported data suggesting that perirhinal lesions led to greater memory impairment for object pairings whereas postrhinal lesions led to greater impairment in memory for the spatial context in which an object was presented.
Hippocampus
Considerable data indicates a strong role for the hippocampus in combining object and spatial information (Eichenbaum et al., 1999; Eichenbaum et al., 2004). Hippocampal neurons in monkeys (Brown & Xiang, 1998), rats (e.g., Otto & Eichenbaum 1992a), and humans (Rutishauser et al. 2006; Davachi & Wagner, 2002) do not show stimulus-selective activations or repetition-related firing patterns during delayed matching and non-matching tasks that test item recognition. Instead, hippocampal neurons show only general responses to novelty or familiarity across a broad range of stimuli, suggesting a role in encoding the outcome of recognition experiences. Results from studies in rats (e.g., Hampson et al. 1993, Moita et al. 2003, Wan et al., 1999; Wood et al. 1999, 2000), monkeys (e.g., Cahusac et al. 1993, Wirth et al. 2003), and humans (e.g., Ekstrom et al. 2003, Kreiman et al. 2000; Henke et al., 1997; Davachi and Wagner, 2002) suggest that hippocampal neurons encode associations between specific stimuli in a unique location or behavioral context. For example, a study that directly examined the coding of object and spatial features of events in rats performing a non-matching to sample task reported that subsets of neurons encoded odor cues, or places where they were sampled. However, the responses of odor selective cells were modulated by the location where the odors were experienced, and the responses of cells encoding the location of sampling events likewise differentiated the odor stimuli that were presented at those locations (Wood et al., 1999). These results indicate that hippocampal neuronal firing patterns reflect unique conjunctions of stimuli with their significance, the animal's specific behaviors, and the places and contexts in which the stimuli occur (Eichenbaum et al., 1999; Eichenbaum, 2004).
Tests of the functional contributions of components of the MTL are prominent in studies on recognition memory, and the results from multiple approaches are mixed in supporting a selective role for the hippocampus in representing items in context. Recent reviews highlight distinctions between two processes that are employed to support recognition, a sense of familiarity with previously experienced stimuli and recollection of the associations and context in which a previously experienced stimulus occurred (Eichenbaum et al., 2007; Davachi, 2006; Diana et al., 2007, but see Squire et al., 2007). These studies provide striking evidence for selective involvement of the perirhinal cortex in familiarity but not recollection, and for the hippocampus and parahippocampal region in recollection, not familiarity. Several studies have reported that transient hypoxia, which more significantly affects the hippocampus than parahippocampal region, results in disproportional deficits in memory for associations or context compared to item recognition (Giovanello et al. 2003; Holdstock et al. 2005; Mayes et al. 2002; Turriziani et al. 2004). One study that used a receiver operating characteristic (ROC) analysis showed that mildly hypoxic patients exhibited severe deficits in recollection, but normal familiarity (Yonelinas et al., 2002). A similar pattern of deficient recollection and preserved familiarity was reported in a patient with relatively selective hippocampal atrophy related to meningitis (Aggleton et al. 2005). However, studies on other hypoxic patients have found equivalent deficits in recollection and familiarity, and associative and item recognition using similar procedures, suggested that the locus or extent of damage may vary across patient groups (Gold et al., 2006, Manns et al., 2003, Stark & Squire 2003; Wais et al., 2006).
Functional imaging studies in humans also have distinguished activation of the hippocampus during recollection but not familiarity judgments. In a recent review of the literature, 84% of contrasts that identified activation related to recollection of items reported hippocampal activation (Eichenbaum et al., 2007). Hippocampal activation was seen in all contrasts related to recollection of associations of items and context. By contrast, only 27% of contrasts that identified neural correlates of familiarity-based recognition revealed hippocampal activation. Thus, while hippocampal activation is more prevalent under conditions where subjects remember stimulus associations and context, and rare when they remember only familiarity with the test items, there remain several exceptions to this rule.
A similar mixture of results exist concerning the role of the hippocampus in recognition memory in animals. In some studies on recognition memory using the delayed nonmatch to sample task, in some studies on monkeys, a statistically significant deficit was observed at a very long delay (Zola et al. 2000). But in other studies no deficit was observed even at long memory delays or when animals were required to remember a long list of stimuli (Murray & Mishkin 1998; Nemanic et al., 2004). In rats, performance on DNMS also is typically intact following selective hippocampal damage (Mumby 2001), although some studies report partial impairment at long delay intervals or when the list of sample objects was numerous (Clark et al. 2001, Dudchencko et al. 2000). In studies that examine recognition by monitoring spontaneous exploration of familiar and novel stimuli, monkeys with selective hippocampal or parahippocampal cortex damage that show little or no deficit in DNMS have severe and rapidly apparent deficits on the spontaneous novelty exploration task (Nemanic et al. 2004; Zola et al. 2000). In rats, some studies report no deficit (e.g., Mumby et al., 2002, Winters et al. 2004, Mumby 2001) whereas other studies have observed impairment at long delays (Clark et al. 2000, Hammond et al. 2004), or following a large amount of damage to the hippocampus (Broadbent et al. 2004). In contrast to the modest and variable deficit observed following damage to the hippocampus, ablation of the perirhinal cortex consistently results in a severe and rapidly developing deficit in both kinds of recognition memory tasks in monkeys (Nemanic et al. 2004) and in rats (Mumby et al. 2002; Norman & Eacott 2005; Winters et al. 2004; Winters & Bussey 2005).
Clarification of these results comes from a study using ROC analyses in rats, where damage could be selectively limited to the hippocampus. In this study rats were trained on a variant of delayed nonmatching to sample in which they initially sampled a series of odors and then judged old and new test stimuli across a range of response criteria. These data were used to derive ROC functions, similar to the approach used in humans (Fortin et al. 2004). Like healthy humans, the ROC curve of normal rats was asymmetrical and curvilinear, indicating both recollection and familiarity components of recognition. By contrast, the ROC curve of rats with selective hippocampal damage was entirely symmetrical and identical to the familiarity component of the ROC of control animals, indicating that recognition was supported primarily by familiarity. Importantly, a strong recollection component was retained in normal rats when the memory delay was lengthened to equate their overall accuracy with that of rats with hippocampal damage at the shorter delay. This observation indicates that deterioration of the recollection component of the ROC curve following hippocampal damage is not a consequence of a generally weakened memory but rather a selective loss of the contribution of recollection. These findings can explain the inconsistent effects of hippocampal damage on recognition memory in previous studies on humans and in animals. In animals, as in humans, the presence and magnitude of deficits depends on the relative contributions of recollection and familiarity processes in each particular task and the selectivity of the deficit in recollection depends on limiting the damage to the hippocampus.
Finally, in variants of recognition tasks where rats must remember places, the hippocampus consistently plays an important role. For example, in a variant of the spontaneous novelty exploration task where an initially presented object is moved to a new location or to a new environment during the test phase, selective hippocampal lesions consistently result in deficits even following relatively small lesions of the hippocampus that have no effect on exploration of novel objects (e.g., Eacott & Norman 2004, Mumby et al. 2002). These findings are consistent with the results of the ROC study in that prototypical aspects of recollective memory involve the combination of “what” and “where” information, such that recollection is reflected in the ability to remember where prior events occurred. In sum, while a final clarification remains on the conditions under which hippocampal representation involves single items as well as items in the context in which they were experienced, there is considerable converging evidence that areas of the MTL make distinct contributions to “what” and “where” processing that may support recollective memory. The mechanisms by which these areas contribute will be discussed next.
The role of the parahippocampal/postrhinal cortex and medial entorhinal area in memory
The combination of findings reviewed above are consistent with an anatomically-guided hypothesis regarding the mechanisms by which different regions of the MTL may interact to support the phenomenology of recollection and familiarity (Eichenbaum et al., 2007). During encoding, information about the specific stimuli to be remembered, processed by the perirhinal cortex and LEC, and information about their spatial context, processed by parahippocampal/postrhinal cortex and MEC, converge onto the hippocampus. When a previously encountered stimulus is processed, retrieval may be supported in part by perirhinal cortex and the LEC, which can identify a match to a pre-existing item representation, observed as suppressed activation. This signal can then be propagated back to neocortical areas to generate the sense of familiarity without participation of the hippocampus. Additionally, during vivid recollection processing of the stimulus may drive the recovery of object-context associations in the hippocampus. These signals, via back projections, may reactivate a representation of the contextual associations in parahippocampal/postrhinal cortex and MEC. These areas, in turn, project back to neocortical areas that processed the context in which the item was previously encountered, thereby eliciting the subjective experience of recollection.
This hypothesis generates the prediction that the parahippocampal/postrhinal cortex and MEC should be activated during recollection of the spatial context in which a cue was previously encountered. Consistent with this prediction, substantial evidence indicates that the parahippocampal/postrhinal cortex typically follows the hippocampus in its selective role in recollection and in memory for places where events occur. In humans, a large proportion of studies report that, along with the hippocampus, activity in the parahippocampal cortex is associated with recollection and not familiarity. In rats, damage to the postrhinal cortex does not affect exploration of novel objects but results in severe impairment in recognizing objects after a change in position or context (Eacott and Norman 2004, Norman & Eacott 2005). In addition, fos is selectively expressed in the postrhinal cortex and hippocampus, but not perirhinal cortex, when the location of familiar stimuli is changed (Wan et al., 1999).
Most directly relevant to the prediction, Bar and Aminoff (2003) compared bold responses to pictures of objects that evoked strong contextual associations in their subjects, such as roulette wheel and a barn, to objects that evoked only weak contextual associations, such as cherries and a windmill. The parahippocampal cortex was activated when objects with strong contextual associations were presented, comparable to the level of activation by presentations of scenes. This finding is consistent with a variety of results showing parahippocampal activation during recollection of items that involve associations with spatial context (Davachi & et al., 2003; Daselaar et al., 2006; Ranganath et al., 2003; Ross and Slotnick, 2008; Ekstrom and Bookheimer, 2007; Hayes et al., 2007; see Eichenbaum et al, 2007 for review).
Furthermore, Bar and Aminoff (2003) observed activation in parahippocampal cortex associated with non-spatial as well as spatial context. Specifically, they observed parahippocampal activation both as subjects viewed objects strongly associated with abstract contexts such as romance, music, and crime, and as they viewed objects that had strong associations with places such as office, street, or playground. Notably, the areas of peak activation within the parahippocampal cortex differed for spatial and non-spatial contexts, with greater activation in the posterior parahippocampal cortex for spatial contextual associations and greater activation in the anterior parahippocampal cortex for abstract contextual associations. This finding subsequently was extended to reveal that the parahippocampal cortex is also activated during viewing of famous faces, wherein the contextual associations are not typically place-specific but rather associated with rich pictorial and abstract contextual information (Bar et al., 2008). In addition, the distinction between spatial and nonspatial contextual activation was clarified in a study that compared activations to simple visual patterns where the contextual associations were explicitly trained (Aminoff et al., 2007). Under the spatial context condition, three patterns were repeatedly presented together in the same spatial configuration on a computer screen. In the non-spatial context condition three patterns were repeatedly presented together but in a variety of spatial configurations. As a control, other patterns were presented singly over several repetitions in different locations. In the test phase where single patterns were presented, stimuli with both spatial and non-spatial contextual associations evoked activity in the parahippocampal cortex, in posterior and anterior areas, respectively, similar to observations where familiar stimuli evoke naturally acquired spatial and abstract contextual associations. Importantly, the non-spatial contextual associations in this study involved only temporal contiguity of associated patterns. Notably, the previous observations of activation of the same area by stimuli associated with “abstract” contexts (e.g., romance, famous faces) can also be characterized by the repeated temporal contiguity of specific items and the associated material (e.g., roses with romance, Angela Jolie with her movies). These findings, therefore, suggest that the parahippocampal cortex may play a role in retrieval of both spatial and temporal context.
The possibility that parahippocampal cortex may play a broad role in contextual association, beyond specifically spatial context, calls into question the current focus on whether “where” stream components of the medial temporal lobe are dedicated to spatial information processing. Is it possible that the MEC, like the parahippocampal cortex, also plays a broader role in temporal as well as spatial contextual processing? Results of a recent study by the present authors are consistent with this suggestion (Lipton et al., 2007). In this study our aim was to determine whether MEC neurons, like hippocampal principal cells, distinguish routes traversed as temporally distinct episodes during performance of a T-maze alternation task. In a previous experiment focused on hippocampal neurons, rats were trained on the classic spatial T-maze alternation task in which successful performance depends on distinguishing left- and right-turn episodes to guide each subsequent choice (Wood et al., 2000). We sought to determine whether hippocampal neurons consistently represent places on the maze or instead are elements of a representation of each distinct left-turn or right-turn type of episode. We reasoned that, if hippocampal neurons encode each sequential behavioral event within one type of episode, then neuronal activity at locations that overlap in left- and right-turn trials should vary according to trial type. Indeed, virtually all cells that were active as the rat traversed these common locations were differentially active on left- versus right-turn trials. These findings suggested a reconciliation of the spatial and episodic memory views of hippocampal function. Place cells represent the series of places where events occur in sequences that compose temporally distinct episodes. The observation of spatial firing patterns in the MEC encouraged us to ask whether the disambiguation of places on the maze by temporal context occurred within the hippocampus or instead occurred earlier in the cortical-hippocampal hierarchy, specifically in the MEC. This possibility is consistent with the suggestion that sequences are bound by a shared temporal context signal that may exist in the entorhinal area (Hasselmo and Eichenbaum, 2005; Hasselmo et al., 2007; Hasselmo, 2007; see also Hasselmo, 2008, in current issue).
We trained rats to perform the spatial alternation task on a T-maze that included return arms that connected the end of each goal arm to the starting end of the central stem (Figure 2). Each trial began as the animal departed one of the two goal areas, and continued as the animal traversed the return arm and the central stem, then turned into the alternate goal area to retrieve a water reward. In this task, each left- or right-turn trial can be considered a unique episode, constructed by connecting sequential behavioral events identified by a series of loci along the maze. Areas that lie along the central stem constitute overlapping elements of both types of episodes. Consistent with previous reports (Fyhn et al., 2004; Hargreaves et al., 2005), MEC neuronal activity was associated with an animal's position along the maze. A strict and consistent periodic spacing in the spatial firing pattern was not observed in these cells, similar to a recent report of a breakdown in the consistent grid pattern (characterized as a “resetting”) of MEC neuronal grid firing when animals' movements on a maze were constrained to make hairpin turns (Derdikman et al., 2006). Nevertheless, a proportion of our medial entorhinal neurons did exhibit a high degree of spatial specificity and multiple spatially distinct areas associated with high firing rates, similar to the characteristics of grid cells. For example, the medial entorhinal cell shown in Figure 3 fired predominantly toward the distal end of the central stem during both left- and right-turn trials, while exhibiting little significant activity through other regions of the maze.
Figure 2.
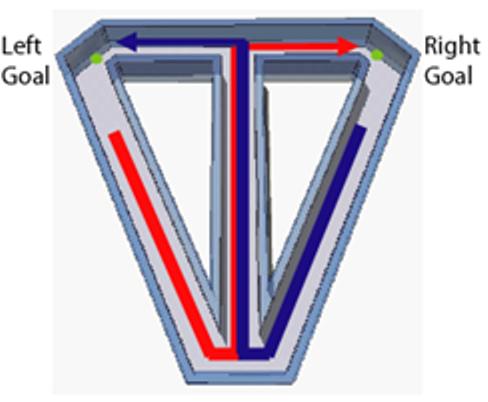
Continuous spatial alternation on modified T-maze. Blue line indicates left-turn trial; red line indicates right-turn trial. Small green circles represent reward sites.
Figure 3.
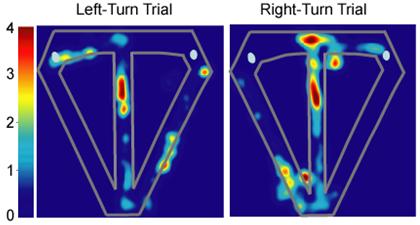
The activity of a medial entorhinal neuron, represented as a false-color rate map, that did not fire differentially on left- versus right-turn trials. Significant main effect of segment (F6,238 = 7.28; p < 0.00001); log-likelihood ratio = 2.35; pcorrect = 0.8; pchance = 0.0002. Color bars indicate firing rate in Hz.
Many of these MEC neurons also exhibited differential firing along the central stem of the maze during left- and right-turn trials, similar to hippocampal neurons (Lipton et al. 2007). The patterns of neuronal activity illustrated in Figure 4 represent typical trial-type specific activity exhibited by medial entorhinal neurons, as well as the range of spatial specificity exhibited by these neurons. Some MEC cells fired selectively during the trial and distinguished left-turn and right-turn trials. For example, the cell shown in Figure 4A was selectively active as the rat traversed the middle portion of the central stem and fired at a higher rate during right-turn compared to left-turn trials. However, most MEC neurons showed only crude spatial specificity. For example, the cells shown in Figures 4B and 4C fired somewhat indiscriminately through different regions of the maze, and although active along the entire central stem, was significantly more active on left-turn and right-turn trials, respectively. In at least some cases, the differences in firing patterns were reminiscent of the shifts in spatial phase and orientation observed when animals move between similarly shaped environments (Fyhn et al., 2007). This pattern of activity was an exclusive feature of MEC neurons, such that we observed no hippocampal units with poorly localized, trial-type specific firing that extended the length of the central stem. Conversely, hippocampal neurons typically exhibited highly localized activity along the maze, exhibited strong spatial specificity, and appeared to distinguish left- and right-turn trials less well than MEC cells (Figure 5).
Figure 4.

Firing patterns of three representative medial entorhinal neurons that reflect both trial disambiguation and the range of spatial specificity A. This unit was significantly more active on right turn trials: Significant main effect of segment (F6,392 = 16.34; p < 0.00001); significant interaction (F6,392 = 4.48; p < 0.0002); log-likelihood ratio = 0.94; pcorrect = 0.64; Pchance = 0.02. B. This unit was significantly more active on left-turn trials: Significant main effect of segment (F6,280 = 3.83; p < 0.001); main effect of trial type (F1,280 = 4.87; p < 0.03); log-likelihood ratio = 1.29; pcorrect = 0.71; pchance = 0.004. C. This unit was significantly more active on right-turn trials: Significant main effect of trial type (F1,280 = 8.36; p = 0.0041); log-likehood ratio = 1.27; pcorrect = 0.71; pchance = 0.004. Color bars indicate firing rate in Hz.
Figure 5.
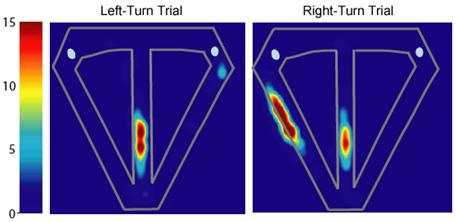
The activity of a hippocampal neuron that differentially represented left-versus right-turn trials; this unit was significantly more active on left-turn trials. Significant main effect of segment (F6,448 = 104.77; p < 0.00001); significant main effect of trial type (F1,448 = 6.61; p < 0.01); significant interaction (F6,448 = 2.52; p < 0.02); log-likelihood ratio = 1.19; pcorrect = 0.58; pchance = 0.13. Color bars indicate firing rate in Hz.
We directly and quantitatively compared the degree of temporal disambiguation and spatial specificity of MEC and hippocampal neurons using a combination of ANOVA and log likelihood analyses. In the ANOVAs, we divided the central stem into seven equal segments, and compared the spatial firing patterns on segments of the central stem between left-turn and right-turn trial types for each cell. A significant main effect of trial type or a trial type by segment interaction qualified a cell as differentiating left- from right-turn trials (Wood et al., 2000). The results of this analysis indicated that 56% of medial entorhinal neurons (23/41 with place fields on the central stem) were significantly more active on either right- or left-turn trials, whereas 33% (16/48) of hippocampal neurons exhibited differential firing on the central stem (Lipton et al., 2007). Our log-likelihood ratio analysis quantified the degree to which firing patterns on left- turn and right-turn trials differed. The log-likelihood ratio was calculated as ln{p[r | L,x]/p[r | R,x]}, where p[r | L,x] is the probability density function of left-turn (L) trials at position x, evaluated at the observed firing rate r, and p[r | R,x] is the equivalent function for right-turn (R) trials (Dayan and Abbott, 2001). For each cell, log-likelihood ratios were summed over all central stem position bins for each trial. The magnitude of average absolute value of the summed log-likelihood ratio reflects how well the representations are distinguished by temporal context (for a more detailed description, see Lipton et al., 2007). This analysis revealed that MEC neurons more robustly distinguished left- from right-turn trials than did hippocampal neurons, such that the average log-likelihood ratio for medial entorhinal neurons was significantly greater than for hippocampal neurons (MEC, 2.82; Hippocampus, 1.7; Wilcoxon rank-sum test, p <0.003). It should be noted that in another study nearly all hippocampal neurons differentiated left- and right-turn trial types (Wood et al., 2000). However, in the current discussed situation in which the differentiation of trial types was weaker in hippocampal neurons (Lipton et al., 2007), MEC neurons showed stronger disambiguation. The combination of these and other analyses showed that both MEC and hippocampal neurons had significant spatial specificity and distinguished left- and right-turn trials. However, the degree of these two types of coding were dissociated in the two areas, such that MEC neurons more strongly distinguished left- and right-turn trial types whereas hippocampal neurons more strongly distinguished the sequence of locations traversed in each type of episode.
Thus, the results of this study suggest that disambiguation of overlapping experiences occurs prior to the hippocampus, and that hippocampal and MEC circuits play distinct and complementary roles in the continuous spatial alternation. Neuronal ensembles in the MEC more strongly distinguished task related episodes in the context of left- versus right-turn trial episodes whereas hippocampal neurons provided a greater degree of spatial specificity. Together these interconnected areas supply requisite elements of a neural code for particular events as they occur within temporally distinct experiences.
A question that remains, however, is how to interpret the observed pattern of MEC trial disambiguation in terms of the findings on contextual retrieval in the human parahippocampal cortex discussed above. Our speculative interpretation that MEC neuronal firing patterns contain a signal for temporal context is based on the observation that MEC spatial firing often extended through a substantial distance on the maze and these firing patterns varied as a function of trial type. The connection between these two properties may be that sustained firing patterns serve as the neural bridge that links sequential events subsequently identified within the hippocampus, and does so differentially for each trial type (Hasselmo and Eichenbaum, 2005; Hasselmo et al., 2007; Hasselmo, 2007; see also Hasselmo, 2008, in current issue). In this way, MEC activity that spans multiple spatial locations signals what is common about those locations in terms of an animal's ongoing behavior, that is, a left- versus right-turn trial in relation to prior or future behavior. These conclusions bring the MEC in line with the findings on the parahippocampal cortex in humans, suggesting that the “where” pathway structures of the MTL system support the encoding and retrieval of temporal as well as spatial context.
Conclusions: What is the role of contextual processing by the “where” pathway in the MTL memory system?
Several papers in this special issue focus on how the MEC may contribute to spatial information processing that underlies navigation. The conclusion of the present paper is different, providing a speculative interpretation of the data suggesting that the MEC and associated cortical areas of the “where” stream may serve a role in the encoding and retrieval of spatial and temporal context. These views are not entirely mutually exclusive, such that one can imagine that navigational computations contain contextual information. Thus, we consider an alternative reconciliation: the functions subserved by the system including the MEC and hippocampus encompass the broader category of memory, upon which navigation is dependent, such that navigation is not in and of itself an exclusive functional attribute of this “where” stream.
It is worth mentioning that the evidence that the MEC and MTL system perform navigational computations is weak. There is substantial evidence, outlined here and elsewhere in this special issue, that neurons in the MEC and hippocampus encode locations, head direction, and movement velocity and are influenced by self motion (Gothard et al., 1996; Hafting et al., 2005). But there also is substantial evidence that the MTL system represents a broad range of events in which these spatial features of representation are prominent examples (Eichenbaum et al., 1999). Furthermore, while the MTL system is essential to remembering places and to making navigational inferences based on combinations of prior experiences (Eichenbaum et al., 1989; Nemani & Whishaw, 2007), it is not likely that the MTL actually serves this role by performing trigonometric computations. Two examples strongly support this point. First, the MTL system is not essential for a rat to learn to solve a spatial problem in a variety of simple mazes, for example, to turn right in a T-maze to find a reward. But this system is essential if the animal must alternate between two turns on the maze or find the location of reward on a multi-arm maze from different starting points (O'Keefe and Nadel, 1978). True navigation involves making trigonometric or other computations that infer novel routes based on known locations and prior traversals of the environment. There is no clear demand for navigational inferences in any of these tasks, and to the extent to which they contribute, the demands for navigational computations are the same in all variants of the tasks. What differs is the demand for remembering and integrating multiple experiences in the form of distinct routes. Second, humans with extensive MTL damage can perform a variety of spatial and navigational computations in environments where prior to MTL damage, they had extensive experience, indicating that the MTL system is not required for spatial computations per se, even as it is required to consolidate the memory of a spatial representation (Teng & Squire, 1999).
If the MEC is not the site of navigational computations, why does it contain grid cells and what is its role in memory? While it is not at all clear why the firing patterns of MEC neurons have a grid-like structure, one suggestion is that this pattern is a consequence of oscillatory interference in the theta rhythms in distinct MEC laminae, combined with spatial signals of head direction and velocity (Burgess et al., 2007; see also Burgess, 2008, in current issue; Giocomo et al., 2007). Notably the strict and consistent grid pattern may be limited to situations where animals continuously move through open fields and linear tracks, and that the multi-peaked spatial firing pattern becomes more complex under circumstances where continuous movements are interrupted by turns and stops (Derdikman et al., 2006) which are prevalent characteristics of behavioral tasks where memory is involved (Lipton et al., 2007). Here we suggest that, whatever the reason for the grid-like pattern that is observed in some situations, it is not essential to the role of MEC in memory. Furthermore, while concurring with the view that the there is a prominent spatial representation within the MEC, we suggest that the MEC also contains a representation of temporal context, and that the combined spatial and temporal context representations serve to link discrete events within memories and at the same time disambiguate memories that contain overlapping events. These functions are essential to episodic memory within spatial as well as non-spatial domains (Eichenbaum et al., 1999; Eichenbaum, 2004).
Acknowledgments
Supported by NIMH MH51570, MH071702 and NSF SBE0354378.
References
- Aggleton JP, Vann SD, Denby C, Dix S, Mayes AR, Roberts N, Yonelinas AP. Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia. 2005;43(12):1810–23. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Alvarado MC, Bachevalier J. Comparison of the effects of damage to the perirhinal and parahippocampal cortex on transverse patterning and location memory in rhesus macaques. J Neurosci. 2005;25:1599–1609. doi: 10.1523/JNEUROSCI.4457-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb Cortex. 2007;17:1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Ishai A. Famous faces activate contextual associations in the parahippocampal cortex. Cereb Cortex. 2008;18:1233–1238. doi: 10.1093/cercor/bhm170. [DOI] [PubMed] [Google Scholar]
- Barry C, Hayman R, Burgess N, Jeffery KJ. Experience-dependent rescaling of entorhinal grids. Nat Neurosci. 2007;10:682–684. doi: 10.1038/nn1905. [DOI] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nature Reviews Neuroscience. 2008;9:182–94. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–20. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Xiang JZ. Recognition memory: Neuronal substrates of the judgment of prior occurrence. Prog Neurobio. 1998;55:149–189. doi: 10.1016/s0301-0082(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Bellgowan PSF, Martin A. Distinct roles for medial temporal lobe structures in memory for objects and their locations. Learning and Memory. 2006;13:638–643. doi: 10.1101/lm.251906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N. Grid cells and theta as oscillatory interference: theory and predictions. Hippocampus. 2008;18(12):xxx–xxx. doi: 10.1002/hipo.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Barry C, O'Keefe J. An oscillatory interference model of grid cell firing. Hippocampus. 2007;17(9):801–812. doi: 10.1002/hipo.20327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: corticocortical connectivity. Ann NY Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Shapiro ML, O'Malley MT, Eichenbaum H. Positional firing properties of perirhinal cortex neurons. Neuroreport. 1998;9:3013–3018. doi: 10.1097/00001756-199809140-00017. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Hafeman DM. Positional firing properties of postrhinal cortex neurons. Neuroscience. 2003;119:577–588. doi: 10.1016/s0306-4522(03)00160-x. [DOI] [PubMed] [Google Scholar]
- Cahusac PMB, Rolls ET, Miyashita Y, Niki H. Modification of the responses of hippocampal neurons in the monkey during the learning of a conditional spatial response task. Hippocampus. 1993;3:29–42. doi: 10.1002/hipo.450030104. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J. Neurosci. 2000;20(23):8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, West AN, Zola SM, Squire LR. Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus. 2001;11:176–86. doi: 10.1002/hipo.1035. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza RE. Triple Dissociation in the Medial Temporal Lobes: Recollection, Familiarity, and Novelty. J. Neurophysiol. 2006;96(4):1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc. Natl. Acad. Sci. U S A. 2003;100:2157–62. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AG. Hippocampal contributions to episodic encoding, Insights from relational and item-based learning. J. Neurophysiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Dayan P, Abbott LF. Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems. MIT Press; Cambridge, MA: 2001. [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Derdikman D, Fyhn M, Hafting T, Moser M-B, Moser EI. Breaking up the entorhinal grid in a hairpin maze. Soc. Neuroscience. 2006 Abs, 68.10. [Google Scholar]
- Dudchencko P, Wood E, Eichenbaum H. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory, but produce significant impairments on spatial span, recognition, and alternation. J. Neurosci. 2000;20:2964–77. doi: 10.1523/JNEUROSCI.20-08-02964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. Oxford University Press; 2001. [Google Scholar]
- Eichenbaum H, Dudchencko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: Is it spatial memory or a memory space? Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;20:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, Fried I. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–87. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Bookheimer SY. Spatial and temporal episodic memory retrieval recruit dissociable functional networks in the human brain. Learn Mem. 2007;14:645–654. doi: 10.1101/lm.575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortial representaion of the local viual environment. Nature. 1998;33392:598601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Epstein RA. The cortical basis of visual scene processing. Visual Cognition. 2005;12:954–978. [Google Scholar]
- Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–64. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- Fyhn M, Hafting T, Treves A, Moser M-B, Moser EI. Hippocampal remapping and grid realignment in entorhinl cortex. Nature. 2007;446:190–194. doi: 10.1038/nature05601. M-B. [DOI] [PubMed] [Google Scholar]
- Gaffan EA, Healey AN, Eacott MJ. Objects and positions in visual scenes: effects of perirhinal and postrhinal cortex lesions in the rat. Behav Neurosci. 2004;118:992–1010. doi: 10.1037/0735-7044.118.5.992. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Zilli EA, Fransen E, Hasselmo ME. Temporal frequency of subthreshold cales with entorhinal grid cell field spacing. Science. 2007;315:1719–1722. doi: 10.1126/science.1139207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanello KS, Verfaellie M, Keane MM. Disproportionate deficit in associative recognition relative to item recognition in global amnesia. Cog.Aff. & Behav.Neurosci. 2003;3:186–94. doi: 10.3758/cabn.3.3.186. [DOI] [PubMed] [Google Scholar]
- Gold JJ, Smith CN, Bayley PJ, Shrager Y, Brewer JB, et al. Item memory, source memory, and the medial temporal lobe: Concordant findings from fMRI and memory-impaired patients. Proc. Natl. Acad. Sci. U S A. 2006;103:9351–56. doi: 10.1073/pnas.0602716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82(1):26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Heyser CJ, Deadwyler SA. Hippocampal cell firing correlates of delayed-match-to-sample performance in the rat. Behav. Neurosci. 1993;107:715–39. doi: 10.1037//0735-7044.107.5.715. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;5729:1792–4. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Arc length coding by interference of theta frequency oscillations may underlie context-dependent hippocampal unit data and episodic memory function. Learning and Memory. 2007;14(11):782–794. doi: 10.1101/lm.686607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. Grid cell mechanisms and function: Contributions of entorhinal persistent spiking and phase resetting. Hippocampus. 2008;18(12):xxx–xxx. doi: 10.1002/hipo.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Eichenbaum H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Networks. 2005;18:1172–1190. doi: 10.1016/j.neunet.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Giocomo LM, Zilli EA. Grid cell firing may arise from interference of theta frequency membrane potential oscillations in single neurons. Hippocampus. 2007;17:1252–1271. doi: 10.1002/hipo.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Nadel L, Ryan L. The effect of scene context on episodic object recognition: parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus. 2007;17:873–889. doi: 10.1002/hipo.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K, Buck A, Weber B, Weisner HG. Human hippocampus establishes associations in memory. Hippocampus. 1997;7:249–256. doi: 10.1002/(SICI)1098-1063(1997)7:3<249::AID-HIPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Henson RN, Cansino S, Herron JE, Robb WG, Rugg MD. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13:259–62. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Gong QY, Roberts N, Kapur N. Item recognition is less impaired than recall and associative recognition in a patient with selective hippocampal damage. Hippocampus. 2005;15(2):203–15. doi: 10.1002/hipo.20046. [DOI] [PubMed] [Google Scholar]
- Kerr KM, Agster KL, Furtak SC, Burwell RD. Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus. 2007;17(9):697–708. doi: 10.1002/hipo.20315. [DOI] [PubMed] [Google Scholar]
- Kreiman K, Kock C, Fried I. Category specific visual responses of single neurons in the human medial temporal lobe. Nature Neurosci. 2000;3:946–953. doi: 10.1038/78868. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10:420–30. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Moser M-B, Moser EI. Place cells, spatial maps, and the population code for memory. Current Opinion in Neurobiology. 2005;15:1–9. doi: 10.1016/j.conb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Lipton PA, White J, Eichenbaum H. Disambiguation of overlapping experiences by neurons the medial entorhinal cortex. Journal of Neuroscience. 2007;27:5787–5795. doi: 10.1523/JNEUROSCI.1063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, Mishkin M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J Neurosci. 2003;23:1956–1965. doi: 10.1523/JNEUROSCI.23-05-01956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16:795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37:171–80. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Hunkin NM, Roberts N. Relative sparing of item recognition memory in a patient with adult-onset damage limited to the hippocampus. Hippocampus. 2002;12:325–40. doi: 10.1002/hipo.1111. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser M-B. Path integration and the neural basis of the ‘cognitive map’. Nature Reviews Neuroscience. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. Activity of neurons in the anterior inferior temporal cortex during a short-term memory task. J. Neurosci. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moita MAP, Moisis S, Zhou Y, LeDoux JE, Blair HT. Hippocampal place cells acquire location specific location specific responses to the conditioned stimulus during auditory fear conditioning. Neuron. 2003;37:485–97. doi: 10.1016/s0896-6273(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser M-B. A metric for space. Hippocampus. 2008;18(12):xxx–xxx. doi: 10.1002/hipo.20483. [DOI] [PubMed] [Google Scholar]
- Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behav Brain Res. 2001;127(1-2):159–81. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J. Neurosci. 1998;18:6568–82. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J Neurosci. 2004;24(8):2013–26. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behav Neurosci. 2005;119:557–566. doi: 10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford University Press; New York: 1978. [Google Scholar]
- Olton DS. Hippocampal function and memory for temporal context. In: Isaacson RL, Pribram KH, editors. The Hippocampus. Vol. 4. Plenum Press; New York: 1986. [Google Scholar]
- Otto T, Eichenbaum H. Neuronal activity in the hippocampus during delayed non-match to sample performance in rats: Evidence for hippocampal processing in recognition memory. Hippocampus. 1992;2:323–34. doi: 10.1002/hipo.450020310. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Kononen M, Hanninen T, Hamalainen A, Soininen H, Aronen HJ. Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe subareas in humans. Eur J Neurosci. 2004;19:1939–49. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Muller RU, Kubie JL, Ranck JB., Jr. The positional firing properties of medial entorhinal neurons: Description and comparison with hippocampal place cells. J Neurosci. 1992;12:1945–1963. doi: 10.1523/JNEUROSCI.12-05-01945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2003;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Ross RS, Slotnick SD. The hippocampus is preferentially associated with memory for spatial context. J Cogn Neurosci. 2008;20:432–446. doi: 10.1162/jocn.2008.20035. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Mamelak AN, Schuman EM. Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron. 2006;49:805–813. doi: 10.1016/j.neuron.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser M-B, Moser EI. Conjuctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nature Reviews Neuroscience. 2007;8:872–83. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Hippocampal damage equally impairs memory for single items and memory for conjunctions. Hippocampus. 2003;13(2):281–92. doi: 10.1002/hipo.10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR. The hippocampal formation participates in novel picture encoding, Evidence from functional MRI. Proceedings of the National Academy of Science, USA. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J. Neurophysiol. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- Suzuki W, Eichenbaum H. The neurophysiology of memory. Ann New York Acad Sci. 2000;911:175–191. doi: 10.1111/j.1749-6632.2000.tb06726.x. [DOI] [PubMed] [Google Scholar]
- Teng E, Squire LR. Memory for places learned long ago is intact after hippocampal damage. Nature. 1999;400:675–677. doi: 10.1038/23276. [DOI] [PubMed] [Google Scholar]
- Turriziani P, Fadda L, Caltagirone C, Carlesimo GA. Recognition memory for single items and associations in amnesia patients. Neuropsychologia. 2004;42:426–33. doi: 10.1016/j.neuropsychologia.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Wais PE, Wixted JT, Hopkins RO, Squire LR. The hippocampus supports both the recollection and the familiarity components of recognition memory. Neuron. 2006;49:459–66. doi: 10.1016/j.neuron.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Aggleton JP, Brown MW. Different contributions of the hippocampus and perirhinal cortex to recognition memory. J Neurosci. 1999;19:1142–1148. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani F, Whishaw IQ. The point of entry contributes to the organization of exploratory behavior of rats on an open field: an example of spontaneous episodic memory. Behav Brain Res. 2007;182:119–128. doi: 10.1016/j.bbr.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J. Neurosci. 2005;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: Heterogeneity of function within the temporal lobe. J. Neurosci. 2004;24(26):5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth S, Yanike M, Frank LM, Smith AC, Brown EN, Suzuki WA. Single neurons in the monkey hippocampus and learning of new associations. Science. 2003;300:1578–81. doi: 10.1126/science.1084324. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog. in Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Lopes da Silva FH. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000;10:398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Wood E, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–16. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- Wood E, Dudchenko P, Robitsek JR, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–33. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–41. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. J. Neurosci. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XO, Brown MW, Aggleton JP. Neuronal signaling of information important to visual recognition memory in rat rhinal and neighboring cortices. Eur. J. Neurosci. 1995;7:753–765. doi: 10.1111/j.1460-9568.1995.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J. Neurosci. 2000;20:451–63. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


