Abstract
Cytomegalovirus (CMV) is the leading transmittable cause of congenital brain abnormalities in children and infection results in fatal ventriculoencephalitis in advanced AIDS patients. Pathology associated with CMV brain infection is seen predominantly in the periventricular region, an area known to harbor neural stem cells (NSCs). In the present study, using an adult model of murine CMV brain infection, we demonstrated that nestin-positive NSCs in the subventricular zone are susceptible to murine CMV infection. Furthermore, primary NSC cultures supported productive murine CMV replication with a 1000-fold increase in viral titers by 5 d post-infection (d p.i). Previous studies from our laboratory demonstrated that CD8 lymphocytes were essential in protecting the brain against murine CMV infection. In the present study we found that interferon (IFN)-gamma treatment increased the expression of MHC class I on NSCs. Viral infection, on the other hand, inhibited this IFN-gamma-induced MHC upregulation. In addition to increasing MHC class I expression, IFN-gamma (but not tumor necrosis factor [TNF]-alpha, interleukin [IL]-1beta, or IL-10) also suppressed NSC proliferation in vitro. This decrease in proliferation was not accompanied by apoptosis or extracellular release of cellular lactate dehydrogenase (LDH), suggesting that the effects were not due to direct cytotoxicity. These studies demonstrate that NSCs are susceptible to murine CMV infection and inflammatory mediators, such as IFN-gamma, alter cellular characteristics which may have an impact on their reparative functions.
INTRODUCTION
Cytomegalovirus (CMV) brain disease is predominantly seen during congenital infection in the developing fetus or as a fatal ventriculoencephalitis in the severely immunocompromised adult with advanced HIV-1 disease. In both congenitally infected children and adult brain infections, CMV preferentially infects cells in the ventricular or subventricular regions (Grassi et al, 1998; Perlman and Argyle, 1992; Schmidbauer et al, 1989). This periventricular predilection has also been recapitulated in mouse models of congenital CMV (Li and Tsutsui, 2000). The subventricular region of the brain is known to harbor neural stem cells (NSCs), a population of undifferentiated cells that have been retained in the adult brain from fetal development which have the ability to migrate, proliferate, and differentiate into neurons, astrocytes, and oligodendrocytes. These cells potentially repopulate damaged brain tissue and aid in the establishment of new neuronal circuits during memory formation in the adult (Gage, 2000; Ni et al, 2004; Temple, 2001).
The neuropathological outcomes associated with CMV brain infection may result from damage or functional modulation of NSCs. Congenital CMV infection is the major cause for birth defects and childhood disorders in the United States. Approximately 8000 children are affected each year with some neurological sequelae related to congenital CMV infection. This statistic surpasses many of the better known childhood disorders, like Down syndrome, fetal alcohol syndrome, or Spinal bifida (Cannon and Davis, 2005). However, little is known about the neuropathogenesis of CMV brain infection. Our laboratory and others have shown that human neural precursor cells are susceptible to CMV infection (Cheeran et al, 2005b; McCarthy et al, 2000; Odeberg et al, 2006). CMV infection has also been shown to alter the cellular differentiation profiles of neural precursor cells (Odeberg et al, 2006; Odeberg et al, 2007). In experimental murine CMV infections, it has been shown that IE gene expression is retained in the cortex of the postnatal brain infected in utero, presumably resulting from the maturation of infected neural stem cells into neurons (Ishiwata et al, 2006). Similar expression of IE in neurons of the cerebellum is associated with delayed maturation and migration of precursors cells (Koontz et al, 2008). A more lucid understanding of the interaction between CMV and NSCs is essential to delineate the neuropathogenic mechanisms of viral infection.
Previously, we have shown that murine CMV brain infection induces a transient increase in proinflammatory cytokine production and leukocyte accumulation that is protective in immunocompetent adult mice (Cheeran et al, 2004). The neuroinflammatory response involves expression of chemokines, consequent trafficking of peripheral immune cells into the brain, subsequent regulation of these responses by anti-inflammatory cytokines, and ultimately resolution of infection. When these inflammatory processes are interrupted, either in immunodeficient animals or in IL-10 deficient animals, murine CMV brain infection turns lethal (Cheeran et al, 2005a; Cheeran et al, 2007). Successful defense against murine CMV brain infection requires CD8 (+) T lymphocytes, via a perforin-mediated mechanism to clear infection. Meanwhile, cells of the central nervous system (CNS) are known to express relatively low MHC class I levels. Viral infection and consequent IFN-gamma expression increase MHC expression on neurons and glia, (Rodriguez et al, 2003) potentially influencing pathogen clearance from the CNS. In the present study, we investigated the role of murine CMV infection and neuroinflammatory mediators in altering MHC class I expression on NSC. Additional studies were performed to investigate the role inflammatory cytokines on NSC self-renewal responses, essential to maintain germinal areas of the brain.
Methods
Viruses
RM461, a recombinant murine CMV expressing E. coli β-galactosidase under the control of the human ie1/ie2 promoter/enhancer (Stoddart et al, 1994), was provided by Edward Mocarski. Viral stocks were passaged in murine salivary glands to retain their virulence. Virus isolated from the salivary glands was then passaged once on NIH3T3 fibroblasts, followed by purification over a sucrose gradient. Sucrose gradient-purified RM461 was used for intracerebroventricular (icv) infections. A recombinant murine CMV expressing green fluorescent protein (GFP) under control of the human elongation factor-1a promoter inserted at the immediate-early gene (IE2) site (strain K181 MC.55 [ie2− GFP+]) was provided by Jon Reuter, (Reuter et al, 2004; van Den Pol et al, 1999). The GFP expressing virus was expanded on NIH 3T3 mouse fibroblasts and purified by centrifugation over a sucrose cushion.
Intracerebroventricular infection
CMV infection of mice was performed via an icv route as described previously (Cheeran et al, 2004). Briefly, female wild-type BALB/c (8–10 weeks) were anesthetized using a combination of Ketamine and Xylazine (100 mg and 10 mg/Kg body weight, respectively) and immobilized on a small animal stereotactic instrument equipped with a Cunningham mouse adapter (Stoelting Co., Wood Dale, IL). The skin and underlying connective tissue were reflected to expose reference sutures (sagittal and coronal) on the skull. The sagittal plane was adjusted such that the bregma and lambda were positioned at the same coordinates on the vertical plane. Salivary gland passaged murine CMV RM461 (1.5×105 TCID50), was injected slowly into the right lateral ventricle at 0.9 mm lateral, 0.5 mm caudal to the bregma and 3.0 mm ventral to the skull surface using a Hamilton syringe fitted to a 28 G cannula. The injection was delivered over a period of 3–5 min. The opening in the skull was sealed with bone wax and the skin closed using 9 mm wound clips (Stoelting Co., Wood Dale, IL).
Immunohistochemistry
Immunofluorescent staining was performed using the protocol previously described (Lokensgard et al, 1999). Briefly, brains were harvested from infected mice that were sacrificed and perfused with serial washes of 2% sodium nitrate and phosphate buffered saline (PBS) to remove contaminating blood cells and pre-fixed with 4% paraformaldehyde. Murine brains were subsequently submerged in 4% paraformaldehyde for 24 h and transferred to 30% sucrose solution for 2 d. Brain tissue slices (30 µm) were stained with a monoclonal antibody to beta-galactosidase (Sigma, St. Louis, MO) or nestin (10 µg/ml; Chemicon, Temecula, CA), followed by a biotin-labeled donkey anti-mouse IgG antibody (Jackson Immunoresearch, West Grove, PA) and a fluorescein-streptavidin conjugate (Vector Laboratory, Burlingame, CA). Dual immunofluorescent staining for beta-galactosidase and nestin was performed as described above and was counter stained with 4’,6-diamidino-2-phenyindole (DAPI), a nucleic acid dye (Chemicon, Temecula, CA).
Murine NSC culture
Murine NSCs were cultured from the brains of E14.5 d fetal mice, using conditions described by Kim et al. (Kim and Morshead, 2003) and our previous studies Ni et al. (Ni et al, 2004) with few modifications. Briefly, timed-pregnant mouse embryos (E14.5) were dissected in Hank’s buffer (Sigma, St. Louis, MO). Embryos were measured and examined for morphological hallmarks characteristic of the correct gestational time. After decapitation and removal of skin, skull, and meninges, cerebral cortices were collected and mechanically triturated in Hank’s buffer. Dissociated cells were collected and resuspended in serum-free medium containing: DMEM/F12, 8 mM glucose, glutamine, 20 mM sodium bicarbonate and N2 Plus supplement (R&D systems, Minneapolis, MN). Dissociated cells (2 × 105) were then plated on 10-cm diameter dishes, pre-coated with 15 µg/ml poly-L-ornithine and 1 µg/ml bovine fibronectin (R&D systems) and cultured in N2 medium supplemented with bFGF (20 ng/ml) and EGF (20 ng/ml, R&D systems). Cultures were maintained at 37°C in a 5% CO2 incubator and monolayers were subcultured at 60–70% confluence in N2 Plus medium with bFGF and EGF. NSCs were induced to differentiate by addition of 2% FBS (Hyclone) and the withdrawal of bFGF and EGF.
Immunostaining of NSCs
Undifferentiated and differentiated NSC cultures were fixed with 4% paraformaldehyde and stained for nestin (NSC), Tuj1 (a neuronal marker), GFAP (astrocytes), and O4 (oligodendrocytes). Anti-nestin, anti-Tuj1, and anti-O4 (R&D Systems) antibodies were used at 10 µg/ml concentrations and anti-GFAP antibody (Dako, Carpentaria, CA) was used at 1:200. Cells were fixed with 4% paraformaldehyde and 0.15% picric acid in PBS at room temperature for 20 min and were then permeated and blocked with 0.1% triton X-100, 1% BSA and 10% normal donkey serum in PBS at room temperature for 45 min. After blocking, cells were incubated with diluted primary antibody overnight at 4° C and sequentially with fluorescence-coupled anti-mouse IgG Ab (Jackson Laboratory, Bar Harbor, Maine) at room temperature in the dark for 1 h. Cells were washed between each step with 0.1% BSA in PBS.
Real-time PCR
Total RNA and DNA were extracted from brain tissue homogenates using the Trizol Reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized using 1 µg of total RNA, SuperScript II reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA) and oligo dT6–12 primers (Sigma-Genosys, The Woodlands, TX). Quantitative real-time PCR was performed using the FullVelocity SYBR Green QPCR master mix (Stratagene, La Jolla, CA) following the manufacturer’s specifications. The 25 µl final reaction volume consisted of pre-made reaction mix (SYBR Green I dye, reaction buffer, Taq DNA polymerase, and dNTPs), 0.3 mM of each primer, and 0.5 ng cDNA in water. Reaction conditions for PCR for the Mx3000P QPCR System (Stratagene) were as follows: polymerase activation at 95° C for 5 min, 40 denaturation cycles of 95° C for 10 s, annealing at 60° C for 10 s and elongation at 72° C for 10 s. Primers for murine CMV were designed from the gene encoding glycoprotein B (gB; GenBank accession no. M86302, 5’-CGCTGGTCGTCTTTCAGTTC-3’ and 5’-CTGTTCGTGTCGCAGTTCTC-3’, 112 bp product). Primers recognizing the housekeeping gene β-actin were designed from the mouse β-actin DNA sequence (GenBank accession no. NM_007393, 5’-GGGCTATGCTCTCCCTCAC-3’ and 5’-GATGTCACGCACGATTTCC-3’, 100 bp product). A melting curve analysis was performed to assess primer specificity and product quality by denaturation at 95 °C, annealing at 65 °C and melting at a rate of 0.1 °C/sec to 95° C. The relative levels of gB expression were quantified using the 2(-Delta Delta CT) method (Livak and Schmittgen, 2001).
Flowcytometry analysis of NSCs
NSCs were stained for nestin expression by fixing cells in 4% paraformaldehyde (in PBS) for 20 min and incubating in SAP buffer (2% FCS, 0.5% saponin, and 0.1% sodium azide in PBS) containing anti-rat nestin-PE monoclonal antibody or IgG-PE isotype antibody (R&D Systems, MN). Following incubation for 30 min at room temperature the cells were washed once with SAP buffer, once with PBS, resuspended in 400 µl of PBS and analyzed on a FACScan flow cytometer (Becton-Dickinson, Mountain View, CA). Five thousand events were collected and analyzed using CELL Quest software. To study the dynamics of MHC class 1 expression on NSCs, monolayer cultures were dissociated into single cell suspension by incubating in Ca++/Mg++ free Hanks balanced salt solution (without trypsin) and pre-incubated with anti- CD32/CD16 monoclonal antibody (2.4G2) to inhibit nonspecific binding. Both infected and uninfected cells were then stained with an anti-H-2Db specific, PE-conjugated monoclonal antibody (BioLegend, San Diego, CA). Data were collected on a BD FACSCanto flow cytometer (Becton-Dickinson, CA) and analyzed using FlowJo (Tree Star Inc, CA).
MTT assay: Analysis of live cell density in NSC cultures
MTT assay is a means of measuring the activity of living cells via mitochondrial dehydrogenase activity. After designated treatment time periods, MTT ([3-(4,5-Dimethylthiazol-2-yl)-2,5- Diphenyltetrazolium Bromide]; final concentration of 1 mg/ml) was added to NSC cultures for 4 h followed by addition of lysis buffer (20% SDS [w/v] in 50% N,N-dimethyl formamide, pH 4.7, adjusted with 2.5% acetic acid and 1N HCl [32:1]) for 16 h. Cell lysates were collected and absorbance will be read at 600 nm (Molecular Devices, Sunnyvale, CA) to reflect uptake of MTT by live cells.
LDH assay: Analysis of cytotoxicity in cytokine-treated cultures
Cytotoxicity of cytokine treatment was assayed using the CytTox96 cell death assay (Promega), which measures the extracellular release of LDH into the media by dead cells, according to the manufacturer’s instructions. Data obtained as absorbance values of treated cells were expressed as a percentage of untreated cells (± SD) after correcting for background from media without cells.
3H-thymidine uptake assay: Analysis of cell proliferation
Interferon-gamma was added 24 h after plating NSCs onto 24-well culture plates for 48 h. 3H-thymidine was added to the culture (1 µci per well) and incubated overnight before being harvested and measured for 3H-thymidine incorporation using a beta-counter.
Results
Infection of neural stem cells in vivo
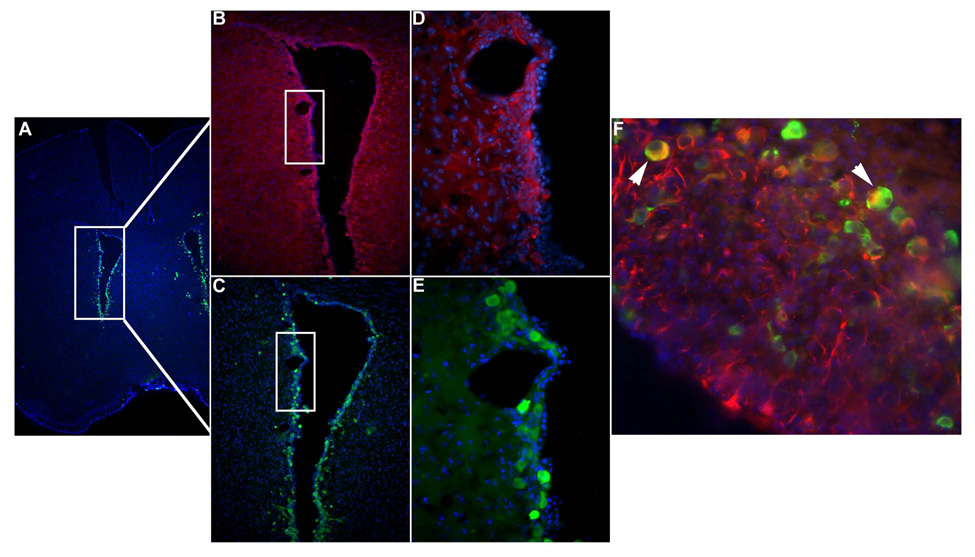
Previous studies performed in our laboratory have shown that subsequent to an icv infection with murine CMV, viral expression was restricted to the periventricular region in immunocompetent animals and spread into the parenchyma only in the absence of an effective CD8 response (Cheeran et al, 2004; Cheeran et al, 2005a). To determine if infected cells around the ventricles were NSCs, we assayed adjacent coronal brain sections from murine CMV infected SCID mice for immunoreactivity to β-galactosidase expression (reporter gene expressed by the virus) and nestin (a stem cell marker) at 5 d p.i. As expected viral gene expression was detected predominantly around the ventricles. The adjacent tissue sections showed overlapping regions of immunoreactivity to nestin (Fig 1). Furthermore, double immunostaining of brain sections for β-galactosidase and nestin showed dual immunoreactivity confirming that the stem cells were susceptible to CMV infection in vivo (Fig 1F).
Figure 1. MCMV-infection of endogenous NSCs in vivo.
Coronal murine brain sections immunostained with anti-β-galactosidase antibody display staining indicative of viral infection with the Lac Z-containing recombinant MCMV RM461. (A & C) Lower magnifications demonstrate that MCMV is localized to cells surrounding the ventricles. (E) Higher power micrographs demonstrate that the infection occurs in periventricular cells (green cells). (B & D) Adjacent serial sections, immunostained to detect the stem cell marker nestin, show that NSCs are co-localized to the infected region (red cells). (F) Double-immunohistochemical staining for β-galactosidase and nestin reveals that MCMV infects NSCs in vivo (white arrows point to dual positive yellow cells).
In vitro infection of NSCs
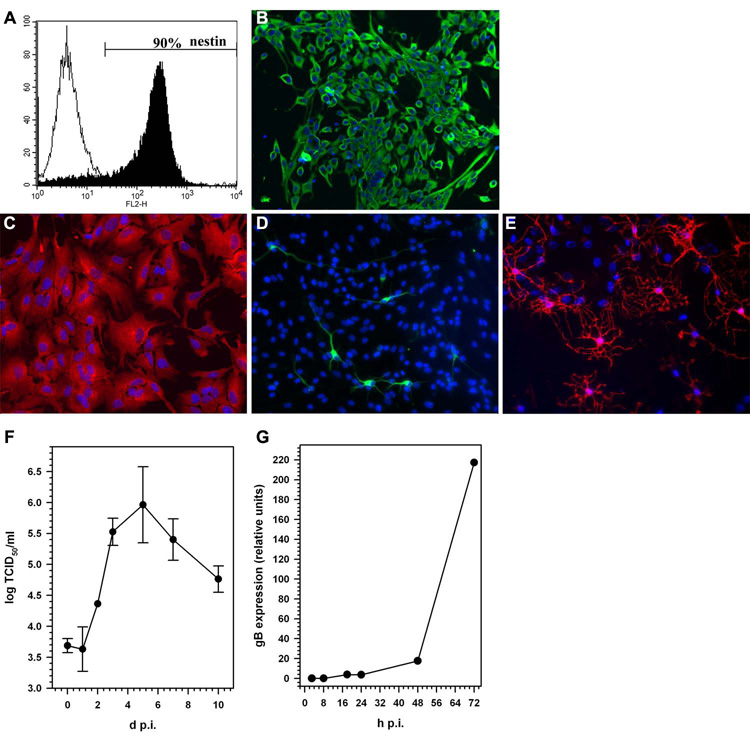
Murine CMV infection of stem cells was further characterized using NSC cultures derived from mouse embryonic brains (E14.5). To ensure purity of the culture system and multipotency of cells in vitro, NSC were analyzed for nestin expression and assayed for their ability to differentiate into astrocytes, neurons, and oligodendrocytes. When grown under serum free conditions, in selective stem cell growth media containing EGF and FGF, >90% of the cultured cells were positive for nestin expression (Fig. 2A and B). When NSC were induced to differentiate using media containing serum, these cells differentiated into astrocytes (expressing glial fibrillary acidic protein, GFAP), neurons (expressing Class III β-tubulin), and oligodendrocytes (immunoreactive for O4) within 7 d (Fig 2C, D and E). The majority of the cells in differentiated cultures were of an astrocyte phenotype, with approx 5% of the cells expressing neuronal or oligodendrocyte markers. The undifferentiated NSC cultures supported productive viral replication. One-step growth kinetics of murine CMV in NSC cultures showed a 2–3 log increases in viral titer, with peak viral growth at 5 d p.i. (Fig 2F). Expression of viral glycoprotein, gB commenced at 48 h p.i. and continued to increase over time (Fig 2G).
Figure 2. Murine NSCs are permissive for MCMV replication.
NSCs obtained from E14.5 d fetal mice were analyzed by immunochemical staining and flow cytometry for a marker of stem cells. (A) Approximately 90% of the cells in the NSC cultures stain positive by flow cytometry using PE-conjugated anti-nestin antibody. (B) Immunohistochemical staining of undifferentiated NSCs for the characteristic stem cell marker nestin (green cells), along with nuclear staining by DAPI (blue). Following in vitro differentiation in medium containing 2% FBS for 7 d, cells were stained with (C) anti-GFAP antibody (red), an astrocyte marker, contrasted with DAPI nuclear staining (blue); (D) anti-Tuj-1 antibody (green), an early neuronal cell marker, contrasted with DAPI nuclear staining (blue); and (E) anti-O4 antibody (red), an oligodendrocyte marker, contrasted with DAPI nuclear staining (blue). (E) One-step growth curve for murine CMV (Smith strain) in NSC monolayer cultures measured by 50% tissue culture infectious dose (TCID50) assay. NSCs were infected with murine CMV (MOI = 1), freeze-thaw cell lysates of infected cultures were harvested at the indicated time points and viral titers were determined by TCID50 assay on NIH 3T3 cells. Viral titers at d 0 represent MCMV detected after 2 h of adsorption and subsequent washing. Data are presented as mean (± SEM) titers pooled from three independent experiments using cells isolated from different brain tissue specimens. (F) Quantitative real-time PCR demonstrating the kinetics of murine CMV glycoprotein B (gB) expression in infected NSCs.
Interferon-gamma stimulates MHC class 1 expression on NSCs
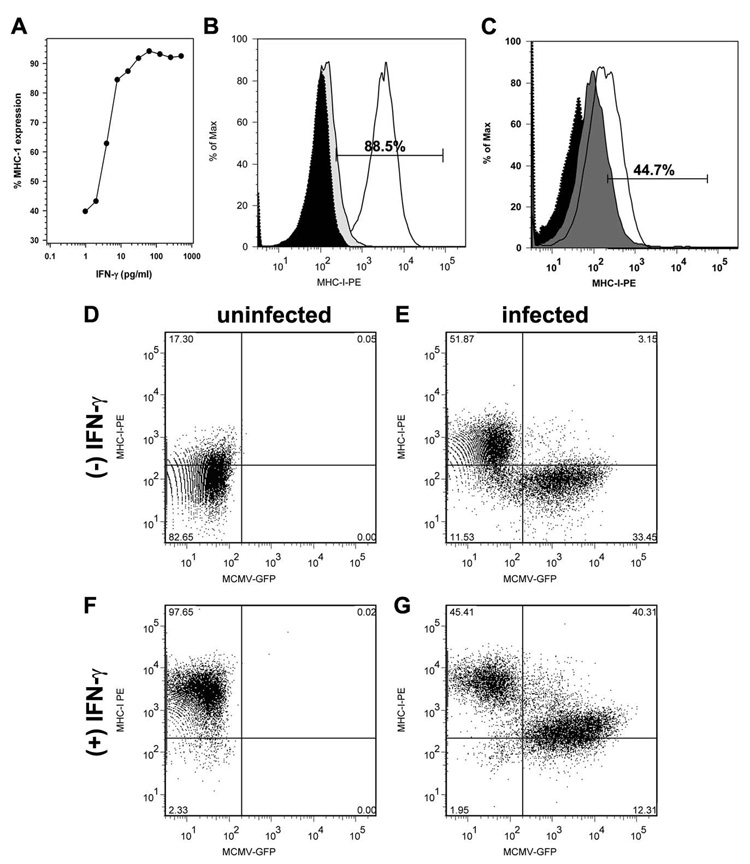
To determine if NSCs could potentially serve as targets for CD8 T-cell recognition, MHC class I expression was evaluated on both infected and uninfected cells. Relatively low levels of expression were detected on culture-derived NSCs by flow cytometry. MHC-1 expression was detected in 18.5% ± 3.7% of untreated NSCs (Fig 3) derived from multiple mouse embryos (ranging from 8%–30%) with mean fluorescence intensity (MFI) of 258 ± 80 (vs. isotype controls = 52.8 ± 12.1). We next asked if inflammatory mediators, like INF-γ, TNF-α, IL-10, and IL-6, that are expressed during murine CMV brain infection (Cheeran et al, 2004; Cheeran et al, 2007), alter MHC-1 expression. Within 24 h of treatment with IFN-γ (25–100 pg/ml; 2–8 IU/ml), MFI for MHC class 1 expression increased about 18 fold to 4941 ± 414 and was expressed on 88 – 95% of the NSCs (Fig 3A and B). At 40 h post-treatment, MFI of class 1 expression further increased reaching 8833 ± 250 (a 30-fold increase over untreated cells). The effect of IFN-γ on NSCs was also dose responsive, inducing saturating levels (>99%; MFI > 9500) class 1 expression at 62 pg/ml (Fig 3A). In addition, after 24 h post-treatment with tumor necrosis factor (TNF)-α the proportion of NSCs expressing MHC class 1increased from to 18.5 ± 3.7% in untreated cultures to 37.9 ± 5.4% with cytokine treatment (p = 0.016; Fig 3C). No further increase in class I expression was observed at 48 h. Finally, interleukin (IL)-6 and IL-10 had no effect on MHC class I expression on NSCs.
Figure 3. Murine CMV downregulates IFN-γ-induced MHC class I expression on NSCs.
NSCs were cultured for 4 d (to 60% confluence) and examined for MHC class 1 expression by flow cytometry. (A) Concentration-response curve showing the effect of increasing amounts of IFN-γ on class I expression. (B) Representative data demonstrating Class I MHC on approximately 88.5% of the NSCs after a 24 h treatment with IFN-γ (30 pg/ml) and (C) on 45% of NSC treated in TNF-α.. NSC cultures were either (D) left untreated, (E) infected with MCMV (30 h), (F) treated with IFN-gamma (30 h), or (G) treated with IFN-gamma (30 h) and simultaneously infected with GFP-expressing recombinant murine CMV. MHC class I expression was assessed using flow cytometry.
Viral infection inhibits MHC class 1 expression on NSCs
We next determined if murine CMV infection would inhibit class I expression on NSC. Using a recombinant murine CMV expressing a GFP reporter (Reuter et al, 2004) to identify infected cells, MHC class 1 expression was analyzed on infected NSCs. MHC expression remained unaltered in GFP-expressing infected cells at 24 h p.i. However, an increase (approx 2 fold) in MHC expression was seen in the uninfected fraction of cultured NSC (Fig 3 D&E). Both MFI values (455 ± 178) and the percentage of MHC-1 expressing cells increased in the GFP negative fraction compared to uninfected cells. In addition, murine CMV infection markedly decreased IFN-γ-induced MHC class 1 expression in NSC (Fig 3 F&G). MFI values decreased significantly with murine CMV infection (from 4941 ± 414 to 1610 ± 538; p<0.001). Conversely, IFN-γ stimulated-uninfected (GFP-negative) cells retained surface MHC expression. Similar proportions of GFP-positive cells were observed in both IFN-gamma-treated and untreated NSC cultures, suggesting that IFN-γ treatment did not alter the ability of CMV to infect NSCs. Additionally, MCMV replication (as measured by a TCID50 assay on NIH 3T3 cells) was not affected by IFN-γ -treatment at various doses between 3 – 80 pg/ml (0.3 –6 IU/ml).
IFN-γ treatment inhibits NSC proliferation
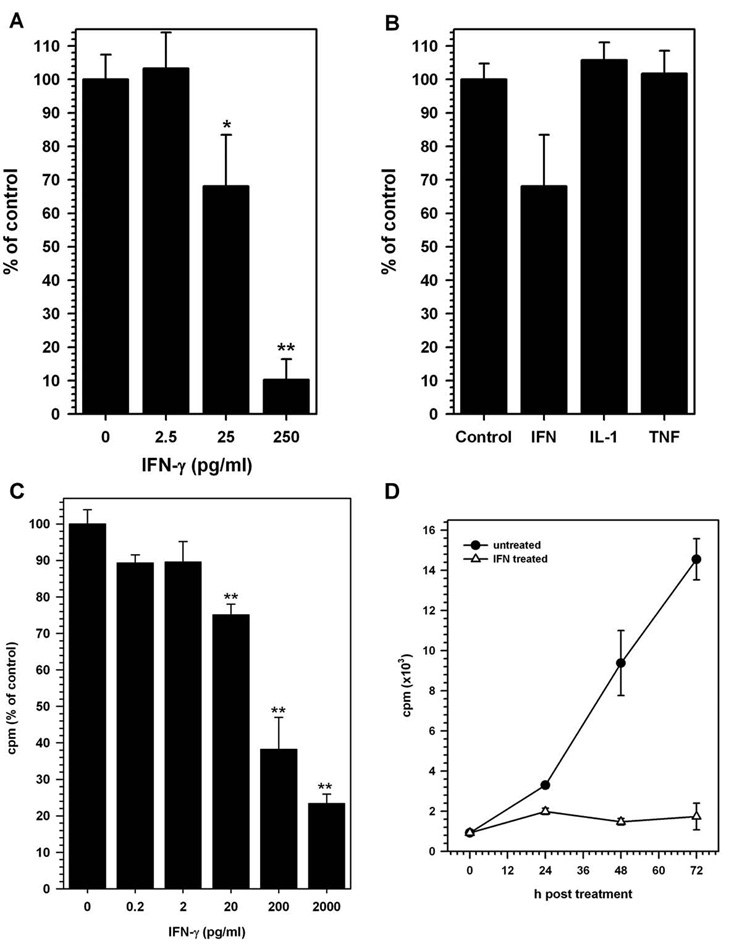
To determine if IFN-γ treatment altered NSC proliferation, varying doses of IFN-γ were added to undifferentiated cultures for 4 d and the cells were assessed for proliferative activity by measuring live cell density using an MTT uptake assay. IFN-γ treatment, using as little as 20 IU (250 pg/ml), suppressed NSC proliferation by 90% compared to untreated cultures (p<0.01). This inhibitory response was dose-responsive in that 2 IU (25 pg/ml) of IFN-γ suppressed proliferation to 30% of untreated cells (p = 0.014) and 2.5 pg/ml (0.2 IU) had no effect (Fig 4 A). Treatment with other pro- and anti-inflammatory cytokines, like IL-1β, TNF-α and IL-10 had no significant effect on NSC proliferation (Fig 4 B). To further confirm that this inhibition of cell proliferation was not due to toxicity and cell death, we tested the supernatants obtained from cytokine-treated NSC cultures for LDH release, indicative of cell death and loss of membrane integrity. No significant increase in LDH release was detected with any of the cytokines used in this study. 3H-thymidine uptake, a direct measure of NSC proliferation, was also significantly decreased with IFN-γ-treatment at 20 pg/ml, 0.2 ng/ml and 2 ng/ml (p<0.001; Fig 4C). Furthermore, this decrease in cell proliferation was evident as early as 24 h post IFN-γ treatment (Fig 4D). Furthermore, analysis of DNA fragmentation by Hoechst staining revealed no increase in apoptotic nuclei in IFN-γ-treated NSC cultures at doses <250 pg/ml (20 IU; data not shown).
Figure 4. IFN-gamma and cytokine effects on NSCs.
NSCs were treated with the indicated cytokine for 4 d. MTT uptake assay was performed on d 4 post-treatment to determine the effects of cytokine treatment on NSC proliferation. (A) Dose-dependent decrease in NSC growth was observed following IFN-gamma treatment (* p=0.014; ** p<0.01). (B) IL-1beta (IL-1) and TNF-alpha (TNF) did not alter NSC proliferation. Data are presented as percent of control (untreated cells) from 3 experiments at 4 d post cytokine treatment (± SD). (C) Similar dose dependent decrease in 3H-thymidine uptake by IFN-γ treated NSC was observed. Data presented as % of untreated control were obtained from 2 separate experiments with duplicate wells for each treatment. (D) This decrease was evident as early as 24 h post treatment with the cytokine as demonstrated by the kinetics of cell proliferation in IFN-treated (open triangles) and untreated (closed circle) cultures.
Discussion
In the present study we demonstrate that murine CMV replicates in neural stem cells both in vivo and in vitro. Previous studies using human neural precursor cells showed similar 1000-fold increases in human CMV replication with detectable immediate early gene expression at 8 h p.i. (Cheeran et al, 2005b). Other published studies have shown that immature neural cells in the subventricular zone of the developing mouse brain are susceptible to CMV infection (Li and Tsutsui, 2000), and their numbers are closely associated with susceptibility of the brain to CMV infection.
CMV brain infection is associated with a transient but robust inflammatory response that includes the expression of soluble mediators and drives the infiltration of systemic immune cells Using an adult murine model of CMV brain infection, we have demonstrated that systemic T lymphocytes, which infiltrate the brain, are responsible for the protection of immunodeficient animals against CMV infection, via a perforin-dependent CD8+ T lymphocyte response (Cheeran et al, 2004; Cheeran et al, 2005a). A similar study reported that murine CMV-specific immune cells, particularly CD4+ T-cells, rapidly cross the blood-brain barrier, congregate at sites of specific MCMV infection, and eliminate acute MCMV within the brain (Reuter et al, 2005). These studies suggest that infiltrating T-cells clear viral brain infection potentially through an MHC dependent, perforin-mediated cytotoxic T lymphocyte (CTL) response.
In the present study, we demonstrated that very low levels of MHC class I molecules are normally expressed on neural stem cells. Lack of MHC expression, in conjunction with the immunosuppressive CNS microenvironment afforded by anti-inflammatory cytokines such as transforming growth factor (TGF)-beta (Mammolenti et al, 2004; Ubiali et al, 2007), may allow this critical cell population to remain unrecognized by the immune system. Lack of MHC expression not only prevents NSC recognition by CTL, but also inhibits natural killer cell activity, keeping these cells immunologically protected in the non-inflamed brain (Hori et al, 2003; Mammolenti et al, 2004). Infection of the brain results in a robust neuroinflammatory response, which includes expression of TNF-alpha, IFN-gamma, and IL-10 (Cheeran et al, 2004; Cheeran et al, 2007). Here, we have shown that treatment with both TNF-α and IFN-γ increases MHC-1 expression on neural stem cells, a finding which indicates that the neuroinflammatory microenvironment induced by infection may abrogate the immune privilege afforded to these cells.
MHC class 1 expression on NSC increased robustly following IFN-gamma treatment, attaining levels of up to 30-fold greater that untreated cells. Similar studies by Mammolenti, et al. have shown that IFN-gamma upregulates class I expression on neural stem cells without altering their multipotent ability to differentiate into various cell types (Mammolenti et al, 2004). In the present study we show that saturating levels of MHC-1 expression are attained at relatively low concentrations (62 pg/ml) of IFN-gamma, 2.5–5 IU /ml (R&D systems, MN). Treatment with IFN-gamma has been demonstrated to make neural stem cells susceptible to CTL activity (Mammolenti et al, 2004), potentially rendering the infected germinal centers of the brain susceptible to depletion by the systemic immune response.
Several viral genes that target MHC class I expression pathways are carried by both murine and human CMV, which are well known for their ability to evade the immune system in an infected host. In the present study, we demonstrate that CMV infection inhibits MHC I expression in the uninfected and IFN-gamma treated NSC. The inhibition of MHC expression was seen in the infected cell population without affecting expression in the bystander/uninfected population. Murine CMV expresses three viral genes (m4, m6 and m152) that inhibit MHC-I expression by either inhibiting the complex from reaching the surface or by associating with the complex at the cell surface (Hengel et al, 1999). Through inhibition of MHC expression, the virus has developed distinct advantages which allow better establishment of infection and latency as well as more efficient transmission (Doom and Hill, 2008). In the developing CNS, inhibition of MHC may have an effect on development of neural circuits, since recent studies have demonstrated novel roles for MHC class I that involve neuronal differentiation, plasticity, establishment of synapses, and modulating synaptic strength (Boulanger, 2004; Goddard et al, 2007; Huh et al, 2000). Viral inhibition of MHC class I expression may thus have a wide-ranging impact on CMV neuropathogenesis.
Interactions between the immune system and neural stem cells are critical not only in brain development, but also in reparative processes during injury. We found that IFN-gamma inhibits NSC proliferation in a dose-dependent manner. This decrease in proliferation was measured both in terms of the total density of live cells post treatmtent IFN-gamma and by 3H-thymidine uptake by cells. Decreased NSC proliferation was neither associated with a concomitant increase in LDH release from the cells, indicative of cell necrosis, nor apoptosis, indicating that IFN alters NSC proliferation without inducing cytotoxicity. In addition to the lack of cytotoxicity in neurosphere cultures, IFN-gamma also has the propensity to induce neurogenesis and inhibit the differentiation of NSCs into astrocytes (Ricci-Vitiani et al, 2006; Wong et al, 2004). Inflammation-associated neurogenesis and oligodendrogenesis have also been found to be blocked by endotoxin-activated microglia, but were induced by microglia activated in response to T-cell produced cytokines (IL-4 and low-level IFN-γ, Butovsky et al, 2006).
In our study TNF-α was not cytotoxic, nor did it have any effect on NSC proliferation. Similar studies using human neural progenitor cells have demonstrated that TNF-α treatment induced apoptosis through the TNF receptor, TNFRI (Sheng et al, 2005). TNF-alpha was also shown to be cytotoxic to murine neurosphere cultures (Wong et al, 2004). This discrepancy in findings may reflect the differences in species, stage of cellular differentiation or the culture system used in the assay. Additionally, in vivo experiments have shown that inflammation-induced proliferation in the sub-ventricular zone was greater in animals that lacked a functional TNFR1, suggesting that TNF may indeed be a negative regulator of NSC proliferation in mouse brains (Iosif et al, 2006). Thus, while in vitro studies provide great insights into the effect of specific cytokines on NSC, their effects in vivo are influenced by numerous additional factors, including by-stander cell responses, soluble factors in the inflammatory milieu, and the antigen specific response of systemic immune cells.
Acknowledgements
The authors thank Thomas Bakken for skillful technical assistance in the conduct of these studies. We would also like to thank Drs. Genya Gekker and Manohar Mutnal for their technical input. This study was supported by the United States Public Health Service grant NS-038836.
References
- Boulanger LM. MHC class I in activity-dependent structural and functional plasticity. Neuron Glia Biol. 2004;1:283–289. doi: 10.1017/S1740925X05000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health. 2005;5:70. doi: 10.1186/1471-2458-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran MC, Gekker G, Hu S, Min X, Cox D, Lokensgard JR. Intracerebral infection with murine cytomegalovirus induces CXCL10 and is restricted by adoptive transfer of splenocytes. Journal of neurovirology. 2004;10:152–162. doi: 10.1080/13550280490441130. [DOI] [PubMed] [Google Scholar]
- Cheeran MC, Gekker G, Hu S, Palmquist JM, Lokensgard JR. T cell-mediated restriction of intracerebral murine cytomegalovirus infection displays dependence upon perforin but not interferon-gamma. Journal of neurovirology. 2005a;11:274–280. doi: 10.1080/13550280590952808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran MC, Hu S, Ni HT, Sheng W, Palmquist JM, Peterson PK, Lokensgard JR. Neural precursor cell susceptibility to human cytomegalovirus diverges along glial or neuronal differentiation pathways. J Neurosci Res. 2005b;82:839–850. doi: 10.1002/jnr.20682. [DOI] [PubMed] [Google Scholar]
- Cheeran MC, Hu S, Palmquist JM, Bakken T, Gekker G, Lokensgard JR. Dysregulated interferon-gamma responses during lethal cytomegalovirus brain infection of IL-10-deficient mice. Virus Res. 2007;130:96–102. doi: 10.1016/j.virusres.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doom CM, Hill AB. MHC class I immune evasion in MCMV infection. Med Microbiol Immunol. 2008 doi: 10.1007/s00430-008-0089-y. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci U S A. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi MP, Clerici F, Perin C, D'Arminio Monforte A, Vago L, Borella M, Boldorini R, Mangoni A. Microglial nodular encephalitis and ventriculoencephalitis due to cytomegalovirus infection in patients with AIDS: two distinct clinical patterns. Clin Infect Dis. 1998;27:504–508. doi: 10.1086/514682. [DOI] [PubMed] [Google Scholar]
- Hengel H, Reusch U, Gutermann A, Ziegler H, Jonjic S, Lucin P, Koszinowski UH. Cytomegaloviral control of MHC class I function in the mouse. Immunol Rev. 1999;168:167–176. doi: 10.1111/j.1600-065x.1999.tb01291.x. [DOI] [PubMed] [Google Scholar]
- Hori J, Ng TF, Shatos M, Klassen H, Streilein JW, Young MJ. Neural progenitor cells lack immunogenicity and resist destruction as allografts. Stem Cells. 2003;21:405–416. doi: 10.1634/stemcells.21-4-405. [DOI] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata M, Baba S, Kawashima M, Kosugi I, Kawasaki H, Kaneta M, Tsuchida T, Kozuma S, Tsutsui Y. Differential expression of the immediate-early 2 and 3 proteins in developing mouse brains infected with murine cytomegalovirus. Archives of virology. 2006;151:2181–2196. doi: 10.1007/s00705-006-0793-0. [DOI] [PubMed] [Google Scholar]
- Kim M, Morshead CM. Distinct populations of forebrain neural stem and progenitor cells can be isolated using side-population analysis. J Neurosci. 2003;23:10703–10709. doi: 10.1523/JNEUROSCI.23-33-10703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koontz T, Bralic M, Tomac J, Pernjak-Pugel E, Bantug G, Jonjic S, Britt WJ. Altered development of the brain after focal herpesvirus infection of the central nervous system. J Exp Med. 2008;205:423–435. doi: 10.1084/jem.20071489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RY, Tsutsui Y. Growth retardation and microcephaly induced in mice by placental infection with murine cytomegalovirus. Teratology. 2000;62:79–85. doi: 10.1002/1096-9926(200008)62:2<79::AID-TERA3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lokensgard JR, Gekker G, Hu S, Chao CC, Simpson M, Schut RL, Peterson PK. CD8+lymphocyte-mediated suppression of human immunodeficiency virus type 1 expression in human brain cells. Immunol Lett. 1999;67:257–261. doi: 10.1016/s0165-2478(99)00014-0. [DOI] [PubMed] [Google Scholar]
- Mammolenti M, Gajavelli S, Tsoulfas P, Levy R. Absence of major histocompatibility complex class I on neural stem cells does not permit natural killer cell killing and prevents recognition by alloreactive cytotoxic T lymphocytes in vitro. Stem Cells. 2004;22:1101–1110. doi: 10.1634/stemcells.22-6-1101. [DOI] [PubMed] [Google Scholar]
- McCarthy M, Auger D, Whittemore SR. Human cytomegalovirus causes productive infection and neuronal injury in differentiating fetal human central nervous system neuroepithelial precursor cells. J Hum Virol. 2000;3:215–228. [PubMed] [Google Scholar]
- Ni HT, Hu S, Sheng WS, Olson JM, Cheeran MC, Chan AS, Lokensgard JR, Peterson PK. High-level expression of functional chemokine receptor CXCR4 on human neural precursor cells. Brain research. 2004;152:159–169. doi: 10.1016/j.devbrainres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Odeberg J, Wolmer N, Falci S, Westgren M, Seiger A, Soderberg-Naucler C. Human cytomegalovirus inhibits neuronal differentiation and induces apoptosis in human neural precursor cells. J Virol. 2006;80:8929–8939. doi: 10.1128/JVI.00676-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg J, Wolmer N, Falci S, Westgren M, Sundtrom E, Seiger A, Soderberg-Naucler C. Late human cytomegalovirus (HCMV) proteins inhibit differentiation of human neural precursor cells into astrocytes. J Neurosci Res. 2007;85:583–593. doi: 10.1002/jnr.21144. [DOI] [PubMed] [Google Scholar]
- Perlman JM, Argyle C. Lethal cytomegalovirus infection in preterm infants: clinical, radiological, and neuropathological findings. Ann Neurol. 1992;31:64–68. doi: 10.1002/ana.410310112. [DOI] [PubMed] [Google Scholar]
- Reuter JD, Gomez DL, Wilson JH, Van Den Pol AN. Systemic immune deficiency necessary for cytomegalovirus invasion of the mature brain. J Virol. 2004;78:1473–1487. doi: 10.1128/JVI.78.3.1473-1487.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter JD, Wilson JH, Idoko KE, van den Pol AN. CD4+ T-cell reconstitution reduces cytomegalovirus in the immunocompromised brain. J Virol. 2005;79:9527–9539. doi: 10.1128/JVI.79.15.9527-9539.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Casalbore P, Petrucci G, Lauretti L, Montano N, Larocca LM, Falchetti ML, Lombardi DG, Gerevini VD, Cenciarelli C, D'Alessandris QG, Fernandez E, De Maria R, Maira G, Peschle C, Parati E, Pallini R. Influence of local environment on the differentiation of neural stem cells engrafted onto the injured spinal cord. Neurol Res. 2006;28:488–492. doi: 10.1179/016164106X115134. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Zoecklein LJ, Howe CL, Pavelko KD, Gamez JD, Nakane S, Papke LM. Gamma interferon is critical for neuronal viral clearance and protection in a susceptible mouse strain following early intracranial Theiler's murine encephalomyelitis virus infection. J Virol. 2003;77:12252–12265. doi: 10.1128/JVI.77.22.12252-12265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidbauer M, Budka H, Ulrich W, Ambros P. Cytomegalovirus (CMV) disease of the brain in AIDS and connatal infection: a comparative study by histology, immunocytochemistry and in situ DNA hybridization. Acta Neuropathol (Berl) 1989;79:286–293. doi: 10.1007/BF00294663. [DOI] [PubMed] [Google Scholar]
- Sheng WS, Hu S, Ni HT, Rowen TN, Lokensgard JR, Peterson PK. TNF-alpha-induced chemokine production and apoptosis in human neural precursor cells. J Leukoc Biol. 2005;78:1233–1241. doi: 10.1189/jlb.0405221. [DOI] [PubMed] [Google Scholar]
- Stoddart CA, Cardin RD, Boname JM, Manning WC, Abenes GB, Mocarski ES. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J Virol. 1994;68:6243–6253. doi: 10.1128/jvi.68.10.6243-6253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- Ubiali F, Nava S, Nessi V, Frigerio S, Parati E, Bernasconi P, Mantegazza R, Baggi F. Allorecognition of human neural stem cells by peripheral blood lymphocytes despite low expression of MHC molecules: role of TGF-beta in modulating proliferation. Int Immunol. 2007;19:1063–1074. doi: 10.1093/intimm/dxm079. [DOI] [PubMed] [Google Scholar]
- van Den Pol AN, Mocarski E, Saederup N, Vieira J, Meier TJ. Cytomegalovirus cell tropism, replication, and gene transfer in brain. J Neurosci. 1999;19:10948–10965. doi: 10.1523/JNEUROSCI.19-24-10948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G, Goldshmit Y, Turnley AM. Interferon-gamma but not TNF alpha promotes neuronal differentiation and neurite outgrowth of murine adult neural stem cells. Exp Neurol. 2004;187:171–177. doi: 10.1016/j.expneurol.2004.01.009. [DOI] [PubMed] [Google Scholar]