Abstract
Substrate rigidity plays crucial roles in regulating cellular functions, such as cell spreading, traction forces, and stem cell differentiation. However, it is not clear how substrate rigidity influences early cell signaling events such as calcium in living cells. Using highly-sensitive Ca2+ biosensors based on fluorescence resonance energy transfer (FRET), we investigated the molecular mechanism by which substrate rigidity affects calcium signaling in human mesenchymal stem cells (HMSCs). Spontaneous Ca2+ oscillations were observed inside the cytoplasm and the endoplasmic reticulum (ER) using the FRET biosensors targeted at subcellular locations in cells plated on rigid dishes. Lowering the substrate stiffness to 1 kPa significantly inhibited both the magnitudes and frequencies of the cytoplasmic Ca2+ oscillation in comparison to stiffer or rigid substrate. This Ca2+ oscillation was shown to be dependent on ROCK, a downstream effector molecule of RhoA, but independent of actin filaments, microtubules, myosin light chain kinase, or myosin activity. Lysophosphatidic acid, which activates RhoA, also inhibited the frequency of the Ca2+ oscillation. Consistently, either a constitutive active mutant of RhoA (RhoA-V14) or a dominant negative mutant of RhoA (RhoA-N19) inhibited the Ca2+ oscillation. Further experiments revealed that HMSCs cultured on gels with low elastic moduli displayed low RhoA activities. Therefore, our results demonstrate that RhoA and its downstream molecule ROCK may mediate the substrate rigidity-regulated Ca2+ oscillation, which determines the physiological functions of HMSCs.
Keywords: FRET, Calcium, RhoA, signal transduction, substrate stiffness, mesenchymal stem cells
Introduction
Emerging evidence demonstrates that mechanical forces play vital roles in regulating cell and tissue functions (Kassab, 2006; Solon et al., 2007). Recently, mechanical factors have also been shown to play important roles in determine the differentiation lineage and commitment of HMSCs. For example, the stiffness of mechanical microenvironment can affect the HMSCs lineage specification (Engler et al., 2006). Intracellular cytoskeletal tension and RhoA have also been reported to regulate HMSCs lineage commitment (McBeath et al., 2004). While substantial progress has been made toward the understanding of how differentiated cells sense mechanical stiffness (Chiu et al., 2008; Discher et al., 2005; Laurent et al., 2002), it remains elusive on the molecular mechanism by which stem cells perceive these mechanical cues to coordinate signaling network to determine their lineage destinations.
Calcium ion (Ca2+) is one of the most important early biological signals (Berridge et al., 2000). Most cells mobilize their Ca2+ signals via the Ca2+ entry across the plasma membrane and/or the Ca2+ traffic between cytoplasm and intracellular stores such as endoplasmic reticulum (ER) or sarcoplasmic reticulum (SR) (Clapham, 2007; Wehrens et al., 2005). Ca2+ entry across the plasma membrane occurs via several distinct pathways, including voltage-operated Ca2+ channels (VOCCs), agonist-dependant and store-operated Ca2+ (SOC) channels (Albert and Large, 2003; Bolotina, 2004; Nilius, 2004). Ca2+ traffic between cytoplasm and intracellular stores, e.g. endoplasmic reticulum (ER), is controlled by two distinct channels, inositol 1,4,5-triphosphate receptor (InsP3R) and ryanodine receptor (RyR) (Berridge, 1993; Berridge et al., 2000; Foskett et al., 2007; Lewis, 2007), as well as the sarcoendoplasmic reticulum calcium transport ATPase (SERCA) serving as a pump that transports Ca2+ ions from cytoplasm into ER (Misquitta et al., 1999; Periasamy and Kalyanasundaram, 2007).
One of the most interesting discoveries in the field of calcium study is the oscillatory Ca2+ signals (Sun et al., 2007). While the role of oscillatory Ca2+ signals is not fully understood, it is clear that oscillatory Ca2+ signals are crucial for a variety of cellular functions (Morita et al., 2003; Sun et al., 2007; Torihashi et al., 2002). For example, spontaneous Ca2+ oscillations were observed in human bone marrow-derived mesenchymal stem cells (HMSCs) to affect differentiation (D'Souza et al., 2001; den Dekker et al., 2001). The regulation of calcium oscillation by electrical stimulation can also promote the differentiation of HMSCs into osteoblasts (Sun et al., 2007). It remains unclear, however, whether and how mechanical microenvironment such as substrate rigidity can affect calcium oscillations.
Fluorescence resonance energy transfer (FRET) is a powerful tool for the detection of protein-protein interaction and enzymatic activities (Zhang et al., 2002). Genetically-encoded biosensors based on FRET have the advantage to be easily targeted to subcellular compartments and have been widely applied to monitor signaling transductions in live cells with high spatiotemporal resolution. In fact, a variety of FRET biosensors using fluorescence proteins (FPs) have been developed, including Ca2+, matrix metalloproteinase, cAMP, phosphor-lipids, membrane receptor integrins, small GTPases Ras and Rap1, RhoA, Rac1, Cdc42, and tyrosine/serine/threonine kinases (Ouyang et al., 2008a; Wang et al., 2008). We have also developed a Calcium biosensor pairing an enhanced cyan FP (ECFP) with a newly developed yellow FP, YPet (Nguyen and Daugherty, 2005). This new Calcium biosensor provides a high dynamic range in monitoring intracellular calcium oscillations (Ouyang et al., 2008b).
In this study, we investigated whether the substrate rigidity can regulate intracellular Ca2+ signaling in HMSCs. Genetically encoded Ca2+ biosensors based on FRET were applied to monitor the Ca2+ signals inside either the cytoplasm or ER in live HMSCs. Our results show that the spontaneous Ca2+ oscillations observed in HMSCs can be regulated by extracellular substrate rigidity via RhoA/ROCK signaling pathway.
Materials and Methods
Gene construction and DNA plasmids
The construct of FRET-based Ca2+ biosensor (ECFP-CaM-M13-EYFP) has been described (Miyawaki et al., 1997). We have replaced EYFP with a recently developed YFP variant YPet to enhance the dynamic range of the biosensor. Briefly, the fragment containing ECFP, CaM, and M13 was fused to YPet and subcloned into pcDNA3.1 for mammalian cell expression by using BamHI/EcoRI sites. To generate an ER-targeted Calcium biosensor, the calreticulin signal sequence MLLPVLLLGLLGAAAD was fused to the N-terminal of ECFP, and an ER retention sequence, KDEL, was added to the end of the C-terminal of YPet (Palmer et al., 2004). The construct of FRET-based RhoA biosensor was a kind gift from Professor Michiyuki Matsuda at Kyoto University, Japan (Yoshizaki et al., 2003). In brief, the RhoA biosensor consists of truncated RhoA (aa 1−189) and the RhoA-binding domain (RBD) of effector, concatenated between a pair of GFP mutants, CFP and YFP. The intramolecular binding of active RhoA (GTP-binding) to the PBD domain is expected to bring CFP in closer proximity to YFP, resulting in an increase of FRET from CFP to YFP. Hence, the FRET signals of the RhoA biosensor can be monitored to assess the RhoA activity. Plasmids encoded RhoA-V14, a constitutive active mutant of RhoA, and RhoA-N19, a negative mutant of RhoA, were previously described (Li et al., 1999).
Cell culture and transfection
Bone marrow-derived HMSCs (from ATCC) were kindly provided by Dr. Shu Chien (University of California, San Diego). The cells were cultured in human mesenchymal stem cell growth medium (MSCGM, PT-3001, Lonza Walkersvile, Inc., MD, USA) containing 10% fetal bovine serum, 2 mM L-glutamine, 100 unit/ml penicillin and 100 μg/ml streptomycin in a humidified incubator of 95% O2 and 5% CO2 at 37°C. The DNA plasmids were transfected into the cells by using Lipofectamine 2000 (Invitrogen) reagent according to the product instructions.
Immunostaining
STRO-1 was used as HMSCs marker (Gronthos et al., 1999; Kassem and Abdallah, 2008; Rosada et al., 2003). After being washed in cold phosphate buffered saline (PBS), the samples were fixed by 4% paraformaldehyde in PBS at RT for 15 min. The cells were incubated with STRO-1 antibody (1:100; Chemicon, Temecula, CA) at 4°C overnight. They were subsequently incubated with Fluorescein (FITC)-conjugated anti-mouse IgG (1:200, Jackson ImmunoResearch Lab. Inc.) at RT for 1 hr.
Solutions and chemicals
Imaging experiments were conducted with CO2-independent medium (Invitrogen, CA, USA). For experiments that requires Ca2+-free conditions, Hanks balanced salt solution (HBSS, Invitrogen) was used containing 20 mM HEPES, 1 mM D-glucose, 2 mM MgCl2, 2 mM MgSO4 (pH 7.4). The chemical reagents 2-Amino-ethoxydiphenyl borate (2-APB, 100 μM), nifedipine (10 μM), thapsigargin (TG, 10 μM), LaCl3 (100 μM), GdCl3 (5 μM), Nocodazole (1 μM), cytochalasin D (2 μM), blebbistatin (10 μM), lysophosphatidic acid (LPA, 1 μM), and ML-7 (5 μM) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The ROCK inhibitor Y-27632 (10 μM) and Jasplakinolide (1 μM) were obtained from Calbiochem (San Diego, CA, USA).
Polyacrylamide gel for cell culture
Polyacrylamide gel solutions were prepared from 40% w/v acrylamide stock solution (5%; Bio-Rad) and 2% w/v bis-acrylamide stock solution (0.03−0.3%; Bio-Rad). To polymerize the solutions, 10% w/v ammonium persulfate (Bio-Rad) and N,N,N9,N9-Tetramethylethylenediamine (TEMED; Bio-Rad) were mixed with distilled water. Sulfo-SANPAH [sulfosuccinimidyl6(4_-azide-2_-nitrophenyl-amino) hexanoate; Pierce] was used to crosslink extracellular matrix molecules onto the gel surface. A detailed protocol about polyacrylamide gels was described previously (Pelham and Wang, 1997).
Microscopy and Imaging
Cells expressing various exogenous proteins were starved with 0.5% FBS for 36−48 hr before imaging experiments. During imaging process, the cells were maintained in CO2-independent medium without serum at 37°C. All images were obtained by using Zeiss Axiovert inverted microscope equipped with a charge-coupled device (CCD) camera (Cascade 512B, Photometrics) and a 440DF20 excitation filter, a 455DRLP dichroic mirror, and two emission filters controlled by a filter changer (480DF30 for CFP and 535DF25 for YFP). Time lapse fluorescence images were acquired at 10 sec interval by MetaFluor 6.2 software (Universal Imaging). The emission ratio images were computed and generated by the MetaFluor software to represent the FRET efficiency before they were subjected to quantification and analysis by Excel (Microsoft).
Statistical analysis
All statistical data are expressed as the mean ± standard error of the mean (S.E.M). Statistical evaluation of the data was performed by the unpaired student's t-test to determine the statistical differences between the two mean values. A significant difference was determined by the p-value (< 0.05).
Results
Genetically encoded FRET Ca2+ biosensors based on ECFP and YPet
Novel FRET Ca2+ biosensors based on ECFP and YPet were developed to visualize and monitor Ca2+ signaling with high sensitivity and spatiotemporal resolution (Fig. 1A). HMSCs were immunostained with anti-STRO-1 antibody, a HMSCs marker, to confirm their undifferentiated state (Fig. 1B). We found that the transfection efficiency of the Ca2+ biosensors was approximately 11.95% (27 of 226 cells) using lipofectamine method in HMSCs. As shown in Fig. 1C and Movie 1, a clear oscillation of cytoplasmic Ca2+ concentration can be observed by the transfected Ca2+ biosensor, which was initiated at a triggering corner and propagated to the whole cell body later. The oscillations of ER Ca2+ concentration can also be detected by a Ca2+ biosensor specifically targeted inside ER (Fig. 1D). These results suggest that our FRET-based biosensor can successfully monitor the spontaneous Ca2+ oscillation at subcellular locations inside HMSCs. This spontaneous Ca2+ oscillation was observed in fifty-nine percentages (26 of 44 cells) of HMSCs.
Figure 1. The application of FRET-based Ca2+ biosensors in human mesenchymal stem cells (HMSCs).
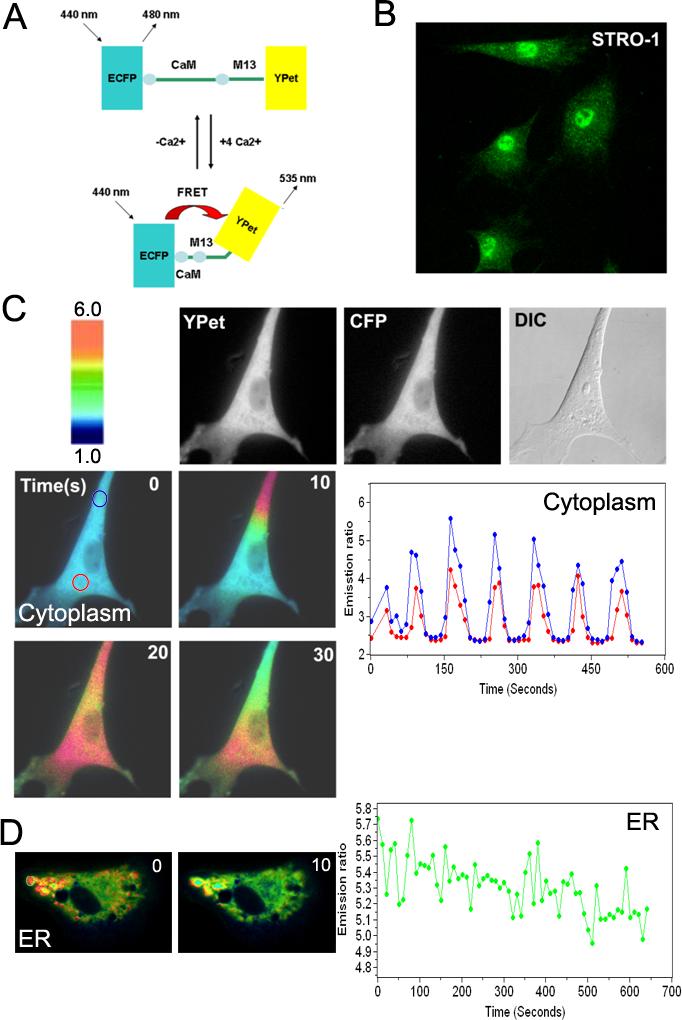
(A): A schematic drawing of the activation mechanism of the Ca2+ FRET biosensor. (B): HMSCs were immunostained by monoclonal antibody against STRO-1, a MSCs marker. (C) The FRET change of the Ca2+ biosensor targeted at cytoplasm. HMSCs were transfected with the cytoplasmic Ca2+ biosensor, which visualizes a spontaneous Ca2+ oscillation. Color images represent the YPet/ECFP emission ratio of the cytoplasmic Ca2+ biosensor. The color scale bar represents the YPet/ECFP emission ratio, with cold and hot colors indicating low and high levels of Ca2+ concentration, respectively. The time course curves represent the YPet/ECFP emission ratio averaged over regions on (blue) and distal (red) to the triggering corner. (D) The color images and time course curves represent the oscillatory FRET changes of the Ca2+ biosensor targeted inside ER.
Ca2+ traffic across both the plasma membrane and ER membrane is important for the spontaneous cytoplasmic Ca2+ oscillation in HMSCs
The cytoplasmic Ca2+ concentration is regulated via the Ca2+ flux across the plasma membrane and/or the exchange with internal calcium stores such as ER. In fact, spontaneous oscillations of Ca2+ can be observed in both cytoplasm and ER (Fig. 1C-D). When the extracellular Ca2+ was eliminated by incubating the cells in the Ca2+-free HBSS, the spontaneous Ca2+ oscillation disappeared (Fig. 2A and Supplementary Fig. 1). The inhibition of Ca2+ channels at the plasma membrane by a selective L-type calcium channel blocker, nifedipine (10 μM), or by inhibitors for SOC, either LaCl3 (100 μM) or GdCl3 (5 μM), blocked the spontaneous cytoplasmic Ca2+ oscillation (Fig. 2B-D). These results suggest that extracellular Ca2+ pool and Ca2+ flux across the plasma membrane are essential for the spontaneous Ca2+ oscillation in HMSCs. We then examined how intracellular Ca2+ store ER contributes to the cytoplasmic Ca2+ oscillation by blocking InsP3R and Ca2+-ATPase (SERCA) pump on ER membrane using 2-APB and thapsigargin (TG), respectively. Both 2-APB (100 μM) and TG (10 μM) blocked the spontaneous Ca2+ oscillation (Fig. 2E-F). TG treatment also caused a transient increase of cytoplasmic Ca2+ concentration, which returned to the basal level after TG treatment for more than 1 hr without the resumption of the Ca2+ oscillation (Supplementary Fig. 2). Hence, although 2-APB may also affect the Ca2+ influx across the plasma membrane (Giambelluca and Gende, 2007), these combined results based on 2-APB and TG suggest that internal calcium stores also play an important role for the spontaneous calcium oscillation. Taken together, these results suggest that the Ca2+ traffic at both the plasma membrane and ER membrane regulate the cytoplasmic Ca2+ oscillation.
Figure 2. The spontaneous Ca2+ oscillations in HMSCs are regulated by Ca2+ traffic across the plasma membrane and ER membrane.
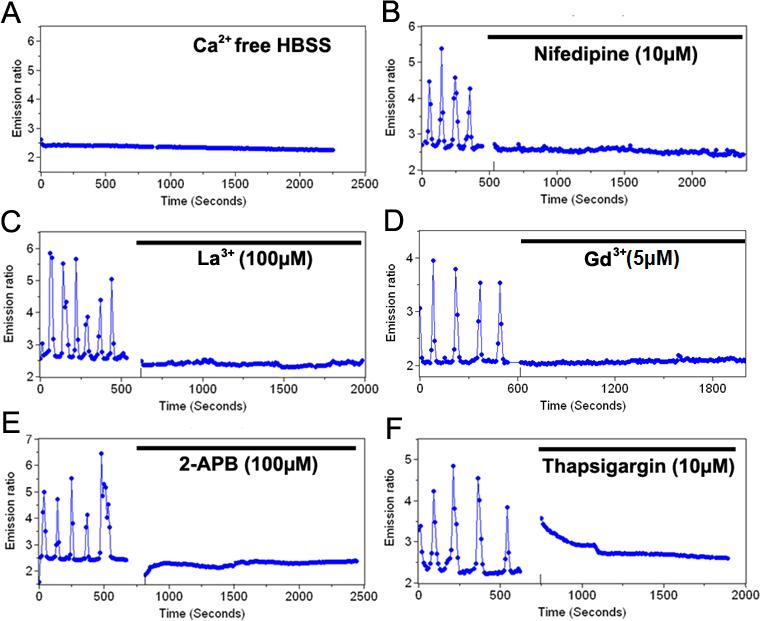
(A): The time course represents the cytoplasmic Ca2+ concentration in Ca2+ free buffer solution. (B-D): The time course represents the cytoplasmic Ca2+ concentration in cells pre-treated with (B) 10 μM nifedipine, a L-type Ca2+ channel blocker, (C) 100 μM LaCl3 or (D) 5 μM GdCl3, SOC blockers. (E) and (F): The time course represents the cytoplasmic Ca2+ concentration in cells pre-treated with (E) 100 μM 2-APB, an InsP3R blocker, or (F) 10 μM thapsigargin, a SERCA pump blocker.
Regulation of the Ca2+ oscillations by substrate rigidity of extracellular environment
Since mechanical environment and Ca2+ signaling have been shown to regulate the stem cell commitment for differentiation (D'Souza et al., 2001; den Dekker et al., 2001; Engler et al., 2006), we then examined whether the mechanical environments can affect this cytoplasmic Ca2+ oscillation in HMSCs. Polyacrylamide gels were applied to control the substrate rigidity of extracellular environment with defined elastic moduli. As shown in Fig. 3A and Movie 2, gels with lower elasticity reduced the Ca2+ oscillation activities in HMSCs. This was further confirmed by statistical analysis results (Fig. 3B-C). Interestingly, 1 kPa gel inhibited both frequency and magnitude of Ca2+ oscillation whereas 8.5 and 5 kPa gels only affected the frequency (Fig. 3B-C). Therefore, the substrate rigidity of extracellular environment can affect Ca2+ oscillation in HMSCs. These results also suggest that the frequency of Ca2+ oscillation may be more sensitive to mechanical environment than the magnitude.
Figure 3. The effect of substrate stiffness on the spontaneous Ca2+ oscillation.

(A): The time courses represent the cytoplasmic Ca2+ concentrations in cells cultured on gels with different rigidity, as indicated. (B) and (C): Bar graphs (mean ± S.E.M.) represent the (B) frequency and (C) magnitude of spontaneous Ca2+ oscillations in cells cultured on gels with different rigidity, as indicated. Error bars indicate standard errors of mean; *p<0.05; ***P<0.001, n=6
The roles of the cytoskeleton and RhoA signaling pathway on the spontaneous cytoplasmic Ca2+ oscillation
We hypothesized that the cytoskeleton and intracellular mechanical tension may mediate the oscillatory Ca2+ signals in sensing the substrate rigidity. In fact, substrate rigidity of extracellular environment was shown to affect cell morphology, cytoskeletal structure and cell adhesion (Yeung et al., 2005). To investigate the roles of cytoskeleton, cytochalasin D (Cyto D) and nocodazole (Noc) were applied to disrupt actin filaments and microtubules, respectively. As shown in Fig. 4A, neither Cyto D nor Noc had significant effects on the spontaneous Ca2+ oscillation in HMSCs, although Cyto D and Noc sufficiently disrupted actin filaments and microtubules, respectively (Supplementary Fig. 3). The spontaneous Ca2+ oscillation was also immune to Jasplakinolide (1 μM), which stabilizes and induces the polymerization of actin filaments (supplementary Fig. 4). Surprisingly, the Ca2+ oscillation was not affected by ML-7, an inhibitor of myosin light chain kinase (MLCK) (Soderling and Stull, 2001), nor by blebbistatin, a myosin II inhibitor which has been shown to mediate the effect of mechanical force on stem cell commitment (Engler et al., 2006) (Fig. 4A-C), . These results suggest that the spontaneous Ca2+ oscillation in HMSCs is independent of cytoskeleton and MLCK/myosin.
Figure 4. The roles of the cytoskeleton and RhoA signaling pathway on the spontaneous cytoplasmic Ca2+ oscillation.
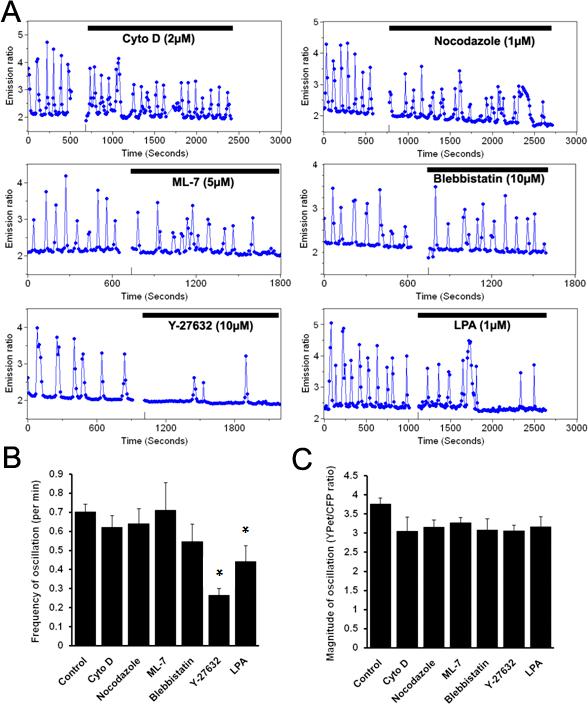
(A): Representative time courses of the cytoplasmic Ca2+ concentration in cells pre-treated with Cyto D (2 μM, n=3), Noc (1 μM, n=3), ML-7 (5 μM, n=5), blebbistatin (10 μM, n=5), Y-27632 (10 μM, n=5), and LPA (1 μM, n=10). (B) and (C): Bar graphs (mean ± S.E.M.) represent the (B) frequency and (C) magnitude of spontaneous Ca2+ oscillations in cells pretreated with different reagents, as indicated. Error bars indicate standard errors of mean; *p<0.05.
Interestingly, Y-27632, an inhibitor of Rho-associated kinase (ROCK), and lysophosphatidic acid (LPA), a stimulator of RhoA, significantly inhibited the frequency, but not the magnitude of Ca2+ oscillation (Fig. 4A-C). These results suggest that RhoA and its downstream molecule ROCK may be involved in the regulation of Ca2+ oscillation in HMSCs.
Substrate rigidity alters the endogenous RhoA activity, which mediates the Ca2+ oscillation in HMSCs
We then examined whether substrate rigidity can regulate the endogenous RhoA activity using a FRET-based RhoA biosensor. The functionality of this RhoA biosensor was first confirmed in HMSCs in response to LPA, an activator of RhoA. As shown in supplementary Fig. 5, LPA significantly increased FRET signals of the RhoA biosensor, representing an elevated RhoA activity (Yoshizaki et al., 2003). Hence, the RhoA biosensor can report the RhoA activity in our system. The application of this RhoA biosensor revealed that 1 kPa gels (n= 6, *p<0.05), but not 5 or 8.5 kPa, led to a significantly reduced RhoA activity (Fig. 5A). These data indicate that the substrate rigidity can directly affect the RhoA activity of HMSCs.
Figure 5. The substrate rigidity affects the endogenous RhoA activity, which mediates the Ca2+ oscillation in HMSCs.
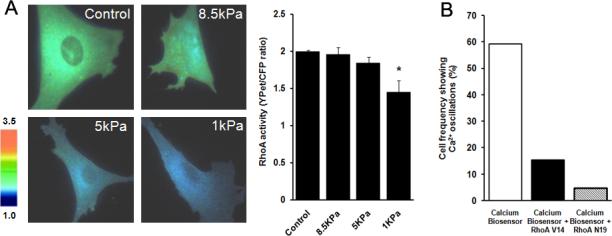
(A): Left: the YFP/CFP emission ratio images of cells transfected with the RhoA biosensor on different substrate gels; Right: bar graphs represent the endogenous RhoA activity (mean ± S.E.M.) measured by the RhoA FRET biosensor. (B): Bar graphs represent the percentile of HMSCs showing Ca2+ oscillations in cells transfected with the control vector, an active RhoA-V14, or a negative RhoA-N19. Error bars indicate standard errors of mean; *p<0.05., n=6
To examine whether substrate rigidities affect the Ca2+ oscillation via RhoA, we first tested whether the spontaneous Ca2+ oscillation can be regulated by the RhoA activity. The FRET Ca2+ biosensor was transfected into HMSCs together with an empty vector, a constitutively active (RhoA-V14) or a dominant-negative (RhoA-N19) mutant of RhoA into HMSCs. Both RhoA-V14 and RhoA-N19 significantly reduced the percentile of HMSCs with oscillating Ca2+ signals (Fig. 5B), with 15.38% (4 of 26 cells) for RhoA-V14 and 4.76% (1 of 21 cells) for RhoA-N19 comparing to the control group (59.09%, 26 of 44 cells). These results suggest that RhoA and possibly its optimized/balanced activity are important for the Ca2+ oscillation in HMSCs.
RhoA is not sufficient to restore the Ca2+ oscillation modulated by substrate rigidity
We then examined whether the restore of RhoA activity is sufficient to rescue the Ca2+ oscillation in HMSCs cultured on soft substrate. The inhibition of the Ca2+ oscillation in HMSCs cultured on the 1kPa gel could not be alleviated when RhoA is activated by incubating the cells with LPA, co-transfection of RhoA-V14, or the combination of LPA and RhoA-V14 (Fig. 6). These results suggest that other factors, independent of RhoA, may also participate in regulating the Ca2+ oscillation in response to substrate rigidity.
Figure 6. Ca2+ oscillations in HMSCs on soft gels were not restored by the activation of RhoA.

Time courses represent the cytoplasmic Ca2+ oscillations before and after LPA stimulation in HMSCs cultured on 1 kPa gels and transfected (A) with or (B) without active RhoA-V14.
Discussion
In this study, we have applied highly-sensitive Ca2+ biosensors containing an ECFP and a YPet as the FRET pair to monitor subcellular Ca2+ concentration in HMSCs. The results indicate that there is a spontaneous cytoplasmic Ca2+ oscillation in HMSCs, which is controlled by the Ca2+ traffic at the plasma membrane and ER membrane. The substrate rigidity can affect the Ca2+ oscillation, which is mediated by RhoA signaling pathway, but not by cytoskeleton and myosin. However, RhoA alone is not sufficient in negating the substrate rigidity effects on the Ca2+ oscillation. Hence, our results suggest that RhoA is essential, but not sufficient in mediating the effect of substrate rigidity on Ca2+ oscillation in HMSCs. These results can also shed new light on the molecular mechanism by which mechanical environment determines the destiny of stem cell commitment and differentiation.
Ca2+ oscillations encode diverse cellular processes such as cell division, differentiation, migration, fertilization and apoptosis (Berridge et al., 2003; Berridge et al., 2000). Both non-excitable and excitable cells display oscillatory Ca2+ signals (Berridge, 1993; Jacob, 1990; Tsien and Tsien, 1990). Spontaneous Ca2+ oscillations have been observed in several cell types, including pancreatic acinar cells, cardiac myocytes, oocytes and fibroblasts (Fewtrell, 1993; Kiselyov et al., 1999; Osipchuk et al., 1990). In this study, we have observed the spontaneous Ca2+ oscillation in 59 % HMSCs, consistent with a previous study (Kawano et al., 2002). The molecular mechanism and biological role of spontaneous Ca2+ oscillation, in particular in HMSCs, are poorly understood. It was shown that the Ca2+ release from ER and Ca2+ influx via plasma membrane in HMSCs are regulated by InsP3Rs and SOCs, respectively, but not by L-type Ca2+ channels (Kawano et al., 2002). The contribution of Ca2+ release from ER to the cytoplasmic Ca2+ oscillation was also supported by the observation of Ca2+ oscillation in both cytoplasm and ER using FRET biosensors targeted at cytoplasm and ER (Fig. 1C-D). Consistently, our results indicate that the inhibition of Ca2+ flux at ER membrane abolished the oscillatory Ca2+ signals in cytoplasm (Fig. 2). However, we found that both SOCs and L-type Ca2+ channels at the plasma membrane are necessary for the spontaneous Ca2+ oscillation (Fig. 2B-D). While the discrepancy of our results and Kawano's on L-type Ca2+ is not clear, we reasoned that different developmental stages of HMSCs may play a role here. In fact, the functional L-type Ca2+ channels can be detected after day 7 of murine embryoid bodies and is dependent on the differentiated state of cells (Gollasch et al., 1998; Kolossov et al., 1998).
Ca2+ oscillations play an important role in regulating cell differentiation (D'Souza et al., 2001; den Dekker et al., 2001). For example, the modulation of Ca2+ oscillation by electrical stimulation can promote osteo-differentiation of HMSCs (Sun et al., 2007). The substrate rigidity can also determine the differentiation fate of HMSCs (Engler et al., 2006). In the process of myofibrillogenesis, differentiation is regulated by substrate compliance and adhesive tension (Engler et al., 2004). In fact, soft gels with well-controlled mechanical properties have been developed for stem cell culture (Dang et al., 2006; Li et al., 2006). Our results showed that Ca2+ oscillations are significantly affected by the rigidity of the substrate gels where HMSCs are seeded (Fig. 3). Hence, the substrate rigidity may determine the commitment and differentiation of HMSCs via the regulation of Ca2+ oscillation. It, however, remains unclear as to the detailed molecular mechanism by which the substrate rigidity affects Ca2+ oscillation. It was postulated that substrates with different rigidity may regulate cellular functions by differentially altering signaling molecules and cytoskeletal structures involved in cell adhesion and force-sensing (Engler et al., 2007). It has also been shown that a remarkable change in cytoskeleton occurs when HMSCs differentiate into osteogenic cell types (Rodriguez et al., 2004) and that the Ca2+ oscillation in HMSCs was not observed in differentiated osteoblasts (Sun et al., 2007). It appears plausible that cytoskeleton may mediate the effects of substrate rigidity on the Ca2+ oscillation in HMSCs, as it is the case for differentiated cells, e.g. human umbilical vein endothelial cells (HUVECs) (Hayakawa et al., 2008). To our surprise, the disruption of neither actin filaments nor microtubules caused significant effect on the Ca2+ oscillation (Fig. 4). These results suggest that the Ca2+ oscillation in HMSCs is independent of cytoskeletal actin filaments and microtubules.
It appears that the substrate rigidity may regulate Ca2+ oscillation via RhoA/ROCK signaling pathway. In fact, a specific ROCK inhibitor, Y-27632, decreased the frequency of the spontaneous Ca2+ oscillation (Fig. 4). LPA, an agonist for G protein coupled receptors (GPCR) and a known stimulator of RhoA/ROCK signaling pathway (Emmert et al., 2004; McBeath et al., 2004; Sun et al., 2007), inhibited the Ca2+ oscillation (Fig. 4). Consistently, the introduction of either active RhoA-V14 or negative RhoA-N19 into HMSCs inhibited the Ca2+ oscillation. These results suggest that a balanced RhoA/ROCK activity is essential for an optimized Ca2+ oscillation in HMSCs. Either the elevation or reduction of endogenous RhoA/ROCK activity may cause significant inhibitory effects on the Ca2+ oscillation. However, the inhibition of myosin light chain kinase (MLCK), a Ca2+/calmodulin dependent kinase downstream to ROCK (Ducibella and Fissore, 2008; Soderling and Stull, 2001), did not have significant effect on the Ca2+ oscillation (Fig. 4). The difference between the effects of ROCK and MLCK on Ca2+ oscillation may be attributed to their different subcellular localization (Totsukawa et al., 2000; Wozniak et al., 2003). In addition, the ROCK may regulate myosin by the direct phosphorylation of MLC as well as the inhibition of MLC phosphatase (Amano et al., 1996; Kimura et al., 1996; Kureishi et al., 1997), bypassing the regulatory effect of MLCK on myosin phosphorylation (Wozniak et al., 2003). Surprisingly, blebbistatin, an inhibitor of myosin II, did not have significant effect on Ca2+ oscillation (Fig. 4). While the exact mechanism is unclear at the current stage, it is possible that molecules other than myosin may function downstream to RhoA and ROCK in regulating Ca2+ oscillation. This central role of RhoA/ROCK in mediating the effects of substrate rigidity on Ca2+ oscillation is consistent with previous reports that the substrate rigidity can affect not only adhesions, but also RhoA/ROCK signaling pathway (Bhadriraju et al., 2007). Indeed, the RhoA/ROCK pathway was also documented to play important roles in Ca2+ sensitization for the contraction of smooth muscle and prostatic tissues (Kimura et al., 1996; Takahashi et al., 2007).
It appears that mechanical environment has more profound impact on the Ca2+ oscillation than simply regulating RhoA/ROCK. In fact, the loss of Ca2+ oscillation on soft gels can not be restored by increasing the intracellular tension via the introduction of RhoA-V14 or the incubation of LPA (Fig. 6). Therefore, some unknown molecules/mechanisms independent of RhoA/ROCK may also participate in the regulation of Ca2+ oscillation in response to mechanical environment. Further studies are warranted to elucidate the underlying mechanism for this Ca2+ regulation by the substrate rigidity.
Supplementary Material
Acknowledgements
This work was supported in part by grants from Wallace H. Coulter Foundation and Beckman Laser Institute, Inc. (Y.W.), and NIH grant GM 072744 (N.W.).
References
- Albert AP, Large WA. Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells. Cell Calcium. 2003;33(5−6):345–356. doi: 10.1016/s0143-4160(03)00048-4. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem. 1996;271(34):20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bhadriraju K, Yang M, Alom Ruiz S, Pirone D, Tan J, Chen CS. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp Cell Res. 2007;313(16):3616–3623. doi: 10.1016/j.yexcr.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotina VM. Store-operated channels: diversity and activation mechanisms. Sci STKE. 2004;2004(243):pe34. doi: 10.1126/stke.2432004pe34. [DOI] [PubMed] [Google Scholar]
- Chiu WT, Tang MJ, Jao HC, Shen MR. Soft Substrate Up-regulates the Interaction of STIM1 with Store-operated Ca2+ Channels That Lead to Normal Epithelial Cell Apoptosis. Mol Biol Cell. 2008;19(5):2220–2230. doi: 10.1091/mbc.E07-11-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- D'Souza SJ, Pajak A, Balazsi K, Dagnino L. Ca2+ and BMP-6 signaling regulate E2F during epidermal keratinocyte differentiation. J Biol Chem. 2001;276(26):23531–23538. doi: 10.1074/jbc.M100780200. [DOI] [PubMed] [Google Scholar]
- Dang JM, Sun DD, Shin-Ya Y, Sieber AN, Kostuik JP, Leong KW. Temperature-responsive hydroxybutyl chitosan for the culture of mesenchymal stem cells and intervertebral disk cells. Biomaterials. 2006;27(3):406–418. doi: 10.1016/j.biomaterials.2005.07.033. [DOI] [PubMed] [Google Scholar]
- den Dekker E, Molin DG, Breikers G, van Oerle R, Akkerman JW, van Eys GJ, Heemskerk JW. Expression of transient receptor potential mRNA isoforms and Ca(2+) influx in differentiating human stem cells and platelets. Biochim Biophys Acta. 2001;1539(3):243–255. doi: 10.1016/s0167-4889(01)00112-4. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Fissore R. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol. 2008;315(2):257–279. doi: 10.1016/j.ydbio.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert DA, Fee JA, Goeckeler ZM, Grojean JM, Wakatsuki T, Elson EL, Herring BP, Gallagher PJ, Wysolmerski RB. Rho-kinase-mediated Ca2+-independent contraction in rat embryo fibroblasts. Am J Physiol Cell Physiol. 2004;286(1):C8–21. doi: 10.1152/ajpcell.00428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166(6):877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sweeney HL, Discher DE, Schwarzbauer JE. Extracellular matrix elasticity directs stem cell differentiation. J Musculoskelet Neuronal Interact. 2007;7(4):335. [PubMed] [Google Scholar]
- Fewtrell C. Ca2+ oscillations in non-excitable cells. Annu Rev Physiol. 1993;55:427–454. doi: 10.1146/annurev.ph.55.030193.002235. [DOI] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87(2):593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollasch M, Haase H, Ried C, Lindschau C, Morano I, Luft FC, Haller H. L-type calcium channel expression depends on the differentiated state of vascular smooth muscle cells. Faseb J. 1998;12(7):593–601. doi: 10.1096/fasebj.12.7.593. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Zannettino AC, Graves SE, Ohta S, Hay SJ, Simmons PJ. Differential cell surface expression of the STRO-1 and alkaline phosphatase antigens on discrete developmental stages in primary cultures of human bone cells. J Bone Miner Res. 1999;14(1):47–56. doi: 10.1359/jbmr.1999.14.1.47. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Tatsumi H, Sokabe M. Actin stress fibers transmit and focus force to activate mechanosensitive channels. J Cell Sci. 2008;121(Pt 4):496–503. doi: 10.1242/jcs.022053. [DOI] [PubMed] [Google Scholar]
- Jacob R. Calcium oscillations in electrically non-excitable cells. Biochim Biophys Acta. 1990;1052(3):427–438. doi: 10.1016/0167-4889(90)90152-4. [DOI] [PubMed] [Google Scholar]
- Kassab GS. Biomechanics of the cardiovascular system: the aorta as an illustratory example. J R Soc Interface. 2006;3(11):719–740. doi: 10.1098/rsif.2006.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem M, Abdallah BM. Human bone-marrow-derived mesenchymal stem cells: biological characteristics and potential role in therapy of degenerative diseases. Cell Tissue Res. 2008;331(1):157–163. doi: 10.1007/s00441-007-0509-0. [DOI] [PubMed] [Google Scholar]
- Kawano S, Shoji S, Ichinose S, Yamagata K, Tagami M, Hiraoka M. Characterization of Ca(2+) signaling pathways in human mesenchymal stem cells. Cell Calcium. 2002;32(4):165–174. doi: 10.1016/s0143416002001240. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 1996;273(5272):245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kiselyov KI, Semyonova SB, Mamin AG, Mozhayeva GN. Miniature Ca2+ channels in excised plasma-membrane patches: activation by IP3. Pflugers Arch. 1999;437(2):305–314. doi: 10.1007/s004240050784. [DOI] [PubMed] [Google Scholar]
- Kolossov E, Fleischmann BK, Liu Q, Bloch W, Viatchenko-Karpinski S, Manzke O, Ji GJ, Bohlen H, Addicks K, Hescheler J. Functional characteristics of ES cell-derived cardiac precursor cells identified by tissue-specific expression of the green fluorescent protein. J Cell Biol. 1998;143(7):2045–2056. doi: 10.1083/jcb.143.7.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997;272(19):12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- Laurent VM, Canadas P, Fodil R, Planus E, Asnacios A, Wendling S, Isabey D. Tensegrity behaviour of cortical and cytosolic cytoskeletal components in twisted living adherent cells. Acta Biotheor. 2002;50(4):331–356. doi: 10.1023/a:1022676903680. [DOI] [PubMed] [Google Scholar]
- Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446(7133):284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- Li Q, Wang J, Shahani S, Sun DD, Sharma B, Elisseeff JH, Leong KW. Biodegradable and photocrosslinkable polyphosphoester hydrogel. Biomaterials. 2006;27(7):1027–1034. doi: 10.1016/j.biomaterials.2005.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Chen BP, Azuma N, Hu YL, Wu SZ, Sumpio BE, Shyy JY, Chien S. Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. J Clin Invest. 1999;103(8):1141–1150. doi: 10.1172/JCI5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Misquitta CM, Mack DP, Grover AK. Sarco/endoplasmic reticulum Ca2+ (SERCA)-pumps: link to heart beats and calcium waves. Cell Calcium. 1999;25(4):277–290. doi: 10.1054/ceca.1999.0032. [DOI] [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388(6645):882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- Morita M, Higuchi C, Moto T, Kozuka N, Susuki J, Itofusa R, Yamashita J, Kudo Y. Dual regulation of calcium oscillation in astrocytes by growth factors and proinflammatory cytokines via the mitogen-activated protein kinase cascade. J Neurosci. 2003;23(34):10944–10952. doi: 10.1523/JNEUROSCI.23-34-10944.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AW, Daugherty PS. Evolutionary optimization of fluorescent proteins for intracellular FRET. Nat Biotechnol. 2005;23(3):355–360. doi: 10.1038/nbt1066. [DOI] [PubMed] [Google Scholar]
- Nilius B. Store-operated Ca2+ entry channels: still elusive! Sci STKE. 2004;2004(243):pe36. doi: 10.1126/stke.2432004pe36. [DOI] [PubMed] [Google Scholar]
- Osipchuk YV, Wakui M, Yule DI, Gallacher DV, Petersen OH. Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+: simultaneous microfluorimetry and Ca2+ dependent Cl- current recording in single pancreatic acinar cells. Embo J. 1990;9(3):697–704. doi: 10.1002/j.1460-2075.1990.tb08162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Lu S, Li XY, Xu J, Seong J, Giepmans BN, Shyy JY, Weiss SJ, Wang Y. Visualization of polarized membrane type 1 matrix metalloproteinase activity in live cells by fluorescence resonance energy transfer imaging. J Biol Chem. 2008a;283(25):17740–17748. doi: 10.1074/jbc.M709872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Sun J, Chien S, Wang Y. Determination of hierarchical relationship of Src and Rac at subcellular locations with FRET biosensors. Proc Natl Acad Sci USA. 2008b doi: 10.1073/pnas.0807537105. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci U S A. 2004;101(50):17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham RJ, Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94(25):13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35(4):430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- Rodriguez JP, Gonzalez M, Rios S, Cambiazo V. Cytoskeletal organization of human mesenchymal stem cells (MSC) changes during their osteogenic differentiation. J Cell Biochem. 2004;93(4):721–731. doi: 10.1002/jcb.20234. [DOI] [PubMed] [Google Scholar]
- Rosada C, Justesen J, Melsvik D, Ebbesen P, Kassem M. The human umbilical cord blood: a potential source for osteoblast progenitor cells. Calcif Tissue Int. 2003;72(2):135–142. doi: 10.1007/s00223-002-2002-9. [DOI] [PubMed] [Google Scholar]
- Soderling TR, Stull JT. Structure and regulation of calcium/calmodulin-dependent protein kinases. Chem Rev. 2001;101(8):2341–2352. doi: 10.1021/cr0002386. [DOI] [PubMed] [Google Scholar]
- Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93(12):4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Liu Y, Lipsky S, Cho M. Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. Faseb J. 2007;21(7):1472–1480. doi: 10.1096/fj.06-7153com. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Nishimura J, Seki N, Yunoki T, Tomoda T, Kanaide H, Naito S. RhoA/Rho kinase-mediated Ca2+ sensitization in the contraction of human prostate. Neurourol Urodyn. 2007;26(4):547–551. doi: 10.1002/nau.20365. [DOI] [PubMed] [Google Scholar]
- Torihashi S, Fujimoto T, Trost C, Nakayama S. Calcium oscillation linked to pacemaking of interstitial cells of Cajal: requirement of calcium influx and localization of TRP4 in caveolae. J Biol Chem. 2002;277(21):19191–19197. doi: 10.1074/jbc.M201728200. [DOI] [PubMed] [Google Scholar]
- Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150(4):797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RW, Tsien RY. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shyy JY-J, Chien S. Fluorescence Proteins, Live Cell Imaging, and Mechanobiology: Seeing is Believing. Annu Rev Biomed Eng. 2008;10 doi: 10.1146/annurev.bioeng.010308.161731. In Press. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol. 2005;67:69–98. doi: 10.1146/annurev.physiol.67.040403.114521. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163(3):583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60(1):24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- Yoshizaki H, Ohba Y, Kurokawa K, Itoh RE, Nakamura T, Mochizuki N, Nagashima K, Matsuda M. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol. 2003;162(2):223–232. doi: 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Campbell RE, Ting AY, Tsien RY. Creating new fluorescent probes for cell biology. Nat Rev Mol Cell Biol. 2002;3(12):906–918. doi: 10.1038/nrm976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


