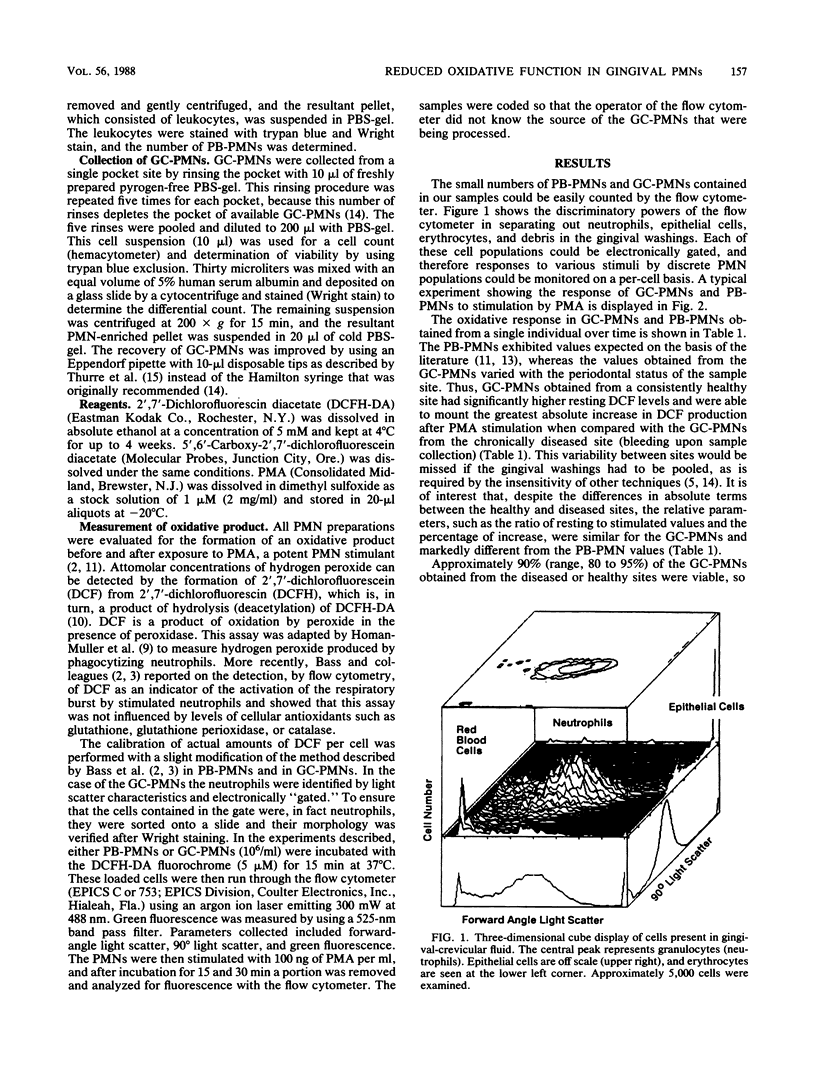
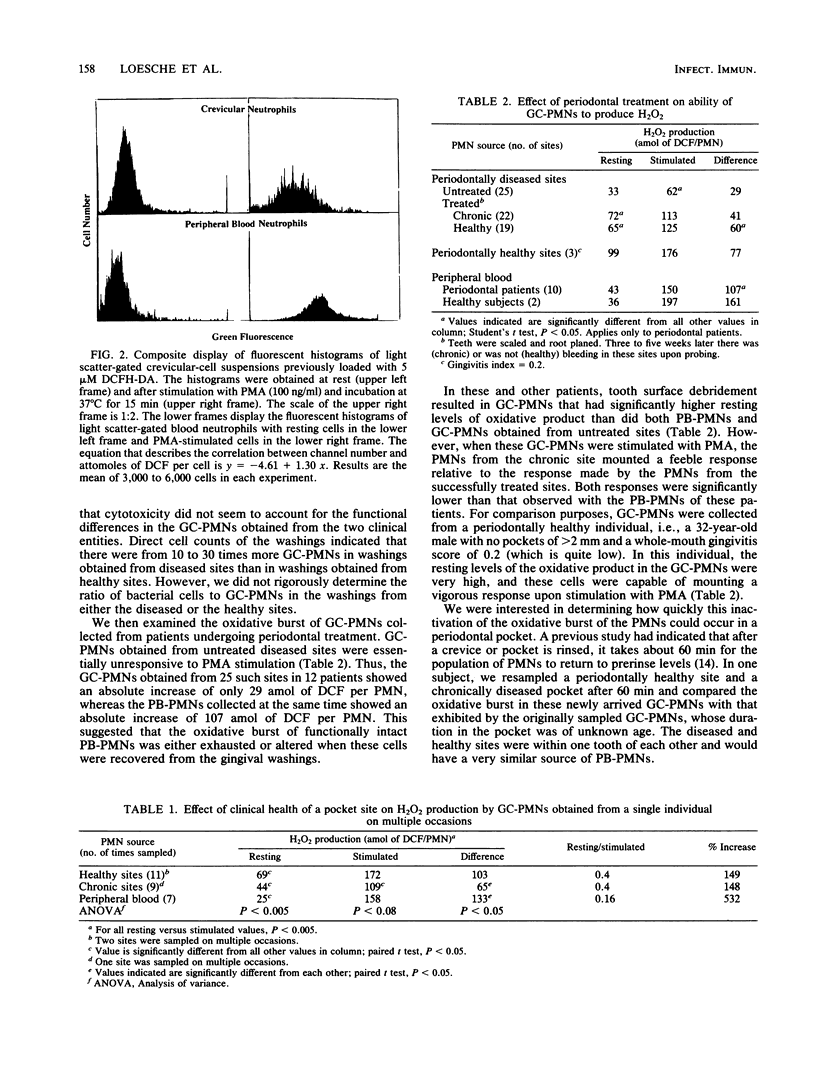
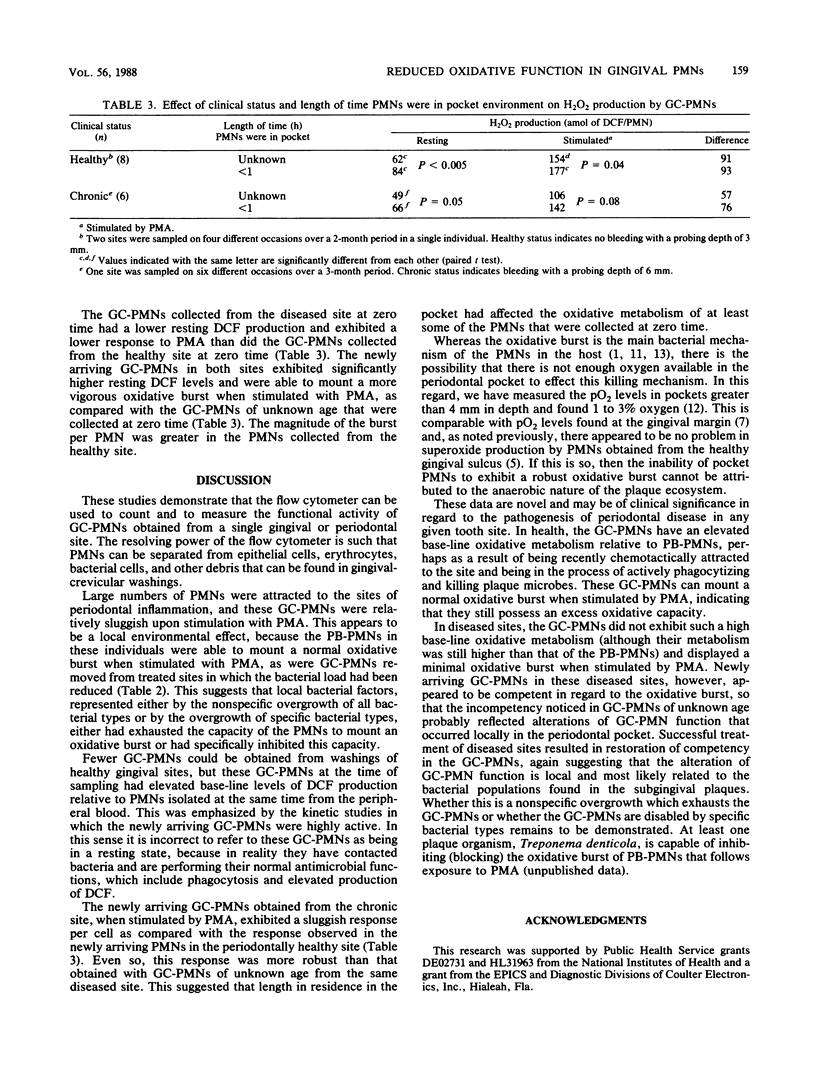
Abstract
Measurable amounts of viable and functional polymorphonuclear neutrophils (PMNs) are recovered from pooled washings of the gingival crevice of healthy individuals. In the present study, we have assessed the ability of the PMNs removed from single healthy or diseased pocket sites to mount an oxidative burst when challenged with phorbol myristate acetate (PMA) and compared these activities with each other and with those obtained with autologous peripheral-blood PMNs. The oxidative burst after PMA stimulation was evaluated by using methods developed for the flow cytometer. The results showed that the PMNs collected from untreated disease sites were minimally responsive to PMA when compared with peripheral-blood PMNs collected at the same time from the same individual. Thus, whereas the peripheral-blood PMNs exhibited significantly lower resting oxidative product formation and a 500% increase when stimulated with PMA, all gingival-crevicular PMNs exhibited significantly higher resting formation of oxidized products but only a 150% increase after PMA stimulation. PMNs obtained from a consistently healthy site had significantly higher resting production of oxidized products and were able to mount the greatest absolute increase in oxidized products after PMA stimulation when compared with PMNs collected from diseases sites. Mechanical debridement of these diseased sites, which both reduced the bacterial numbers and restored clinical health, resulted in the recovery of gingival-crevicular PMNs that exhibited an oxidative burst more typical of that observed in PMNs obtained from healthy gingival sites and from the peripheral blood. This suggested that the PMNs collected from the diseased sites either had been exhausted by the large numbers of bacteria present in these sites or had been specifically inhibited by these bacteria.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Bass D. A., Olbrantz P., Szejda P., Seeds M. C., McCall C. E. Subpopulations of neutrophils with increased oxidative product formation in blood of patients with infection. J Immunol. 1986 Feb 1;136(3):860–866. [PubMed] [Google Scholar]
- Bass D. A., Parce J. W., Dechatelet L. R., Szejda P., Seeds M. C., Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983 Apr;130(4):1910–1917. [PubMed] [Google Scholar]
- Charon J. A., Metzger Z., Hoffeld J. T., Oliver C., Gallin J. I., Mergenhagen S. E. An in vitro study of neutrophils obtained from the normal gingival sulcus. J Periodontal Res. 1982 Nov;17(6):614–625. doi: 10.1111/j.1600-0765.1982.tb01183.x. [DOI] [PubMed] [Google Scholar]
- Duque R. E., Phan S. H., Hudson J. L., Till G. O., Ward P. A. Functional defects in phagocytic cells following thermal injury. Application of flow cytometric analysis. Am J Pathol. 1985 Jan;118(1):116–127. [PMC free article] [PubMed] [Google Scholar]
- Eskow R. N., Loesche W. J. Oxygen tensions in the human oral cavity. Arch Oral Biol. 1971 Sep;16(9):1127–1128. doi: 10.1016/0003-9969(71)90218-4. [DOI] [PubMed] [Google Scholar]
- Genco R. J., Slots J. Host responses in periodontal diseases. J Dent Res. 1984 Mar;63(3):441–451. doi: 10.1177/00220345840630031601. [DOI] [PubMed] [Google Scholar]
- Homan-Müller J. W., Weening R. S., Roos D. Production of hydrogen peroxide by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):198–207. [PubMed] [Google Scholar]
- KESTON A. S., BRANDT R. THE FLUOROMETRIC ANALYSIS OF ULTRAMICRO QUANTITIES OF HYDROGEN PEROXIDE. Anal Biochem. 1965 Apr;11:1–5. doi: 10.1016/0003-2697(65)90034-5. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Gusberti F., Mettraux G., Higgins T., Syed S. Relationship between oxygen tension and subgingival bacterial flora in untreated human periodontal pockets. Infect Immun. 1983 Nov;42(2):659–667. doi: 10.1128/iai.42.2.659-667.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Skapski H., Lehner T. A crevicular washing method for investigating immune components of crevicular fluid in man. J Periodontal Res. 1976 Feb;11(1):19–24. doi: 10.1111/j.1600-0765.1976.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Thurre C., Robert M., Cimasoni G., Baehni P. Gingival sulcular leukocytes in periodontitis and in experimental gingivitis in humans. J Periodontal Res. 1984 Sep;19(5):457–468. doi: 10.1111/j.1600-0765.1984.tb01301.x. [DOI] [PubMed] [Google Scholar]


