Abstract
The asthmatic response to the common cold is highly variable and early characteristics that predict worsening of asthma control following a cold have not been identified.
In this prospective multi-center cohort study of 413 adult subjects with asthma, we used the mini-Asthma Control Questionnaire (mini-ACQ) to quantify changes in asthma control and the Wisconsin Upper Respiratory Symptom Survey-21 (WURSS-21) to measure cold severity. Univariate and multivariable models examined demographic, physiologic, serologic, and cold-related characteristics for their relationship to changes in asthma control following a cold.
We observed a clinically significant worsening of asthma control following a cold (increase in mini-ACQ of 0.69 ± 0.93). Univariate analysis demonstrated season, center location, cold length, and cold severity measurements all associated with a change in asthma control. Multivariable analysis of the covariates available within the first 2 days of cold onset revealed the day 2 and the cumulative sum of the day 1 and 2 WURSS-21 scores were significant predictors for the subsequent changes in asthma control.
In asthmatic subjects the cold severity measured within the first 2 days can be used to predict subsequent changes in asthma control. This information may help clinicians prevent deterioration in asthma control following a cold.
Keywords: asthma, asthma control, common cold
INTRODUCTION
Respiratory tract infections (including the common cold) have been associated with increased asthma symptoms, exacerbations, and hospitalizations [1-4]. Recent prospective longitudinal cohort studies in asthmatic adults have also documented an association between respiratory tract infection and worsening asthma symptoms, declines in lung function, and asthma exacerbations [5-9]. For each of these endpoints there was a highly variable clinical response to the respiratory tract infection. In the controlled research setting of an experimental rhinoviral infection, asthmatic subjects also demonstrated a variable clinical, pathologic, and physiologic response [10-18]. Despite documentation of an acute upper respiratory infection, the asthma symptoms ranged from none to severe. Although these studies observed a highly variable post-cold clinical course, none have attempted to identify patient or cold episode characteristics that predict a subsequent change in asthma control. Given the current emphasis on measuring asthma control in the NAEPP Expert Panel Report 3 guidelines [2], improved understanding of the effect of a cold on asthma control is needed.
To identify early characteristics that predict subsequent worsening of asthma control following a cold, we designed the Post-cold Asthma Control and Exacerbation (PAX) study. This was a multi-center prospective cohort study in adult subjects with mild to moderate persistent asthma aimed at defining the prospective incidence of natural colds in asthmatics and identifying early predictors for the changes in asthma control following a cold. We postulated patient and cold episode characteristics would predict a subsequent worsening of asthma control. To examine this hypothesis, we utilized the Asthma Control Questionnaire without lung function, referred to as the mini-ACQ [19], to quantitate the cold-induced change in asthma control and multivariable modeling to identify unique predictors for the change in asthma control following the onset of a cold.
MATERIAL AND METHODS
Details are provided in the online supplement.
Study Design
The PAX study was a multi-center prospective cohort study that recruited subjects with mild to moderate persistent asthma from January 2005 to April 2006. This study recruited subjects that were screened for the Long Acting Beta Agonist Response by Genotype (LARGE) study performed by the Nation Institute of Health Asthma Clinical Research Network (ACRN). At study entry, informed consent was obtained and this was followed by acquisition of demographic information, completion of questionnaires, spirometry, serum collection, and administration of the entry mini-ACQ questionnaire [19]. At the onset of a self-identified cold, the subject notified the study coordinator so that the mini-ACQ could be obtained 7 days after cold onset (Post-cold day 7 mini-ACQ) and 14 days after cold onset (Post-cold day 14 mini-ACQ). Since asthma control and the mini-ACQ may fluctuate over time, and we planned on following subjects for over one year we acquired periodic mini-ACQ scores (approximately every 8 weeks) to serve as a recent Pre-cold mini-ACQ score for comparison to subsequent post-cold mini-ACQ scores. The mini-ACQ was used to quantitate asthma control with the minimally important difference (MID) being a change of 0.5 (Supplement Figure S1 and S2) [19-22]. The WURSS-21 questionnaire was used to quantitate severity of cold symptoms and functional impairment with the MID being a change of 9.48 (Supplement Figure S3) [23, 24]. The PAX Cold questionnaire was used to assess a subject’s prior cold history and consisted of four questions related to previous cold frequency per year, severity of colds, the frequency of cold-induced asthma symptoms, and the severity of cold-induced asthma symptoms (Supplement Figure S4). Each center’s Institutional Review Board approved the protocol.
Statistical Analysis
To compare WURSS-21 scores by day an independent sample t-test was used. The Pre-cold, Post-cold day 7 and Post cold day 14 mini-ACQ scores were compared using repeated measure analysis of variance. Covariates associated with the change between Pre-cold and Post-cold day 7 mini-ACQ scores were identified using independent or paired t-test, repeated measures ANOVA, and Pearson’s correlation. For all comparisons a p-value of < 0.05 was considered significant. The covariates with an association (p < 0.05) with a change in asthma control that could be obtained within the first 2 days of the cold onset were then analyzed using generalized estimating equations [25]. Additional details regarding statistical analysis are included in the online supplement. Data were analyzed with SAS 9.1.2 and SPSS 13.0.
RESULTS
Characteristics of the cold cohort
The entire PAX study population included 413 subjects with mild to moderate persistent asthma. Of these 413 subjects, 134 (32.4%) had a least one self-reported cold and constituted the Cold cohort. The baseline demographic and clinical characteristics of the Cold cohort are shown in Table 1. The Cold cohort was indistinguishable from those that did not have a cold (the Non-cold cohort) in terms of gender, race or ethnicity, age, center location of enrollment, pre-bronchodilator lung function, serum IgE concentrations, atopic status, and prior tobacco use. Compared to the Non-cold cohort, the Cold cohort had a lower initial mini-ACQ score (1.04 ± 0.77 vs 1.22 ± 0.79, mean ± SD, p=0.04) indicating the Cold cohort had slightly better asthma control at study entry.
Table 1.
Demographic Characteristics of Cold Cohort*
| Cold Cohort (n =134) | |
|---|---|
| Female, n (%) | 93 (69.4) |
|
| |
| Race or ethnicity, n (%)† | |
| White, non-Hispanic | 100 (74.6) |
| Black, non-Hispanic | 19 (14.2) |
| Hispanic | 8 (6.0) |
| Other | 7 (5.2) |
|
| |
| Age, years | 38.7 ± 11.7 |
|
| |
| Subjects per center, n (%) | |
| Boston | 25 (18.7) |
| Denver | 18 (13.4) |
| Madison | 29 (21.6) |
| San Diego | 11 (8.2) |
| San Francisco | 17 (12.7) |
| Saint Louis | 16 (11.9) |
| Winston-Salem | 18 (13.4) |
|
| |
| Pre-bronchodilator FEV1 | |
| Liters | 2.69 ± 0.73 |
| % predicted | 80.0 ± 14.5 |
|
| |
| Total serum IgE, IU/ml‡ | 99.6, 115 |
|
| |
| Atopic, n (%)§ | 56 (96.6) |
|
| |
| Prior tobacco use, n (%)† | 26 (19.4) |
|
| |
| Entry mini-ACQ score ** | 1.04 ± 0.77 |
Plus-minus values are means ± standard deviation (SD)
Race, ethnicity, and prior tobacco use were self-reported
n = 132, reported as geometric mean and coefficient of variation (%)
n = 58
n = 125, scores can range from 0 (no symptoms) to 6 (severe symptoms)
Abbreviations: FEV1, forced expiratory volume in one second; IU, International Unit; ACQ, asthma control questionnaire.
Further characterization of the 134 subjects in the Cold cohort demonstrated that 89 (66.4%) subjects had only one cold and 45 (33.6%) had multiple colds (Table 2). To assess the relationship between prior cold episodes and asthma symptoms, we administered the PAX Cold Questionnaire. Subjects recalled a history of two and a half colds every year (range 1-8), that their colds were of “moderate” severity, that colds “sometimes” to “usually” made their asthma symptoms worse, and that their asthma was of “moderate” severity when a cold worsened their asthma.
Table 2.
Characteristics of the Cold Cohort*
| Cold Cohort (n=134) | |
|---|---|
| Reported colds per subject, n (%) | |
| 1 | 89 (66.4) |
| 2 | 24 (17.9) |
| 3 | 13 (9.7) |
| 4 | 6 (4.5) |
| 5 | 1 (0.7) |
| 6 | 1 (0.7) |
|
| |
| PAX Cold Questionnaire responses† | |
| Colds per year | 2.51 ± 1.55 |
| Severity of previous colds‡ | 3.05 ± 0.76 |
| Frequency of cold-induced asthma symptoms§ | 3.64 ± 1.10 |
| Severity of cold-induced asthma symptoms∥ | 3.01 ± 0.87 |
Plus-minus values are means ± SD
n=76
Scores can range from 1 (extremely mild) to 6 (extremely severe)
Scores can range from 1 (never) to 5 (always)
Scores can range from 1 (extremely mild) to 6 (extremely severe)
Incidence of cold episodes
For all 413 subjects in the PAX study there were a total of 211 cold episodes in 149.4 total subject-years of follow-up. The overall incidence of colds per subject-year of follow-up for the entire study population was 1.41 (range from 1.11 in Winston-Salem, NC to 1.85 in Madison, WI). To determine the incidence of cold episodes by season, we chose four-month intervals so that the November through February interval would correspond to a typical cold season. About half (44%) of the 211 cold episodes occurred in the winter season when the cold incidence was 2.05 colds per subject-year of follow-up (Table 3). The average time from study entry to onset of a cold was 4 months and the length of cold was approximately one week (7.2 ± 4.4 days, mean ± SD).
Table 3.
Incidence of Cold Episodes*
| Cold Episodes | ||
|---|---|---|
| Per center, n (%) | Incidence* | |
| Total | 211 | 1.41 |
| Boston | 39 (18.5) | 1.30 |
| Denver | 31 (14.7) | 1.48 |
| Madison | 50 (23.7) | 1.85 |
| San Diego | 16 (7.6) | 1.13 |
| San Francisco | 28 (13.3) | 1.47 |
| Saint Louis | 23 (10.9) | 1.40 |
| Winston-Salem | 24 (11.4) | 1.11 |
|
| ||
| Season | Season, n (%) | Incidence* |
|
|
||
| November-February | 93 (44.1) | 2.05 |
| March-June | 61 (28.9) | 1.15 |
| July-October | 57 (27.0) | 1.12 |
per subject-year of follow-up
Measurement of cold severity
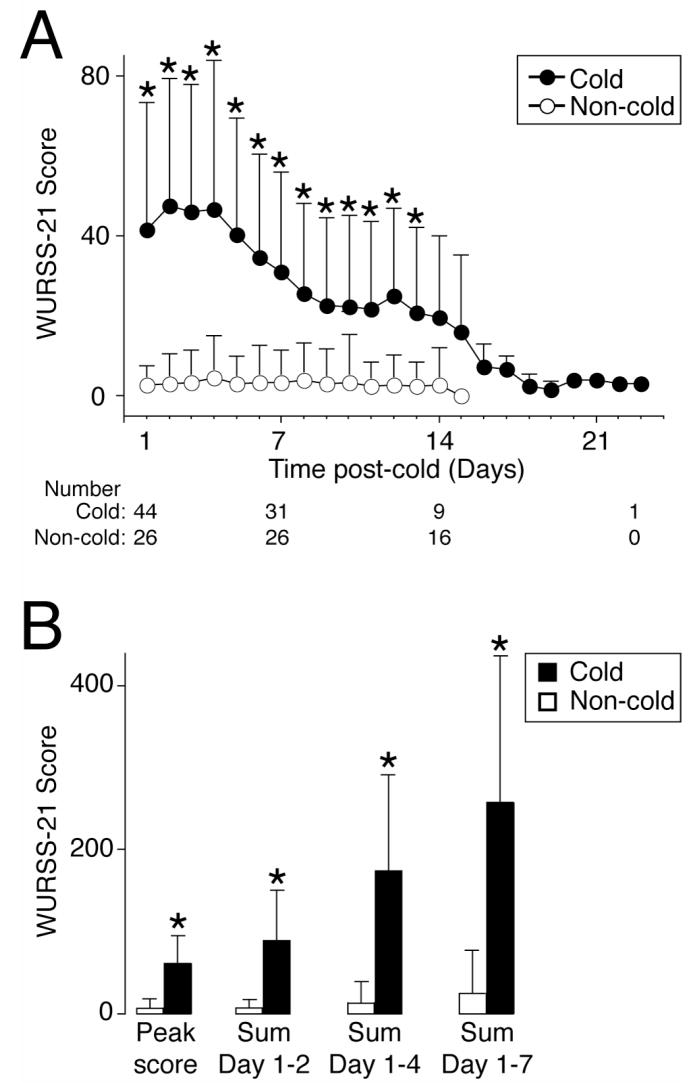
We used the WURSS-21 as an illness-specific quality of life instrument to measure severity of cold symptoms (range 0 to 140 with higher scores indicating more symptoms and functional impairment) [23, 24]. Since asthma subjects may have symptoms that could elevate the WURSS-21 score independent of a cold, we compared the WURSS-21 scores between asthmatics with and without colds. Compared to asthmatics without a cold, those with a cold demonstrated a significant increase in their daily WURSS-21 scores through day 13 (Figure 1A). Furthermore, the magnitude of this increase was greater than the minimally important difference (MID) for the WURSS-21 (MID = 9.48). The subjects with a cold also had a significantly higher peak, cumulative sum of days 1 to 2, days 1 to 4, and days 1 to 7 WURSS-21 scores (Figure 1B). Compared to the baseline rescue albuterol use (0.57 ± 0.94 puffs/day), there was a significant increase on days 1 through 7 following the cold onset with the highest mean daily use occurring 4 days after the onset of the cold (2.02 ± 2.56 puffs/day).
Figure 1. Asthmatics with a cold demonstrate an increased WURSS-21 score.
In A-B, WURSS-21 scores for asthmatics with a cold (Cold) and without a cold (Non-cold). Day 1 indicates the day of cold onset. All values represent mean ± SD and a significant difference between cohorts using the independent groups t-test is indicated (*).
Measurement of asthma control following a cold
To quantify the change in asthma control following the onset of a cold we used the mini-ACQ, an instrument used to measure asthma control with higher scores indicating worse asthma control [19-22]. The mean change between the Pre-cold mini-ACQ and the Post-cold day 7 mini-ACQ was 0.69 ± 0.93 and represents a change greater than the MID for the mini-ACQ (MID = 0.5). Although the Post-cold day 14 mini-ACQ remained statistically increased over the Pre-cold mini-ACQ the difference was below the MID (0.26 ± 0.84) (Figure 2). In a subgroup of cold episodes that were associated with a subsequent asthma exacerbation (n=27), the mean change between the Pre-cold and both the Post-cold day 7 and day 14 mini-ACQ was even more pronounced and remained greater than the MID at both time points (1.41 ± 1.08 and 1.09 ± 1.26 respectively). For complete analysis of the demographic and cold episode characteristics for the subjects without and with a post-cold exacerbation see the online supplement (Results section and Tables S1, S2 and S3).
Figure 2. Asthmatics with a cold demonstrate worsening asthma control.
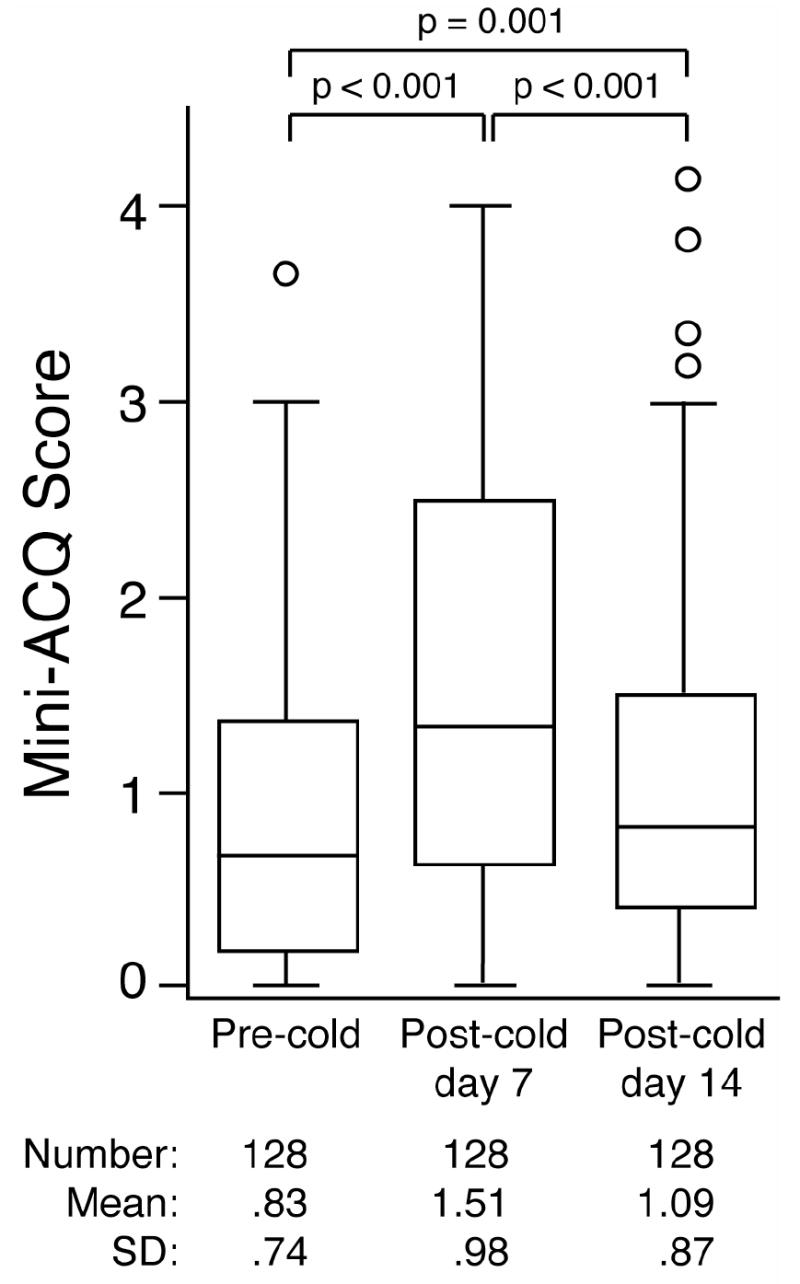
Pre-cold, Post-cold day 7, and Post-cold day 14 mini-ACQ scores are shown for the cohort of cold subjects with complete mini-ACQ data. For each box plot the horizontal line represents the median, the box stretches from the 25th to the 75th percentile of values, the whiskers end at the 95% confidence intervals and outliers are indicated by individual circles. p-values for each comparison (using repeated measures ANOVA) are shown.
Characteristics that Predict Changes in Asthma Control
We examined demographic, physiologic, serologic and cold episode characteristics, to identify characteristics that were associated with a change in asthma control following a cold. Season of cold onset, center location, and duration of cold were associated with a change in asthma control. In addition, peak rescue albuterol use, cumulative summary of rescue albuterol use on days 1-4 and 1-7, and all WURSS-21 scores, except for Day 1, were associated with a change in asthma control following a cold (Table 4). We found no associations with gender, race or ethnicity, age, FEV1, FEV1 % predicted, IgE concentration, atopic status, prior tobacco use, previous cold frequency, severity of previous cold, frequency of cold-induced asthma symptoms, severity of cold-induced asthma symptoms, Day 1 rescue albuterol, Day 2 rescue albuterol, cumulative rescue albuterol use on Days 1-2, or concurrent treatment regimens (inhaled corticosteroids with or without long acting β-agonists) (p > 0.10).
Table 4.
Characteristics of Colds Associated With a Change in Asthma Control*
| Explanatory Variable | p |
|---|---|
| Characteristics of Current Colds | |
| Season of onset† | 0.05 |
| Center† | 0.005 |
| Length of cold‡ | <0.001 |
|
| |
| Rescue Albuterol Use After Cold Onset§ | |
| Peak | 0.001 |
| Sum Day 1-4 | 0.029 |
| Sum Day 1-7 | 0.004 |
|
| |
| WURSS-21 Assessment of Current Cold∥ | |
| Day 1 | 0.0571 |
| Day 2 | <0.0009 |
| Peak | <0.0001 |
| Sum Day 1-2 | 0.0066 |
| Sum Day 1-4 | 0.0009 |
| Sum Day 1-7 | <0.0001 |
Asthma control defined as the change in mini-ACQ between Pre-cold and Post-cold Day 7
n = 143, p-values are associated with repeated measures ANOVA
n = 127, p-value is associated with Pearson’s correlation
n = 67, p-value is associated with Pearson’s correlation
n=51, p-values are associated with the generalized estimating equation parameter estimates
Since our goal was to identify early characteristics that predict a change in post-cold asthma control, we limited the covariates in our multivariable models to those that could be collected in the first 48 hours of a cold: season of onset, center location, Day 1 WURSS-21 score, Day 2 WURSS-21 score, and Sum Day 1-2 WURSS-21 score. Season of onset and center location were collapsed into fewer categories (season: March-October vs November-February; center location: Eastern vs Central/Mountain vs Pacific). Accordingly, we generated three multivariable models for Day 1, Day 2, or Sum Day 1-2 WURSS-21 scores, each also included season of onset, and center location. The Day 1 WURSS-21 score, season of onset, and center location covariates were not significant predictors of a change in asthma control (p>0.05). The Day 2 WURSS-21 score was a significant predictor of a change in the post-cold day 7 mini-ACQ (p=0.0009, estimate=0.012, standard error 0.004, 95% confidence limits 0.005-0.019) indicating that for every 1 point increase in the Day 2 WURSS-21 score there is a predicted 0.01 increase in the change in mini-ACQ score from Pre-cold to Post-cold day 7. The Sum Day 1-2 WURSS-21 score was also a significant predictor (p=0.009, estimate=0.005, standard error 0.002, 95% confidence limits 0.001-0.009) indicating that for every 1 point increase in the Sum Day 1-2 WURSS-21 score there is a predicted 0.005 increase in the change in mini-ACQ score from Pre-cold to Post-cold day 7.
We were interested in selecting the best early cold characteristics for predicting a change in mini-ACQ using season of onset, center location, Day 1 WURSS-21 score, Day 2 WURSS-21 score, and Sum Day 1-2 WURSS-21 score. Therefore, we used a stepwise approach and after creating the marginal models, only Day 2 and Sum Day 1-2 WURSS-21 scores were significant predictors (p-value=0.0009 and 0.0038 respectively). Due to the high correlation between Day 2 and Sum Day 1-2 WURSS-21 scores, both were not included in the same model. The quasi-likelihood under the independence model criterion (QICu) statistics for both the Day 2 and Sum Day 1-2 models were 53.0 indicating both models fit the data equally well. A correlation structure that makes no assumptions about the relationship between pairs of observations from the same participant was selected for both models using the QIC statistic. Again, both models performed equally. We conclude that the Day 2 (p=0.0003, estimate=0.0129, 95%CI 0.0059-0.0198) and the Sum Day 1-2 (p=0.0030, estimate=0.0061, 95%CI 0.0021-0.0101) WURSS-21 scores were early cold characteristics that predicted changes in asthma control.
DISCUSSION
The results from this multi-center prospective cohort study indicate that severity of the cold measured within the first two days following cold-onset is a unique predictor for subsequent worsening of asthma control. We identified that the Day 2 and cumulative Sum Day 1-2 WURSS-21 scores predicted post-cold worsening of asthma control. The WURSS-21 score is the cumulative sum of 20 Likert scale answers that can be obtained from the asthma subject relatively quickly. Although we propose that both WURSS-21 scores can be used in the future, the Day 2 WURSS-21 score may be more practical since it requires only one collection time point. Our multivariate modeling indicated that a Day 2 WURSS-21 score of 50 predicted a 0.5-point increase in the mini-ACQ. Since a change of 0.5 is the MID for the mini-ACQ, asthma subjects with a Day 2 WURSS-21 score greater than 50 are at increased risk for a clinically significant worsening of their asthma control.
To our knowledge the current study represents the largest prospective cohort study of colds in adult asthmatics and is the first multi-center study performed. The seven centers enrolled 413 asthmatics and accrued a total of 149.3 subject-years of follow-up. Our overall cold incidence per subject-year of follow-up was 1.4 which is similar to previous prospective reports (range 1.2-6.7) [5-9, 26]. The variability in these rates likely reflects different geographic locations and season of follow-up as well as different criteria for cold identification. To avoid over-reporting of cold episodes, we specifically instructed the subjects not to report a cold if they were only experiencing asthma or allergy symptoms. To avoid under-reporting of colds, we did not require meeting any additional criteria other than the subject replying “yes” to the cold question, “Do you have a cold today?”. Even though the study coordinators regularly queried the subjects regarding cold episodes, we may have under reported cold episodes due to incomplete diary information. Given the multi-center nature of the current study, the large sample size, long follow-up times that spanned all seasons, and our efforts to avoid under- and over-reporting, we feel our incidence of about one and a half prospectively identified colds per year is accurate.
Since the current study is the first to identify predictors for a change in asthma control following a naturally occurring cold, direct comparison to the literature is not possible. Three experimental rhinoviral inoculation studies attempted to identify characteristics of asthmatics that were associated with a more severe post-viral course and two found no distinguishing factors [12, 16]. In the third study, asthmatic subjects with high pre-inoculation IgE concentration (greater than 124 IU/ml) had higher cold-induced lower respiratory tract symptoms [18]. This study was limited by a small sample size (n=6 and n=10 in the high and low IgE cohorts respectively) and post-hoc dichotomization of individuals according to a relatively arbitrary IgE concentration. We did not identify an association between IgE concentrations and changes in asthma control in the univariate analysis (p=0.83). We measured IgE concentrations at study entry, on average of 4 months prior to cold onset. Accordingly, we cannot exclude the possibility that IgE concentrations measured closer to cold onset could predict changes in asthma control. However, IgE concentrations are relatively stable over this time frame [27, 28], so it is unlikely that additional measurements would identify IgE concentrations as a significant predictor of a change in asthma control following a cold.
The clinical responsiveness of the mini-ACQ to measure changes in asthma control has been validated in asthmatics with and without stable disease [19, 21]. Compared to the Pre-cold mini-ACQ score, the increase in the Post-cold day 7 mini-ACQ score was significant and greater than the MID for the mini-ACQ. This clinically significant worsening of asthma control was transient as the increase in the Post-cold day 14 mini-ACQ was less than the MID. The temporal change in asthma control corresponds with the temporal change in asthma symptoms, lung function, and airway inflammatory parameters in previous reports following a natural cold or experimental rhinoviral infection [10-16, 18, 29]. The change in mini-ACQ following a cold was higher in the cohort with a post-cold asthma exacerbation and the magnitude of this increase was greater than the MID for the mini-ACQ. Collectively, this data suggests the mini-ACQ is responsive to changes in asthma control following the onset of a cold and supports its use as an endpoint in future studies.
Cold severity in asthmatics was measured with the WURSS-21 questionnaire, a survey that has been validated in 151 subjects [24]. Compared to the WURSS-21 scores in this previous report, the WURSS-21 scores in our cohort of asthmatics rose and fell in an identical fashion; however, the Day 1 and peak WURSS-21 scores appear about 20% lower and the dispersion approximately two times larger. This may be related to our smaller sample size or the inclusion of subjects with less severe colds as we purposely employed less stringent cold identification criteria. Alternatively, the validation study WURSS-21 scores may be somewhat overestimated if, as stated by the authors, their colds were longer and more severe compared to previous studies [24]. To support the use of the WURSS-21 survey in asthmatics, we have demonstrated that the WURSS-21 scores in asthmatics changed significantly following a self-reported cold and the WURSS-21 scores in asthmatics without colds are very low and display minimal day-today variability. Accordingly, we feel the WURSS-21 can serve as a valuable tool to measure severity of a cold in asthmatics.
There are limitations in the current study regarding selection criteria, study design, and availability of data. The current study excluded subjects with severe disease, significant smoking history, use of high dose inhaled corticosteroids, or a history of life-threatening asthma. Therefore, our results cannot be generalized to asthmatics with these conditions. The PAX study occurred within the ongoing LARGE parent trial in which asthmatic subjects receiving inhaled corticosteroids were randomized to either placebo or long acting beta-agonists, hence a confounding treatment effect may exist. The PAX Cold and WURSS-21 questionnaire data was incomplete due to missing data and introduction of these questionnaires after the onset of some cold episodes. External validation of the identified predictors could not be performed because we are unaware of an existing dataset that includes the required predictors and outcome variables. Finally, we did not collect microbiologic specimens during the cold episode so it is not possible to determine the role of viral or bacterial respiratory tract infections on post-cold asthma control.
In conclusion, we identified that the Day 2 and cumulative Sum Day 1-2 WURSS-21 scores predicted a post-cold worsening of asthma control. These cold severity measurements may provide an easy-to-use tool to provide important prognostic information to the clinician. Thus, clinicians may want to include an assessment of cold severity together with a measure of asthma control and adjust therapy according to the NAEPP guidelines. Furthermore, utilization of these predictors will aid in identifying patients at high risk for subsequent worsening of asthma control and thus may be used to develop stratification criteria for clinical or interventional studies designed to modify asthma control following a cold.
Supplementary Material
Acknowledgments
The authors thank all the clinical coordinators and administrators at each center for their dedication and effort in completing this study: Mary Ellen Scheipeter, Steve DeMartino, Elise Bender, and Joy Kiviat (Saint Louis); Ronald Zimmerman, Kelly Bixler, Kerrie Sheaffer (Hershey); Peggy Cadbury (San Francisco); William Kahn, Susan M. Trahan, Cheryl Wilmoth, Lauren Imboden, Jeffrey Krings, Kathryn Calloway and Bob Hmieleski (Wake Forest); Mary Gill (Denver); Jenny Matthews, Barb Miller, Ann Sexton (Madison); Sreedhar Bunga and Gautham Marigowda (Boston);
Support statement: U10-HL74227, U10-HL74231, U10-HL074204, U10-HL74212, U10-HL74073, U10-HL074206, U10-HL074208, U10-HL74225, U10-HL74218, M01-RR00036, M01-RR07122 from the National Heart, Lung, and Blood Institute.
References
- 1.Global strategy for asthma management and prevention. 2006:1–109. http://wwwginasthmaorg.
- 2.National Heart, Lung, and Blood Institute National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007:1–440. http://wwwnhlbinihgov/guidelines/asthma/asthgdlnpdf.
- 3.Lambert HP, Stern H. Infective factors in exacerbations of bronchitis and asthma. Br Med J. 1972;3:323–327. doi: 10.1136/bmj.3.5822.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minor TE, Dick EC, DeMeo AN, Ouellette JJ, Cohen M, Reed CE. Viruses as precipitants of asthmatic attacks in children. Jama. 1974;227:292–298. [PubMed] [Google Scholar]
- 5.Atmar RL, Guy E, Guntupalli KK, Zimmerman JL, Bandi VD, Baxter BD, Greenberg SB. Respiratory tract viral infections in inner-city asthmatic adults. Arch Intern Med. 1998;158:2453–2459. doi: 10.1001/archinte.158.22.2453. [DOI] [PubMed] [Google Scholar]
- 6.Beasley R, Coleman ED, Hermon Y, Holst PE, O’Donnell TV, Tobias M. Viral respiratory tract infection and exacerbations of asthma in adult patients. Thorax. 1988;43:679–683. doi: 10.1136/thx.43.9.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, Johnston SL. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. Bmj. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudgel DW, Langston L, Jr, Selner JC, McIntosh K. Viral and bacterial infections in adults with chronic asthma. Am Rev Respir Dis. 1979;120:393–397. doi: 10.1164/arrd.1979.120.2.393. [DOI] [PubMed] [Google Scholar]
- 10.Bardin PG, Fraenkel DJ, Sanderson G, van Schalkwyk EM, Holgate ST, Johnston SL. Peak expiratory flow changes during experimental rhinovirus infection. Eur Respir J. 2000;16:980–985. doi: 10.1183/09031936.00.16598000. [DOI] [PubMed] [Google Scholar]
- 11.Cheung D, Dick EC, Timmers MC, de Klerk EP, Spaan WJ, Sterk PJ. Rhinovirus inhalation causes long-lasting excessive airway narrowing in response to methacholine in asthmatic subjects in vivo. Am J Respir Crit Care Med. 1995;152:1490–1496. doi: 10.1164/ajrccm.152.5.7582282. [DOI] [PubMed] [Google Scholar]
- 12.Fleming HE, Little FF, Schnurr D, Avila PC, Wong H, Liu J, Yagi S, Boushey HA. Rhinovirus-16 colds in healthy and in asthmatic subjects: similar changes in upper and lower airways. Am J Respir Crit Care Med. 1999;160:100–108. doi: 10.1164/ajrccm.160.1.9808074. [DOI] [PubMed] [Google Scholar]
- 13.Fraenkel DJ, Bardin PG, Sanderson G, Lampe F, Johnston SL, Holgate ST. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med. 1995;151:879–886. doi: 10.1164/ajrccm/151.3_Pt_1.879. [DOI] [PubMed] [Google Scholar]
- 14.Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 15.Grunberg K, Sharon RF, Sont JK, In ’t Veen JC, Van Schadewijk WA, De Klerk EP, Dick CR, Van Krieken JH, Sterk PJ. Rhinovirus-induced airway inflammation in asthma: effect of treatment with inhaled corticosteroids before and during experimental infection. Am J Respir Crit Care Med. 2001;164:1816–1822. doi: 10.1164/ajrccm.164.10.2102118. [DOI] [PubMed] [Google Scholar]
- 16.Halperin SA, Eggleston PA, Beasley P, Suratt P, Hendley JO, Groschel DH, Gwaltney JM., Jr Exacerbations of asthma in adults during experimental rhinovirus infection. Am Rev Respir Dis. 1985;132:976–980. doi: 10.1164/arrd.1985.132.5.976. [DOI] [PubMed] [Google Scholar]
- 17.Mosser AG, Vrtis R, Burchell L, Lee WM, Dick CR, Weisshaar E, Bock D, Swenson CA, Cornwell RD, Meyer KC, Jarjour NN, Busse WW, Gern JE. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med. 2005;171:645–651. doi: 10.1164/rccm.200407-970OC. [DOI] [PubMed] [Google Scholar]
- 18.Zambrano JC, Carper HT, Rakes GP, Patrie J, Murphy DD, Platts-Mills TA, Hayden FG, Gwaltney JM, Jr, Hatley TK, Owens AM, Heymann PW. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol. 2003;111:1008–1016. doi: 10.1067/mai.2003.1396. [DOI] [PubMed] [Google Scholar]
- 19.Juniper EF, O’Byrne PM, Roberts JN. Measuring asthma control in group studies: do we need airway calibre and rescue beta2-agonist use? Respir Med. 2001;95:319–323. doi: 10.1053/rmed.2001.1034. [DOI] [PubMed] [Google Scholar]
- 20.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 21.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99:553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Barrett B, Locken K, Maberry R, Schwamman J, Brown R, Bobula J, Stauffacher EA. The Wisconsin Upper Respiratory Symptom Survey (WURSS): a new research instrument for assessing the common cold. J Fam Pract. 2002;51:265–273. [PubMed] [Google Scholar]
- 24.Barrett B, Brown R, Mundt M, Safdar N, Dye L, Maberry R, Alt J. The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid. J Clin Epidemiol. 2005;58:609–617. doi: 10.1016/j.jclinepi.2004.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 26.Tarlo S, Broder I, Spence L. A prospective study of respiratory infection in adult asthmatics and their normal spouses. Clin Allergy. 1979;9:293–301. doi: 10.1111/j.1365-2222.1979.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 27.Boulet LP, Chapman KR, Cote J, Kalra S, Bhagat R, Swystun VA, Laviolette M, Cleland LD, Deschesnes F, Su JQ, DeVault A, Fick RB, Jr, Cockcroft DW. Inhibitory effects of an anti-IgE antibody E25 on allergen-induced early asthmatic response. Am J Respir Crit Care Med. 1997;155:1835–1840. doi: 10.1164/ajrccm.155.6.9196083. [DOI] [PubMed] [Google Scholar]
- 28.Fahy JV, Fleming HE, Wong HH, Liu JT, Su JQ, Reimann J, Fick RB, Jr, Boushey HA. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155:1828–1834. doi: 10.1164/ajrccm.155.6.9196082. [DOI] [PubMed] [Google Scholar]
- 29.Pizzichini MM, Pizzichini E, Efthimiadis A, Chauhan AJ, Johnston SL, Hussack P, Mahony J, Dolovich J, Hargreave FE. Asthma and natural colds. Inflammatory indices in induced sputum: a feasibility study. Am J Respir Crit Care Med. 1998;158:1178–1184. doi: 10.1164/ajrccm.158.4.9712082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.