Abstract
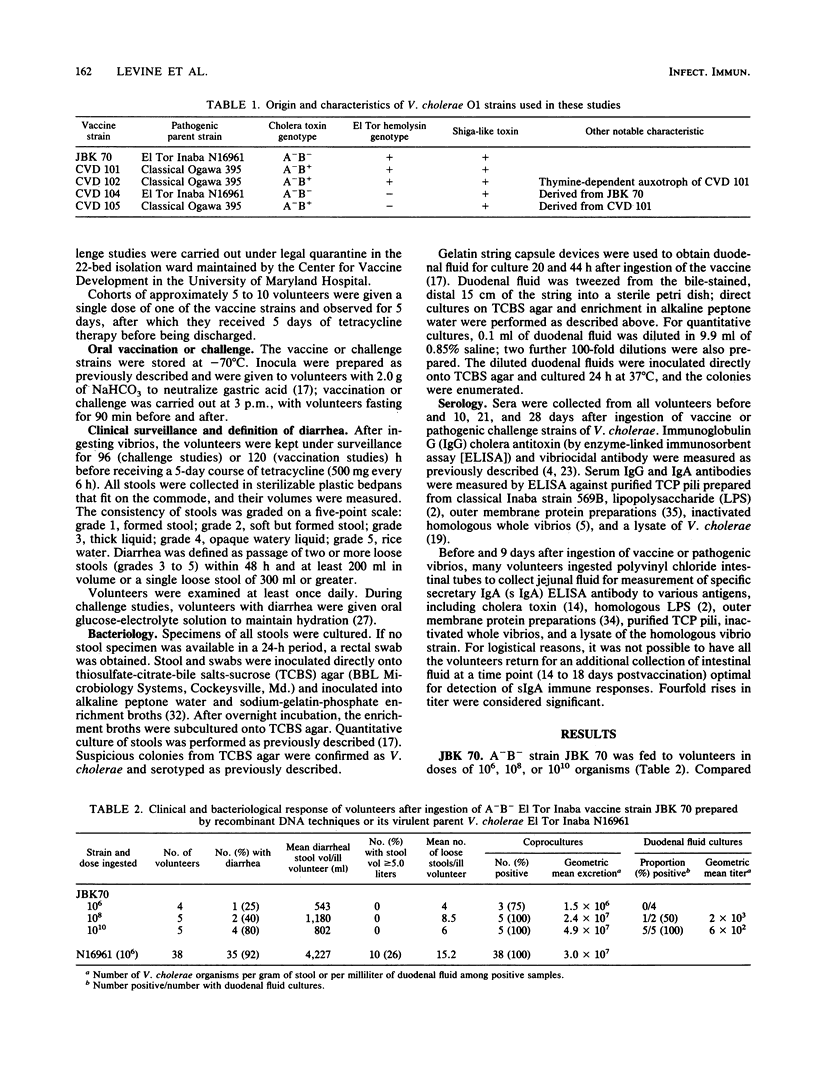
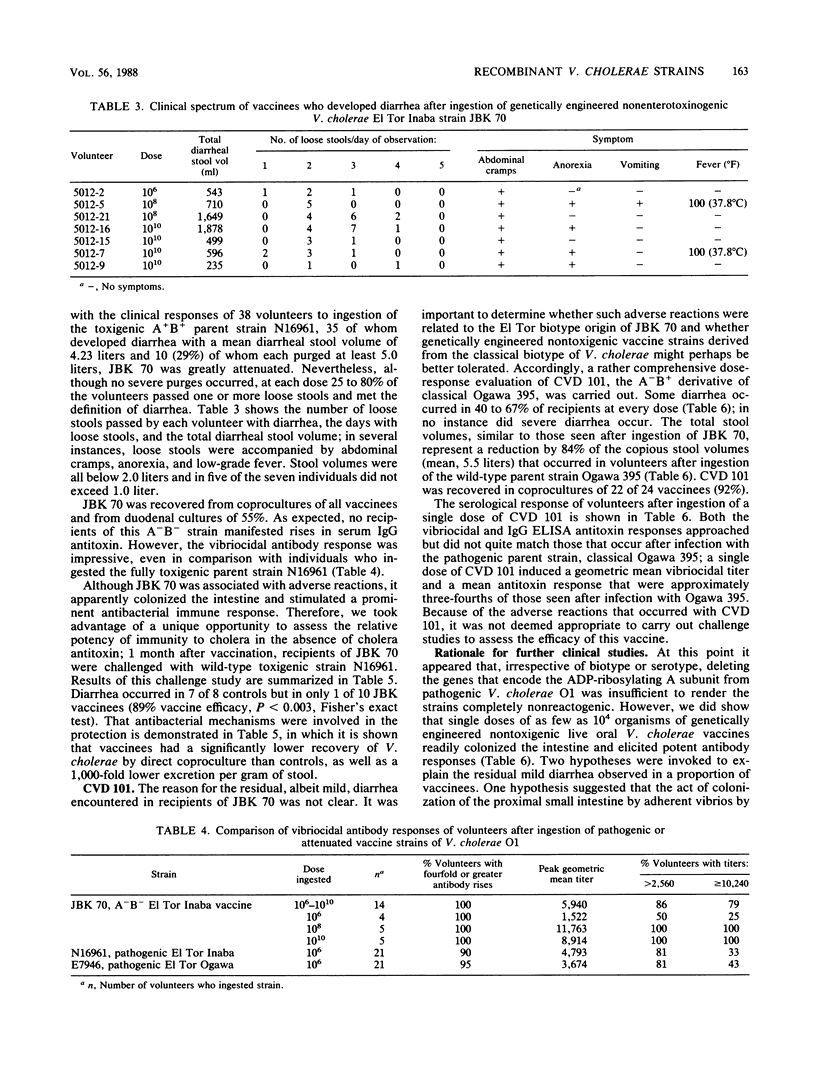
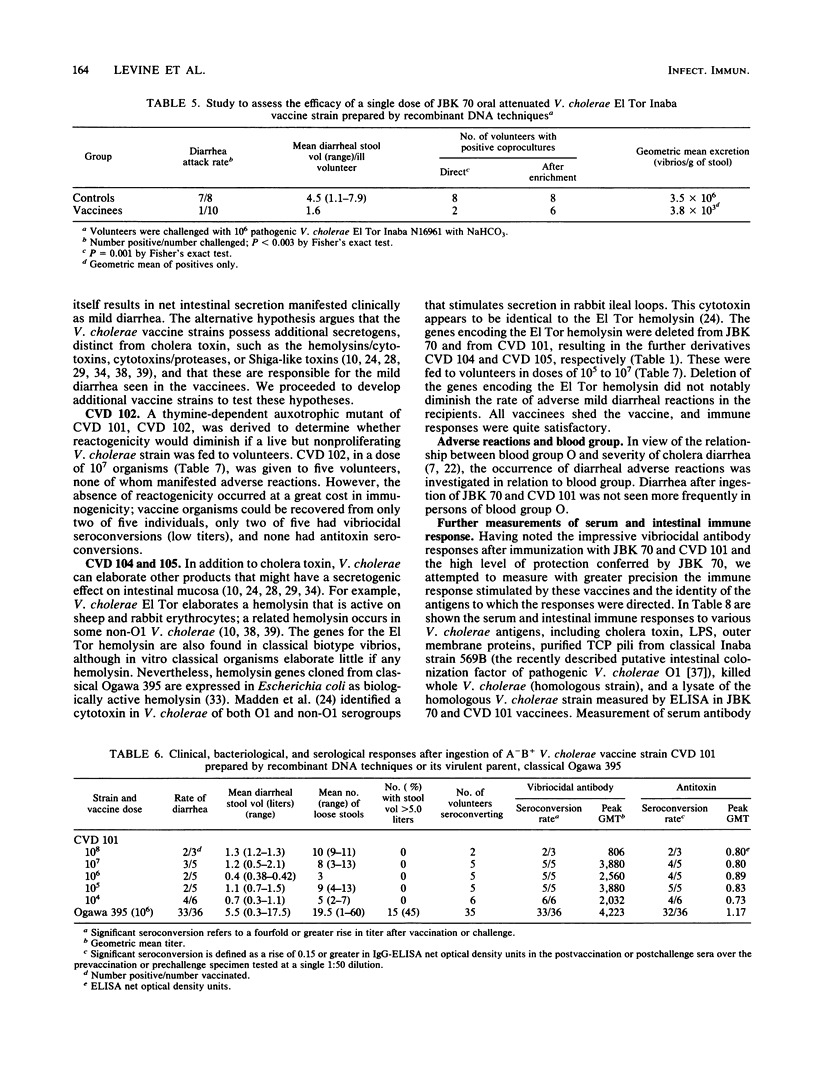
Vibrio cholerae O1 A-B- vaccine strain JBK 70 and A-B+ CVD 101 prepared by recombinant DNA techniques from pathogenic EI Tor Inaba N16961 and classical Ogawa 395, respectively, were fed to 38 volunteers in single doses of 10(4) to 10(10). Although severe diarrhea did not occur in any vaccine, more than one-half developed mild diarrhea. These attenuated strains colonized well and elicited prominent vibriocidal and antitoxic (CVD 101) antibody responses. Recipients of a single dose of JBK 70 were significantly protected when challenged with 10(6) wild-type N16961. Diarrhea occurred in 7 of 8 controls but in only 1 of 10 vaccines (P less than 0.003, 89% vaccine efficacy), demonstrating the potency of immune mechanisms that do not involve cholera antitoxin. Further derivatives were prepared to explore the pathogenesis of the residual diarrhea, considering that either intestinal colonization by the vaccine itself or accessory toxins might be responsible. CVD 102, an auxotrophic mutant of CVD 101, did not cause diarrhea but colonized poorly and elicited feeble immune responses. Derivatives of JBK 70 and CVD 101 (CVD 104 and 105) deleted of genes encoding the EI Tor hemolysin still caused mild diarrhea. Genetically engineered strains can be colonizing, highly immunogenic, and protective single-dose oral vaccines, but they must be further attenuated before they can be considered for use as public health tools.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attridge S. R., Rowley D. Prophylactic significance of the nonlipopolysaccharide antigens of Vibrio cholerae. J Infect Dis. 1983 Nov;148(5):931–939. doi: 10.1093/infdis/148.5.931. [DOI] [PubMed] [Google Scholar]
- Black R. E., Levine M. M., Clements M. L., Young C. R., Svennerholm A. M., Holmgren J. Protective efficacy in humans of killed whole-vibrio oral cholera vaccine with and without the B subunit of cholera toxin. Infect Immun. 1987 May;55(5):1116–1120. doi: 10.1128/iai.55.5.1116-1120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Craig J. P., Pierce N. F., Hornick R. B. Response of man to infection with Vibrio cholerae. II. Protection from illness afforded by previous disease and vaccine. J Infect Dis. 1974 Oct;130(4):325–333. doi: 10.1093/infdis/130.4.325. [DOI] [PubMed] [Google Scholar]
- Clements M. L., Levine M. M., Young C. R., Black R. E., Lim Y. L., Robins-Browne R. M., Craig J. P. Magnitude, kinetics, and duration of vibriocidal antibody responses in North Americans after ingestion of Vibrio cholerae. J Infect Dis. 1982 Apr;145(4):465–473. doi: 10.1093/infdis/145.4.465. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Development of an enzyme-linked immunosorbent assay for studying Vibrio cholerae cell surface antigens. J Clin Microbiol. 1982 Jul;16(1):41–45. doi: 10.1128/jcm.16.1.41-45.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R. I., Becker S., Huq M. I., Stoll B. J., Khan M. U., Merson M. H., Lee J. V., Black R. E. Endemic cholera in rural Bangladesh, 1966-1980. Am J Epidemiol. 1982 Dec;116(6):959–970. doi: 10.1093/oxfordjournals.aje.a113498. [DOI] [PubMed] [Google Scholar]
- Glass R. I., Holmgren J., Haley C. E., Khan M. R., Svennerholm A. M., Stoll B. J., Belayet Hossain K. M., Black R. E., Yunus M., Barua D. Predisposition for cholera of individuals with O blood group. Possible evolutionary significance. Am J Epidemiol. 1985 Jun;121(6):791–796. doi: 10.1093/oxfordjournals.aje.a114050. [DOI] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Ichinose Y., Yamamoto K., Nakasone N., Tanabe M. J., Takeda T., Miwatani T., Iwanaga M. Enterotoxicity of El Tor-like hemolysin of non-O1 Vibrio cholerae. Infect Immun. 1987 May;55(5):1090–1093. doi: 10.1128/iai.55.5.1090-1093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jertborn M., Svennerholm A. M., Holmgren J. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J Clin Microbiol. 1986 Aug;24(2):203–209. doi: 10.1128/jcm.24.2.203-209.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Lockman H., Baldini M. M., Levine M. M. Recombinant nontoxinogenic Vibrio cholerae strains as attenuated cholera vaccine candidates. Nature. 1984 Apr 12;308(5960):655–658. doi: 10.1038/308655a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Cisneros L., Nalin D. R., Young C. R. Duration of infection-derived immunity to cholera. J Infect Dis. 1981 Jun;143(6):818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Cisneros L., Saah A., Nalin D. R., Gill D. M., Craig J. P., Young C. R., Ristaino P. The pathogenicity of nonenterotoxigenic Vibrio cholerae serogroup O1 biotype El Tor isolated from sewage water in Brazil. J Infect Dis. 1982 Mar;145(3):296–299. doi: 10.1093/infdis/145.3.296. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Lanata C., Sears S., Honda T., Young C. R., Finkelstein R. A. Evaluation in humans of attenuated Vibrio cholerae El Tor Ogawa strain Texas Star-SR as a live oral vaccine. Infect Immun. 1984 Feb;43(2):515–522. doi: 10.1128/iai.43.2.515-522.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Herrington D., Murphy J. R., Morris J. G., Losonsky G., Tall B., Lindberg A. A., Svenson S., Baqar S., Edwards M. F. Safety, infectivity, immunogenicity, and in vivo stability of two attenuated auxotrophic mutant strains of Salmonella typhi, 541Ty and 543Ty, as live oral vaccines in humans. J Clin Invest. 1987 Mar;79(3):888–902. doi: 10.1172/JCI112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Nalin D. R., Craig J. P., Hoover D., Bergquist E. J., Waterman D., Holley H. P., Hornick R. B., Pierce N. P., Libonati J. P. Immunity of cholera in man: relative role of antibacterial versus antitoxic immunity. Trans R Soc Trop Med Hyg. 1979;73(1):3–9. doi: 10.1016/0035-9203(79)90119-6. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Nalin D. R., Rennels M. B., Hornick R. B., Sotman S., Van Blerk G., Hughes T. P., O'Donnell S., Barua D. Genetic susceptibility to cholera. Ann Hum Biol. 1979 Jul-Aug;6(4):369–374. doi: 10.1080/03014467900003751. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Young C. R., Black R. E., Takeda Y., Finkelstein R. A. Enzyme-linked immunosorbent assay to measure antibodies to purified heat-labile enterotoxins from human and porcine strains of Escherichia coli and to cholera toxin: application in serodiagnosis and seroepidemiology. J Clin Microbiol. 1985 Feb;21(2):174–179. doi: 10.1128/jcm.21.2.174-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden J. M., McCardell B. A., Shah D. B. Cytotoxin production by members of genus Vibrio. Lancet. 1984 Nov 24;2(8413):1217–1218. doi: 10.1016/s0140-6736(84)92777-6. [DOI] [PubMed] [Google Scholar]
- Majumdar A. S., Dutta P., Dutta D., Ghose A. C. Antibacterial and antitoxin responses in the serum and milk of cholera patients. Infect Immun. 1981 Apr;32(1):1–8. doi: 10.1128/iai.32.1.1-8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley W. H., Benenson A. S., Barui R. A serological survey for cholear antibodies in rural east Pakistan. 1. The distribution of antibody in the control population of a cholera-vaccine field-trial area and the relation of antibody titre to the pattern of endemic cholera. Bull World Health Organ. 1968;38(3):327–334. [PMC free article] [PubMed] [Google Scholar]
- Nalin D. R., Levine M. M., Hornick R. B., Bergquist E. J., Hoover D., Holley H. P., Waterman D., VanBlerk J., Matheny S., Sotman S. The problem of emesis during oral glucose-electrolytes therapy given from the onset of severe cholera. Trans R Soc Trop Med Hyg. 1979;73(1):10–14. doi: 10.1016/0035-9203(79)90120-2. [DOI] [PubMed] [Google Scholar]
- Nishibuchi M., Seidler R. J., Rollins D. M., Joseph S. W. Vibrio factors cause rapid fluid accumulation in suckling mice. Infect Immun. 1983 Jun;40(3):1083–1091. doi: 10.1128/iai.40.3.1083-1091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Chen M. E., Holmes R. K., Kaper J., Levine M. M. Environmental and human isolates of Vibrio cholerae and Vibrio parahaemolyticus produce a Shigella dysenteriae 1 (Shiga)-like cytotoxin. Lancet. 1984 Jan 14;1(8368):77–78. doi: 10.1016/s0140-6736(84)90006-0. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Pierce N. F., Apple R. T., Cray W. C., Jr M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986 Jun;153(6):1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Kaper J. B., Mekalanos J. J., Cray W. C., Jr, Richardson K. Determinants of the immunogenicity of live virulent and mutant Vibrio cholerae O1 in rabbit intestine. Infect Immun. 1987 Feb;55(2):477–481. doi: 10.1128/iai.55.2.477-481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennels M. B., Levine M. M., Daya V., Angle P., Young C. Selective vs. nonselective media and direct plating vs. enrichment technique in isolation of Vibrio cholerae: recommendations for clinical laboratories. J Infect Dis. 1980 Sep;142(3):328–331. doi: 10.1093/infdis/142.3.328. [DOI] [PubMed] [Google Scholar]
- Richardson K., Michalski J., Kaper J. B. Hemolysin production and cloning of two hemolysin determinants from classical Vibrio cholerae. Infect Immun. 1986 Nov;54(2):415–420. doi: 10.1128/iai.54.2.415-420.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S. C., Neogi P. K., Alam K., Huq M. I., Al-Mahmud K. A. A new enterotoxin produced by Vibrio cholerae O1. J Diarrhoeal Dis Res. 1984 Mar;2(1):3–12. [PubMed] [Google Scholar]
- Sears S. D., Richardson K., Young C., Parker C. D., Levine M. M. Evaluation of the human immune response to outer membrane proteins of Vibrio cholerae. Infect Immun. 1984 May;44(2):439–444. doi: 10.1128/iai.44.2.439-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Observations on the pathogenic properties of the K88, Hly and Ent plasmids of Escherichia coli with particular reference to porcine diarrhoea. J Med Microbiol. 1971 Nov;4(4):467–485. doi: 10.1099/00222615-4-4-467. [DOI] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Al-Omani M., Honda T., Takeda Y., Miwatani T. Non-O1 Vibrio cholerae hemolysin: purification, partial characterization, and immunological relatedness to El Tor hemolysin. Infect Immun. 1984 Jul;45(1):192–196. doi: 10.1128/iai.45.1.192-196.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Ichinose Y., Nakasone N., Tanabe M., Nagahama M., Sakurai J., Iwanaga M. Identity of hemolysins produced by Vibrio cholerae non-O1 and V. cholerae O1, biotype El Tor. Infect Immun. 1986 Mar;51(3):927–931. doi: 10.1128/iai.51.3.927-931.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


