Abstract
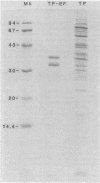
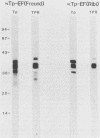
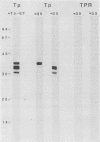
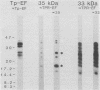
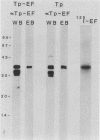
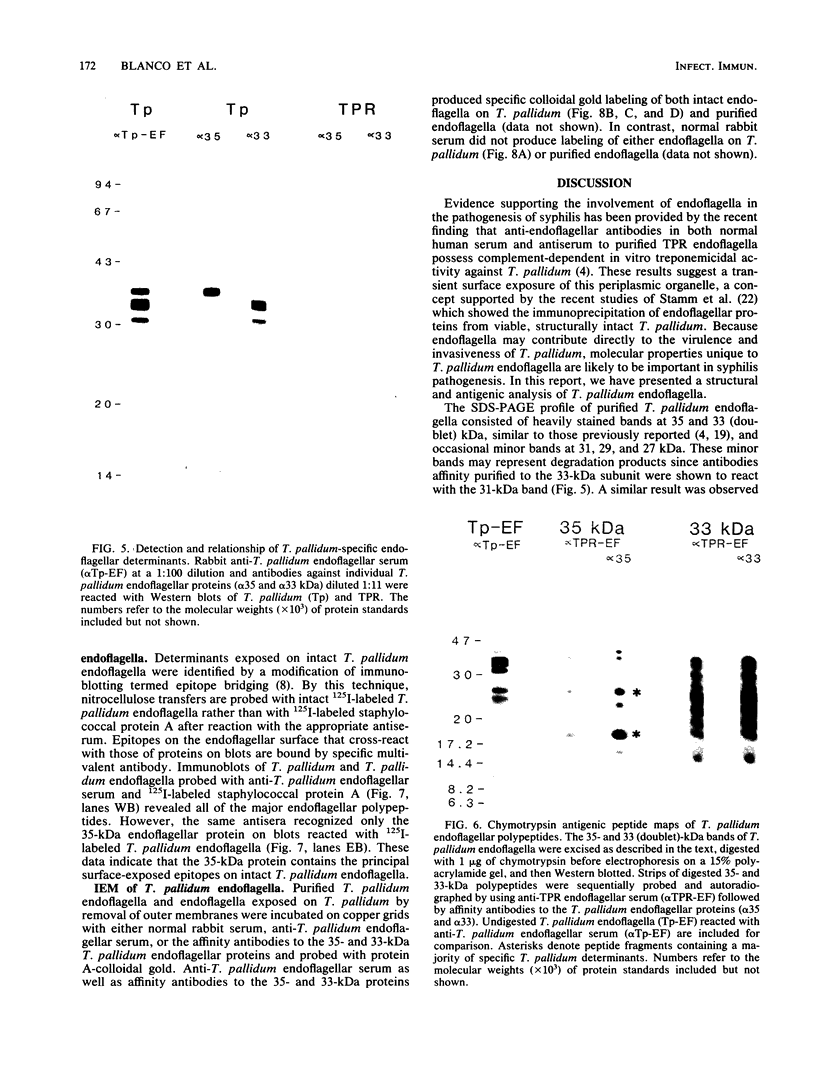
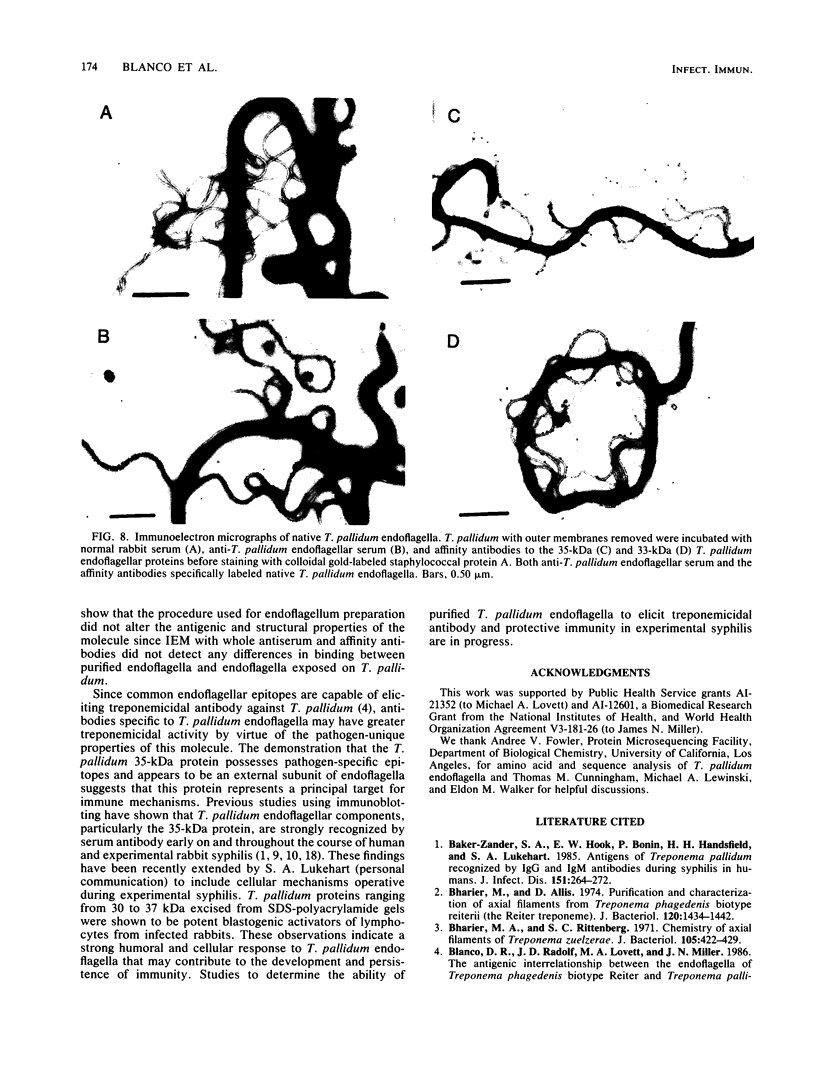
Purified endoflagella from Treponema pallidum, Nichols strain, were characterized both structurally and antigenically. Structural analysis showed T. pallidum endoflagella are composed of 35- and 33-kilodalton (kDa) subunits which lack cysteine and do not share N-terminal amino acid sequence homology (20 residues). Intact endoflagella were dissociated into the composite subunits by incubation, which disrupts noncovalent bonds. Antiserum raised against purified T. pallidum endoflagella identified shared epitopes on the endoflagellar polypeptides of the nonpathogen, Treponema phagedenis biotype Reiter. Pathogen-specific epitopes were also found on the 35- and 33-kDa polypeptides by using affinity-purified endoflagellar antibodies. The pathogen-specific epitopes were localized by immunoblotting analysis of chymotryptic digests of the endoflagellar subunits; 18- and 26-kDa fragments derived from the 35-kDa subunit were found to possess a majority of the pathogen-specific epitopes. Both the 35- and 33-kDa subunits had surface exposure, as determined by immunoelectron microscopy, although additional immunochemical data indicated that the surface exposure of the 35-kDa subunit was greater.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker-Zander S. A., Hook E. W., 3rd, Bonin P., Handsfield H. H., Lukehart S. A. Antigens of Treponema pallidum recognized by IgG and IgM antibodies during syphilis in humans. J Infect Dis. 1985 Feb;151(2):264–272. doi: 10.1093/infdis/151.2.264. [DOI] [PubMed] [Google Scholar]
- Bharier M. A., Rittenberg S. C. Chemistry of axial filaments of Treponema zuezerae. J Bacteriol. 1971 Jan;105(1):422–429. doi: 10.1128/jb.105.1.422-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharier M., Allis D. Purification and characterization of axial filaments from Treponema phagedenis biotype reiterii (the Reiter treponeme). J Bacteriol. 1974 Dec;120(3):1434–1442. doi: 10.1128/jb.120.3.1434-1442.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco D. R., Radolf J. D., Lovett M. A., Miller J. N. The antigenic interrelationship between the endoflagella of Treponema phagedenis biotype Reiter and Treponema pallidum Nichols strain. I. Treponemicidal activity of cross-reactive endoflagellar antibodies against T. pallidum. J Immunol. 1986 Nov 1;137(9):2973–2979. [PubMed] [Google Scholar]
- Canale-Parola E. Motility and chemotaxis of spirochetes. Annu Rev Microbiol. 1978;32:69–99. doi: 10.1146/annurev.mi.32.100178.000441. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Doetsch R. N., Sjoblad R. D. Flagellar structure and function in eubacteria. Annu Rev Microbiol. 1980;34:69–108. doi: 10.1146/annurev.mi.34.100180.000441. [DOI] [PubMed] [Google Scholar]
- Fehniger T. E., Walfield A. M., Cunningham T. M., Radolf J. D., Miller J. N., Lovett M. A. Purification and characterization of a cloned protease-resistant Treponema pallidum-specific antigen. Infect Immun. 1984 Nov;46(2):598–607. doi: 10.1128/iai.46.2.598-607.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff P. A., Bishop N. H., Miller J. N., Lovett M. A. Humoral immune response in experimental syphilis to polypeptides of Treponema pallidum. J Immunol. 1983 Oct;131(4):1973–1977. [PubMed] [Google Scholar]
- Hanff P. A., Fehniger T. E., Miller J. N., Lovett M. A. Humoral immune response in human syphilis to polypeptides of Treponema pallidum. J Immunol. 1982 Sep;129(3):1287–1291. [PubMed] [Google Scholar]
- Hanff P. A., Norris S. J., Lovett M. A., Miller J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984 Oct-Dec;11(4):275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- Hardy P. H., Jr, Fredericks W. R., Nell E. E. Isolation and antigenic characteristics of axial filaments from the Reiter Treponeme. Infect Immun. 1975 Feb;11(2):380–386. doi: 10.1128/iai.11.2.380-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978 Mar;42(1):114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Gubish E. R., Jr Identification of Treponema pallidum antigens: comparison with a nonpathogenic treponeme. J Immunol. 1982 Aug;129(2):833–838. [PubMed] [Google Scholar]
- MILLER J. N., WHANG S. J., FAZZAN F. P. STUDIES ON IMMUNITY IN EXPERIMENTAL SYPHILIS. I. IMMUNOLOGIC RESPONSE OF RABBITS IMMUNIZED WITH REITER PROTEIN ANTIGEN AND CHALLENGED WITH VIRULENT TREPONEMA PALLIDUM. Br J Vener Dis. 1963 Sep;39:195–198. doi: 10.1136/sti.39.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison M. Lactoperoxidase-catalyzed iodination as a tool for investigation of proteins. Methods Enzymol. 1980;70(A):214–220. doi: 10.1016/s0076-6879(80)70051-4. [DOI] [PubMed] [Google Scholar]
- Moskophidis M., Müller F. Molecular analysis of immunoglobulins M and G immune response to protein antigens of Treponema pallidum in human syphilis. Infect Immun. 1984 Jan;43(1):127–132. doi: 10.1128/iai.43.1.127-132.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn C. W., Bailey M. J., Cockayne A. The axial filament antigen of Treponema pallidum. Immunology. 1985 Apr;54(4):635–641. [PMC free article] [PubMed] [Google Scholar]
- Petersen C. S., Pedersen N. S., Axelsen N. H. A simple method for the isolation of flagella from Treponema Reiter. Acta Pathol Microbiol Scand C. 1981 Dec;89(6):379–385. doi: 10.1111/j.1699-0463.1981.tb02716.x. [DOI] [PubMed] [Google Scholar]
- Radolf J. D., Blanco D. R., Miller J. N., Lovett M. A. Antigenic interrelationship between endoflagella of Treponema phagedenis biotype Reiter and Treponema pallidum (Nichols): molecular characterization of endoflagellar proteins. Infect Immun. 1986 Dec;54(3):626–634. doi: 10.1128/iai.54.3.626-634.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Hodinka R. L., Wyrick P. B., Bassford P. J., Jr Changes in the cell surface properties of Treponema pallidum that occur during in vitro incubation of freshly extracted organisms. Infect Immun. 1987 Sep;55(9):2255–2261. doi: 10.1128/iai.55.9.2255-2261.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Putative Treponema pallidum cytadhesins share a common functional domain. Infect Immun. 1985 Sep;49(3):833–835. doi: 10.1128/iai.49.3.833-835.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]