Abstract
Objective
To assess sexual function in women with spontaneous 46,XX primary ovarian insufficiency after at least 3 months of a standardized hormone replacement regimen.
Design
Cross-sectional cohort, controlled
Setting
National Institutes of Health Clinical Research Center
Patient(s)
Women with primary ovarian insufficiency (N=143) and regularly menstruating controls (N=70).
Interventions
Self-administered questionnaires; 100 micrograms/day estradiol patch, oral medroxyprogesterone acetate 10 mg for 12 days each month for patients
Main Outcome Measure(s)
Derogatis Interview for Sexual Function Self Report (DISF-SR)
Result(s)
Women with primary ovarian insufficiency had significantly lower DISF-SR composite scores as compared to control women. Their serum total testosterone levels were significantly correlated with DISF-SR composite score (r=0.20, P=0.03), although this accounted for only 4% of the variance in this measure. Patients with testosterone levels below normal tended to have lower DISF-SR composite scores. Of patients with primary ovarian insufficiency 9/127 (7%) scored below the 2nd centile on the composite sexual function score compared to 1/49 (2%) of control women (difference not statistically significant).
Conclusion(s)
As assessed by the DISF-SR, sexual function is in the normal range for most young women with 46,XX spontaneous primary ovarian insufficiency who are receiving physiologic estradiol replacement. However, as a group, these young women score significantly lower on this sexual function scale than control women.
Capsule
Sexual function is in the normal range for most young women with 46,XX spontaneous primary ovarian insufficiency who are receiving physiologic estradiol replacement.
Keywords: sexual function, primary ovarian insufficiency, premature ovarian failure, premature menopause, primary hypogonadism, hypergonadotropic hypogonadism, hypergonadotropic amenorrhea, hormone replacement therapy, estrogen, testosterone
Introduction
Premature ovarian failure, previously known as premature menopause, has been defined as the development of hypergonadotropic hypogonadism before the age of 40 years, which is two standard deviations below the mean age of natural menopause (1–3). This condition is associated with amenorrhea, symptoms of estrogen deficiency, and gonadotropin levels in the menopausal range. The prevalence is approximately 1 in 250 by age 35 and 1 in 100 by age 40 (4).
The term “premature ovarian failure” is problematic because it implies permanent cessation of ovarian function. In fact, many women with this condition experience intermittent ovarian function that may last for decades. Pregnancy may occur in some women many years after the diagnosis (5). Our preferred term for the condition is “primary ovarian insufficiency” as first introduced by Fuller Albright et al. in 1942 (6).
Sexual dysfunction can have harmful effects on relationships, self-esteem, and quality of life, yet sexual dysfunction commonly receives little attention in clinical practice. Sexual dysfunction in women involves the interaction of emotional, cultural, personal, interpersonal, contextual, and medical factors (7–10). In one national survey approximately 25% of women reported marked distress about their sexual relationship and/or their own sexuality (7). The study found that the best predictors of sexual distress were markers of general emotional well-being and emotional relationship with the partner during sexual activity. Physical aspects of sexual response in women, including arousal, vaginal lubrication, and orgasm, were poor predictors (7).
Young women find the diagnosis of spontaneous 46,XX primary ovarian insufficiency particularly traumatic (11). Disorders of mental health, especially depression, frequently underlie the presentation of sexual dysfunction (12). The traditional separatist notion that sexual dysfunction has either psychological or organic origins has been replaced by an understanding that the two are closely linked and must be taken into consideration together (13).
There is little evidence specifically analyzing the relationship between sexual dysfunction and spontaneous 46,XX primary ovarian insufficiency (14). To our knowledge no controlled studies to date have specifically evaluated sexual function in women with this condition. We undertook this study to determine if women who have spontaneous 46,XX primary ovarian insufficiency and are estradiol replete have sexual dysfunction as compared to young women of similar age who have normal ovarian function.
Subjects and Methods
Study population
The study was approved by the Institutional Review Board of the National Institute of Child Health and Human Development, National Institutes of Health (Bethesda, MD). All women provided written informed consent. We recruited women with spontaneous 46,XX primary ovarian insufficiency by the Internet and by clinician referral. We recruited controls by local advertisement.
From January 2000 to November 2005 we recruited 143 women with spontaneous 46,XX primary ovarian insufficiency and 70 control women of similar age who had normal ovarian function. We recruited patients and controls as part of a screening process for enrollment in a 3-year prospective double-blind randomized placebo-controlled trial (designed to evaluate the effect of transdermal estradiol/testosterone therapy on bone density). The 3-year study was conducted under a cooperative research and development agreement (CRADA) between the National Institutes of Health and Procter and Gamble Pharmaceuticals, Mason, OH). So as to obtain a representative spectrum of sexual function in this population there was no a priori requirement that patients or controls have a sexual partner or be sexually active (13). No reference to sexual function was made as part of recruitment efforts.
Study design
This was a controlled cross-sectional study designed to compare sexual function of women with spontaneous 46,XX primary ovarian insufficiency who receive a standardized hormone regimen for at least 3 months and women of similar age who have normal ovarian function. Women with spontaneous primary ovarian insufficiency were seen for two visits: 1) a baseline screening evaluation at which time they had been off any estrogen/progestogen hormone therapy for at least two weeks, and 2) after receiving a standardized hormone regimen for at least 3 months consisting of a 100 μg estradiol patch and cyclic oral medroxyprogesterone acetate (10 mg for 12 days each month).
Women with primary ovarian insufficiency
To be eligible for the study patients had to meet the following inclusion criteria: 1) diagnosis of spontaneous 46,XX primary ovarian insufficiency before the age of 40 years (i.e., at least 4 months of oligo/amenorrhea and two FSH levels in the menopausal range, as defined by the local assay employed, and confirmed on two separate occasions, at least 1 month apart); 2) age between 18 and 42; 3) no iatrogenic cause of the ovarian insufficiency or known chromosomal abnormality; 4) normal thyroid and adrenal function; and 5) no contraindication for hormone therapy. Screening baseline evaluation was as previously described (15).
Controls
Control women were healthy, non pregnant, and regularly menstruating (cycles between 21 and 35 days). They did not smoke more than 2 cigarettes per day or use alcohol (<2 drinks a day). They were taking no chronic medications, and were not using hormonal contraception. They were compensated according to NIH guidelines.
Evaluation of sexual function
We employed a self-report questionnaire; the Derogatis Interview for Sexual Function (DISF-SR - Female Version) is a reliable and well-validated measure of sexual functioning designed for use in women (16). The DISF-SR has five domain scores: Cognition/Fantasy, Sexual Arousal, Sexual Behavior/Experiences, Orgasm, and Drive & Relationship. In addition there is the composite score, which is computed and summarizes sexual functioning across the five primary DISF-SR domains. The DISF-SR consists of 25 items measuring level of sexual activity and overall sexual functioning during the past 30 days. A combination of 9-point frequency scales (e.g., 0=not at all and 8=4 or more times per day) and 4-point frequency scales (e.g., 0=not at all and 4=extremely) are used.
Assessment of each domain is computed as a scale score in terms of area T-score (generated through a normalizing transformation); a T-score of 50 places the respondent in the 50th centile of the norm, a T-score of 30 places the respondent in the 2nd centile, and a T-score of 70 places her in the 98th centile (16). Sexual function was evaluated in patients during the estradiol only phase of the hormone replacement cycle (not on progestin). In control women this was evaluated during the mid-follicular phase of their menstrual cycle (days 5–12).
Hormonal assays
Serum total testosterone, free testosterone, and estradiol were measured as previously reported (17). We measured serum total testosterone by RIA after extraction chromatography (Esoterix Endocrinology, Calabassa Hills, CA)(18). We determined serum free testosterone concentrations using equilibrium dialysis (19). Estradiol was measured by competitive chemiluminescence immunoassay using the Immulite 2000 analyzer (Diagnostic Products Corporation, Los Angeles, USA).
Statistical analysis
For categorical variables with more than two levels we compared groups with the exact likelihood ratio test using StatXact 4 for Windows 2000 (CYTEL Software Corporation, Cambridge, MA. All other analyses were conducted using the Statistical Analysis System 2002 (SAS Institute Inc., Cary, NC). Continuous variables were compared using Wilcoxon’s rank sum test. Proportions were compared using Fisher’s exact test. Correlation was assessed using Spearman’s rank correlation. All p-values are two-tailed and P<.05 was considered significant.
Results
One hundred thirty-eight women with spontaneous 46,XX primary ovarian insufficiency and 62 controls completed the self-report questionnaire DISF-SR -Female Version. Five patients and 8 controls declined to complete the instrument. The median (SD) age at diagnosis of women with spontaneous 46,XX primary ovarian insufficiency was 29±6.9 years.
Table 1 summarizes the demographic and clinical characteristics of the women. The two groups did not differ significantly with regard to age, body mass index, race, and education. However, significantly more patients than controls were married. This difference may be due to the fact that women with primary ovarian insufficiency represent a selected population interested in fertility. Because marital status is a significant parameter of sexual function, comparisons were made among the subsets of married and single patients and controls as well as all patients and all controls.
Table 1.
Comparison of demographic and clinical characteristics between women with primary ovarian insufficiency (n=143) and controls (n=70)
| Characteristic | Women with Primary Ovarian Insufficiency | Control Women | P |
|---|---|---|---|
| Age (median, SD) | 32.0 (5.5) years | 28.5 (7.3) years | NS |
|
| |||
| BMI (kg/m2) (median, SD) | 22.9 (3.1) | 23.0 (2.7) | NS |
|
| |||
| Race (%) | NS | ||
| White | 121 (84.6%) | 50 (71.4%) | |
| Black | 13 (9.1%) | 11 (15.7%) | |
| Hispanic | 4 (2.8%) | 8 (11.4%) | |
| Asian | 5 (3.5%) | 1 (1.4%) | |
|
| |||
| Age at menarche (median, SD) | 13.0 (1.5) | 13.0 (1.3) | NS |
|
| |||
| Marital status | 0.02 | ||
| Married | 87 (60.8%) | 27 (38.6%) | |
| Single/Divorced | 56 (39.2%) | 43 (61.4%) | |
|
| |||
| Highest education (n,%) | NS | ||
| High School | 5 (3.6%) | 2 (3.2%) | |
| Graduate school or college | 135 (96.4%) | 60 (96.8%) | |
BMI : body mass index
NS: non-significant
Table 2 summarizes the comparison of the domains of sexual function in patients and controls. The mean composite sexual function scores did not differ significantly by marital status in either the patients or controls (comparisons not shown). Overall, as represented by the DISF-SR composite score of sexual function, when the patients and controls were compared without regard to marital status estradiol-replete women with primary ovarian insufficiency scored significantly lower than control women (P= 0.001). Women with primary ovarian insufficiency scored significantly lower on the Sexual Cognition/Fantasy scale (P=0.002), the Orgasm scale (P=0.003), and the Drive & Relationship scale (P=0.02). No significant differences between the two groups were found in the domains of Sexual Arousal and Sexual Behavior/Experiences. In direct comparisons of patients and controls segregated by marital status, only the composite scores remained statistically significant (p=0.03 for married and p=0.01 for single).
Table 2.
Comparison of sexual function in women with primary ovarian insufficiency (POI) (n=138) and controls (n=62)
| Area T-score | Married women with POI (n=84) | Single women with POI (n=54) | All women with POI (n=138) | Married controls (n=24) | Single controls (n=38) | All controls (n=62) | P1 Between married POI and controls | P2 Between single POI and controls |
|---|---|---|---|---|---|---|---|---|
| Composite score | 41.0 (12.5) | 45.0 (11.2) | 43.0 (12.0) | 53.0 (12.3) | 55.0 (11.5) | 53.0 (11.7) | 0.03 | 0.01 |
| Sexual cognition/fantasy | 51.0 (8.9) | 54.0 (10.2) | 52.0 (8.9) | 51.0 (9.5) | 60.0 (10.2) | 56.0 (9.9) | NS | 0.03 |
| Sexual arousal | 46.0 (11.2) | 49.5 (12.1) | 46.6 (11.5) | 55.0 (11.7) | 50.0 (13.6) | 54 (10.9) | NS | NS |
| Sexual behavior/experiences | 49.0 (9.6) | 45.0 (11.2) | 49.0 (10.2) | 50.0 (12.1) | 47.0 (13.2) | 49.0 (11.1) | NS | NS |
| Orgasm | 43.0 (10.2) | 40.0 (9.7) | 40.0 (9.9) | 50.0 (7.7) | 44.0 (11.5) | 47.0 (9.9) | 0.002 | NS |
| Drive & Relationship | 43.0 (10.3) | 43.0 (10.3) | 43.0 (10.1) | 48.0 (8.9) | 48.0 (12.2) | 48.0 (11.1) | NS | NS |
Values are presented as median (SD)
NS: non-significant
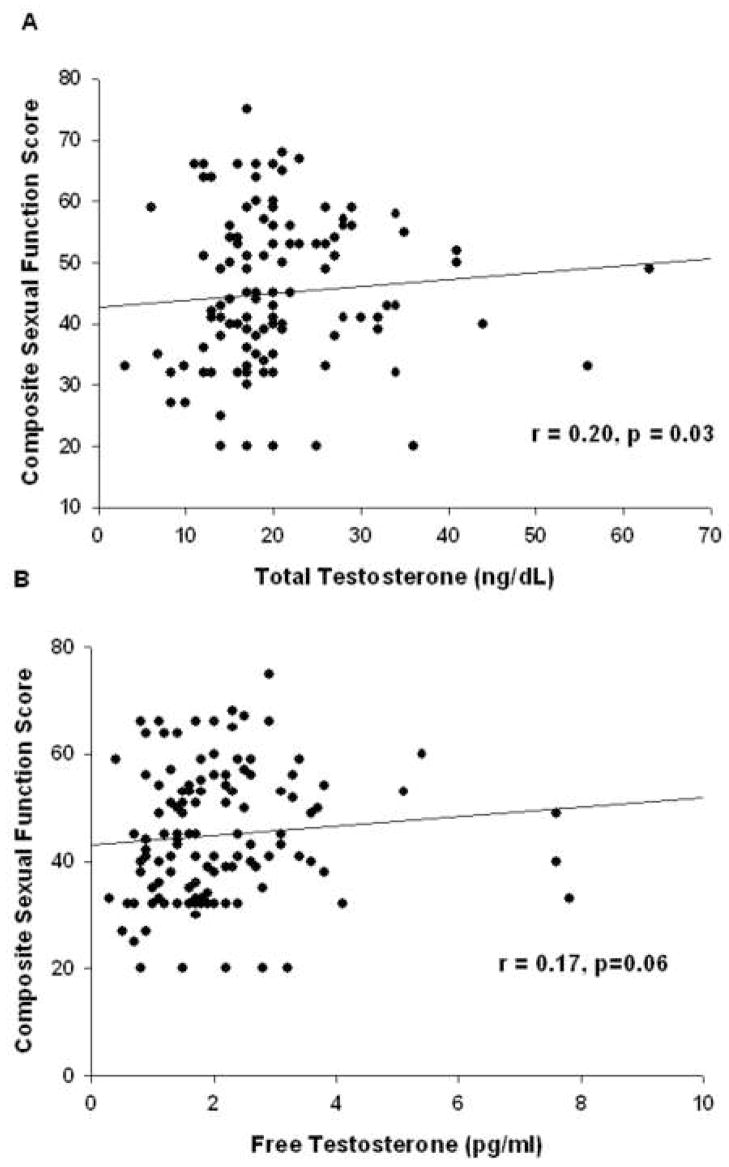
A significant correlation was found between composite sexual function score and serum total testosterone in women with primary ovarian insufficiency (r=0.20, p=0.03) and a trend toward a correlation with serum free testosterone (r=0.17, p=0.06) (Figure 1). No such correlation was found in normal control women. In women with primary ovarian insufficiency composite sexual function score did not significantly correlate with age, age of onset of menstrual irregularity, time since diagnosis, level of education, or serum estradiol level.
Figure 1.
Correlation in women with primary ovarian insufficiency between sexual function composite score as assessed by the Derogatis Interview for Sexual Function Self Report (DISF-SR) and (A) serum total testosterone (n= 121), (B) serum free testosterone (n=120).
Of patients with primary ovarian insufficiency 9/127 (7%) scored below the 2nd centile on the composite sexual function score compared to 1/49 (2%) of control women (p=0.287). Eighteen women (18/120, 15%) with primary ovarian insufficiency had a serum free testosterone level below the normal lower limit for the assay utilized (1.1pg/ml). Six women (6/121, 5%) had serum total testosterone below the normal limit of 10 ng/dL. Women with an abnormally low serum free testosterone level had a trend toward lower mean composite sexual function scores compared to women who had these values in the normal range (50.4 vs 67.3 respectively, p=0.052), as did women with a low serum total testosterone (35.9 vs 62.3 respectively, p=0.075).
Discussion
We have demonstrated that despite physiologic estradiol replacement therapy young women with 46,XX spontaneous primary ovarian insufficiency as a group score significantly lower on the DISF-SR sexual function composite score as compared to control women. To our knowledge this is the first report to investigate sexual function in women with this condition in a controlled manner. Although we did not employ separate instruments to evaluate psychosocial factors that may affect sexual function, such as mood, self-image and feelings for the partner, we find it reassuring that only a minority of women (7%) with primary ovarian insufficiency had scores that placed them clearly in the abnormal range of sexual function (at or below the 2nd percentile on the composite score). Most women with 46,XX spontaneous primary ovarian insufficiency (93%) have sexual function scores in the normal range. Additional research is needed to assess the relative contributions of psychosocial factors and testosterone deficiency on the sexual function of these women.
Many sexual function studies include only patients in stable relationships or those who are sexually active, and thus exclude those for whom sexual dysfunction has precluded sexual activities or relationships (13). Excluding these women would cause an underestimation of the degree of sexual dysfunction. We did not exclude women on these criteria and our findings are thus more generally applicable to women with 46,XX spontaneous primary ovarian insufficiency.
Our data demonstrate that women with 46,XX spontaneous primary ovarian insufficiency do not differ from control women in the domains of Sexual Arousal and Sexual Behavior/Experiences. Thus, patients did not differ from controls with regard to the frequency of sexual arousal, the adequacy of vaginal lubrication, or the frequency of sexual activities. Patients did differ from controls in the domains of Sexual Cognition/Fantasy, Orgasm, and Drive & Relationship. Thus, patients as a group were more likely to report less frequent sexual thoughts, dreams or fantasies, less satisfaction with orgasm, and in general a less satisfying sexual relationship as compared to controls, although when controlled for marital status the differences were modest.
In the present study, women with primary ovarian insufficiency received cyclic hormone therapy, using transdermal estradiol (100 mcg) and oral medroxyprogesterone acetate (10 mg). We have previously shown that this regimen results in physiological serum estradiol levels compared to controls (20). Evaluation of sexual function was performed during the estrogen-only phase of the cycle in order to avoid the progestin-induced dysphoria, which could affect the results (21).
There are multiple mechanisms by which sexual function might be impaired in young women with 46,XX spontaneous primary ovarian insufficiency. These women are living with a medical disorder that causes premature loss of ovarian function and fertility. The integrated psychosocial effects of a medical disorder can have a more significant effect on sexual function than do the biomedical factors (13,22). A controlled study and a previous meta-analysis have both demonstrated that there are strong links between depression and impaired sexual function in women with diabetes (23,24). It has been suggested that evidence-based treatment of diabetes-associated sexual dysfunction in women should include attention to depression, interpersonal issues, and the psychological aspects of living with diabetes, since these are known to correlate with sexual dysfunction (25). Possibly similar correlations with sexual function exist in women with 46,XX primary ovarian insufficiency but we did not assess this specifically in the present study. This is an area in need of further research.
We have previously demonstrated that women with primary ovarian insufficiency have significantly reduced serum free and total testosterone levels as compared to control women (17). Here we show that serum testosterone level is significantly correlated with the composite sexual function score in women with primary ovarian insufficiency and that there is a trend for women with serum testosterone levels below the normal limit to score lower on the composite sexual function score. These findings suggest that the testosterone deficiency found in some of these women may be a contributing factor to sexual dysfunction. However, the correlation between serum testosterone and composite sexual function score is weak; serum testosterone accounts for only 4% of the variance in composite sexual function score.
Large clinical studies have failed to demonstrate a correlation between testosterone levels and sexual function in women (26,27). Similarly, a study that evaluated hormonal changes after oophorectomy in conjunction with perimenopausal hysterectomy found no significant association with 1 year postoperative sexual or psychological well-being (28). Other prospective studies of oophorectomy are in agreement (29,30). The Endocrine Society has concluded that the female androgen deficiency syndrome is not presently well defined and that clinicians should not make this diagnosis in women until normative data on total and free testosterone levels have been established across the lifespan (31). The Endocrine Society also points out that serum testosterone levels may not reflect total androgen tissue exposure (31). Indeed, it is well known that androgens may exert their effects in target cells in which they are synthesized from inactive precursors without release in the circulation (32).
When assessed by functional brain MRI, surgically menopausal women who watch erotic stimuli have reduced limbic system activity compared to premenopausal control women; furthermore, as compared to estrogen treatment alone, treatment with estrogen and testosterone caused a greater increase in limbic activation (33). A systematic Cochrane review concluded that the addition of testosterone to hormone replacement in menopausal women has a beneficial effect on sexual function (34). Several trials conducted in women with sexual dysfunction after surgical or natural menopause have shown improvements in some sexual function domains (35–38). It is possible that in some specific clinical situations in which clear evidence of testosterone deficiency can be established, and in which other psychosocial aspects of female sexual function are not a prominent component of the dysfunction, replacement of testosterone might significantly improve sexual function. The challenge is to be able to identify those specific patients and gather long-term safety data on testosterone administration.
A study by Gerber et al. assessed the effect of free testosterone levels on sexual function during the natural menopause transition (39). Importantly, the study addressed other psychosocial variables, which may also contribute to change in sexual function. These factors included participant’s job satisfaction, satisfaction with financial resources, confidence in ability to manage symptoms, stressful life events, exercise, body image, and quality of personal relationships. The study showed no correlation between testosterone levels and sexual function, whereas exercise was clearly associated with sexual satisfaction. The authors make the point that a focus on the hormonal aspects of menopause has promoted a disregard of other important psychosocial factors affecting sexual function (39). It will be important to avoid making the same mistake while investigating sexual function in young women with spontaneous 46,XX primary ovarian insufficiency.
Other studies are in agreement that factors such as general well-being, relationship to partner and sexual attitudes are more important than hormone levels with regard to female sexual function. A community based study by Cawood et al. involving 141 women who were aged 40 to 60 years showed that none of the hormonal parameters significantly predicted measures of sexuality; the most important predictors were other aspects of the sexual relationship, sexual attitudes and measures of well-being (40). A similar but larger study by Dennerstein et al. came to similar conclusions (41).
In summary, as assessed by the DISF-SR, sexual function is in the normal range for most young women with 46,XX spontaneous primary ovarian insufficiency who are receiving physiologic estradiol replacement. However, as a group, these young women score significantly lower on this sexual function scale than control women.
Acknowledgments
Supported by the Intramural Research Program, National Institute of Child Health and Human Development, National Institutes of Health. VHV and LMN are Commissioned Officers in the United States Public Health Service.
Footnotes
Where the work was done: National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MacMahon B, Worcester J. Age at menopause. United States--1960–1962. Vital Health Stat. 1966;11:1–20. [PubMed] [Google Scholar]
- 2.Luoto R, Kaprio J, Uutela A. Age at natural menopause and sociodemographic status in Finland. Am J Epidemiol. 1994;139:64–76. doi: 10.1093/oxfordjournals.aje.a116936. [DOI] [PubMed] [Google Scholar]
- 3.van Noord PA, Dubas JS, Dorland M, Boersma H, te Velde E. Age at natural menopause in a population-based screening cohort: the role of menarche, fecundity, and lifestyle factors. Fertil Steril. 1997;68:95–102. doi: 10.1016/s0015-0282(97)81482-3. [DOI] [PubMed] [Google Scholar]
- 4.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–606. [PubMed] [Google Scholar]
- 5.Nelson LM, Covington SN, Rebar RW. An update: spontaneous premature ovarian failure is not an early menopause. Fertil Steril. 2005;83:1327–1332. doi: 10.1016/j.fertnstert.2004.11.059. [DOI] [PubMed] [Google Scholar]
- 6.Albright F, Smith PH, Fraser R. A syndrome characterized by primary ovarian insufficiency and decreased stature. American Journal of the Medical Sciences. 1942;204:625–648. [Google Scholar]
- 7.Bancroft J, Loftus J, Long JS. Distress about sex: a national survey of women in heterosexual relationships. Arch Sex Behav. 2003;32:193–208. doi: 10.1023/a:1023420431760. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann U, Philippsohn S, Heiser K, Ruffer-Hesse C. Low sexual desire in midlife and older women: personality factors, psychosocial development, present sexuality. Menopause. 2004;11:726–740. doi: 10.1097/01.gme.0000143705.42486.33. [DOI] [PubMed] [Google Scholar]
- 9.Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS) Menopause. 2006;13:46–56. doi: 10.1097/01.gme.0000172596.76272.06. [DOI] [PubMed] [Google Scholar]
- 10.Basson R. Clinical practice. Sexual desire and arousal disorders in women. N Engl J Med. 2006;354:1497–1506. doi: 10.1056/NEJMcp050154. [DOI] [PubMed] [Google Scholar]
- 11.Groff AA, Covington SN, Halverson LR, Fitzgerald OR, Vanderhoof V, Calis K, et al. Assessing the emotional needs of women with spontaneous premature ovarian failure. Fertil Steril. 2005;83:1734–1741. doi: 10.1016/j.fertnstert.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 12.van Lankveld JJ, Grotjohann Y. Psychiatric comorbidity in heterosexual couples with sexual dysfunction assessed with the composite international diagnostic interview. Arch Sex Behav. 2000;29:479–498. doi: 10.1023/a:1001995704034. [DOI] [PubMed] [Google Scholar]
- 13.Basson R, Schultz WW. Sexual sequelae of general medical disorders. Lancet. 2007;369:409–424. doi: 10.1016/S0140-6736(07)60197-4. [DOI] [PubMed] [Google Scholar]
- 14.Graziottin A, Basson R. Sexual dysfunction in women with premature menopause. Menopause. 2004;11:766–777. doi: 10.1097/01.gme.0000139926.02689.a1. [DOI] [PubMed] [Google Scholar]
- 15.Kim TJ, Anasti JN, Flack MR, Kimzey LM, Defensor RA, Nelson LM. Routine endocrine screening for patients with karyotypically normal spontaneous premature ovarian failure. Obstet Gynecol. 1997;89:777–779. doi: 10.1016/s0029-7844(97)00077-x. [DOI] [PubMed] [Google Scholar]
- 16.Derogatis LR. The Derogatis Interview for Sexual Functioning (DISF/DISF-SR): an introductory report. J Sex Marital Ther. 1997;23:291–304. doi: 10.1080/00926239708403933. [DOI] [PubMed] [Google Scholar]
- 17.Kalantaridou SN, Calis KA, Vanderhoof VH, Bakalov VK, Corrigan EC, Troendle JF, et al. Testosterone deficiency in young women with 46,XX spontaneous premature ovarian failure. Fertil Steril. 2006;86:1475–1482. doi: 10.1016/j.fertnstert.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 18.Furuyama S, Mayes DM, Nugent CA. A radioimmunoassay for plasma testosterone. Steroids. 1970;16:415–428. doi: 10.1016/s0039-128x(70)80124-6. [DOI] [PubMed] [Google Scholar]
- 19.Nelson JC, Tomei RT. Direct determination of free thyroxin in undiluted serum by equilibrium dialysis/radioimmunoassay. Clin Chem. 1988;34:1737–1744. [PubMed] [Google Scholar]
- 20.Popat VB, Vanderhoof VH, Calis KA, Troendle JF, Nelson LM. Normalization of serum luteinizing hormone levels in women with 46,XX spontaneous primary ovarian insufficiency. Fertil Steril. 2007 doi: 10.1016/j.fertnstert.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherwin BB. The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal women. J Clin Endocrinol Metab. 1991;72:336–343. doi: 10.1210/jcem-72-2-336. [DOI] [PubMed] [Google Scholar]
- 22.DeLamater JD, Sill M. Sexual desire in later life. J Sex Res. 2005;42:138–149. doi: 10.1080/00224490509552267. [DOI] [PubMed] [Google Scholar]
- 23.Enzlin P, Mathieu C, Van den BA, Bosteels J, Vanderschueren D, Demyttenaere K. Sexual dysfunction in women with type 1 diabetes: a controlled study. Diabetes Care. 2002;25:672–677. doi: 10.2337/diacare.25.4.672. [DOI] [PubMed] [Google Scholar]
- 24.de GM, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369:597–611. doi: 10.1016/S0140-6736(07)60280-3. [DOI] [PubMed] [Google Scholar]
- 26.Davis SR, Davison SL, Donath S, Bell RJ. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294:91–96. doi: 10.1001/jama.294.1.91. [DOI] [PubMed] [Google Scholar]
- 27.Santoro N, Torrens J, Crawford S, Allsworth JE, Finkelstein JS, Gold EB, et al. Correlates of circulating androgens in mid-life women: the study of women’s health across the nation. J Clin Endocrinol Metab. 2005;90:4836–4845. doi: 10.1210/jc.2004-2063. [DOI] [PubMed] [Google Scholar]
- 28.Aziz A, Brannstrom M, Bergquist C, Silfverstolpe G. Perimenopausal androgen decline after oophorectomy does not influence sexuality or psychological well-being. Fertil Steril. 2005;83:1021–1028. doi: 10.1016/j.fertnstert.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Farquhar CM, Harvey SA, Yu Y, Sadler L, Stewart AW. A prospective study of 3 years of outcomes after hysterectomy with and without oophorectomy. Am J Obstet Gynecol. 2006;194:711–717. doi: 10.1016/j.ajog.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 30.Teplin V, Vittinghoff E, Lin F, Learman LA, Richter HE, Kuppermann M. Oophorectomy in premenopausal women: health-related quality of life and sexual functioning. Obstet Gynecol. 2007;109:347–354. doi: 10.1097/01.AOG.0000252700.03133.8b. [DOI] [PubMed] [Google Scholar]
- 31.Wierman ME, Basson R, Davis SR, Khosla S, Miller KK, Rosner W, et al. Androgen therapy in women: an Endocrine Society Clinical Practice guideline. J Clin Endocrinol Metab. 2006;91:3697–3710. doi: 10.1210/jc.2006-1121. [DOI] [PubMed] [Google Scholar]
- 32.LaBrie F, Luu-The V, Labrie C, Belanger A, Simard J, Lin SX, et al. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003;24:152–182. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- 33.Archer JS, Love-Geffen TE, Herbst-Damm KL, Swinney DA, Chang JR. Effect of estradiol versus estradiol and testosterone on brain-activation patterns in postmenopausal women. Menopause. 2006;13:528–537. doi: 10.1097/01.gme.0000188737.46746.cd. [DOI] [PubMed] [Google Scholar]
- 34.Somboonporn W, Davis S, Seif MW, Bell R. Testosterone for peri- and postmenopausal women. Cochrane Database Syst Rev. 2005:CD004509. doi: 10.1002/14651858.CD004509.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Braunstein GD, Sundwall DA, Katz M, Shifren JL, Buster JE, Simon JA, et al. Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Arch Intern Med. 2005;165:1582–1589. doi: 10.1001/archinte.165.14.1582. [DOI] [PubMed] [Google Scholar]
- 36.Buster JE, Kingsberg SA, Aguirre O, Brown C, Breaux JG, Buch A, et al. Testosterone patch for low sexual desire in surgically menopausal women: a randomized trial. Obstet Gynecol. 2005;105:944–952. doi: 10.1097/01.AOG.0000158103.27672.0d. [DOI] [PubMed] [Google Scholar]
- 37.Simon J, Braunstein G, Nachtigall L, Utian W, Katz M, Miller S, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005;90:5226–5233. doi: 10.1210/jc.2004-1747. [DOI] [PubMed] [Google Scholar]
- 38.Shifren JL, Davis SR, Moreau M, Waldbaum A, Bouchard C, Derogatis L, et al. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 Study. Menopause. 2006;13:770–779. doi: 10.1097/01.gme.0000243567.32828.99. [DOI] [PubMed] [Google Scholar]
- 39.Gerber JR, Johnson JV, Bunn JY, O’Brien SL. A longitudinal study of the effects of free testosterone and other psychosocial variables on sexual function during the natural traverse of menopause. Fertil Steril. 2005;83:643–648. doi: 10.1016/j.fertnstert.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 40.Cawood EH, Bancroft J. Steroid hormones, the menopause, sexuality and well-being of women. Psychol Med. 1996;26:925–936. doi: 10.1017/s0033291700035261. [DOI] [PubMed] [Google Scholar]
- 41.Dennerstein L, Lehert P, Burger H, Dudley E. Factors affecting sexual functioning of women in the mid-life years. Climacteric. 1999;2:254–262. doi: 10.3109/13697139909038085. [DOI] [PubMed] [Google Scholar]