Abstract
Ecological validity of neuropsychological assessment includes the ability of tests to predict real-world functioning and/or covary with brain structures. Studies have examined the relationship between adaptive skills and test performance, with less focus on the association between regional brain volumes and neurobehavioral function in healthy children. The present study examined the relationship between temporal lobe gray matter volumes and performance on two neuropsychological tests hypothesized to measure temporal lobe functioning (Visual Perception-VP; Peabody Picture Vocabulary Test, Third Edition-PPVT-III) in 48 healthy children ages 5-18 years. After controlling for age and gender, left and right temporal and left occipital volumes were significant predictors of VP. Left and right frontal and temporal volumes were significant predictors of PPVT-III. Temporal volume emerged as the strongest lobar correlate with both tests. These results provide convergent and discriminant validity supporting VP as a measure of the “what” system; but suggest the PPVT-III as a complex measure of receptive vocabulary, potentially involving executive function demands.
Keywords: Neuropsychological Tests, Visual Perception, Receptive Language, MRI, Brain Volumes, Temporal Lobe, Normal Development, PPVT
INTRODUCTION
Ecological validity of pediatric neuropsychological assessment is considered the ability of laboratory tests to predict real world functioning and/or to correlate with underlying brain structures. Although a few studies have examined the relationship between “real life” skills and intelligence (Kahn, 1992), attention (Price, Joschko, & Kerns, 2003), and executive functions (Isquith, Gioia, & Espy, 2004), there has been less focus on the relationship between these daily life skills and neuropsychological measurement of skills considered to be dependent on temporal lobe integrity (e.g., visual perception or receptive language skills).
Functional neuroimaging studies and research involving patients with known lesions or specific disorders have contributed to our understanding of temporal lobe functioning. Studies involving patients with lesions of the left temporal lobe (e.g., middle temporal gyrus, superior temporal gyrus, superior temporal sulcus, angular gyrus) have documented language comprehension deficits (Boatman, Lesser, & Gordon, 1995; Dronkers, Wilkins, Van Valin, Redfern, & Jaeger, 2004). Research with specific clinical populations (e.g., autism and dyslexia) has also documented temporal lobe abnormalities. Specifically in autism, a disorder characterized by primary deficits in language and social interaction, abnormalities in the function of the superior aspect of the temporal lobes have been identified (Abell et al., 1999; Boddaert et al., 2004; Meresse et al., 2005; Ohnishi et al., 2000; Zilbovicius et al., 2000). In healthy individuals a leftward asymmetry of the temporal lobe has been documented (Geschwind & Levitsky, 1968); whereas, in developmental dyslexia the volume of the temporal lobes has been shown to be symmetrical (Rumsey et al., 1986). Recent studies have not consistently found symmetrical temporal lobe volumes in dyslexic individuals, but have documented exaggerated asymmetries for those with dyslexia. Specifically, smaller left temporal lobe volumes, particularly in the planum temporale, and decreased overall temporal lobe volumes have been found when compared to control participants (e.g., Eliez et al., 2000; Hugdahl et al., 2003; Leonard et al., 1993; Vinckenbosch, Robichon, & Eliez, 2005). Similarly, neuroanatomical findings from Livingstone and colleagues indicate that poor readers have smaller lateral (LGN) and medial (MGN) geniculate nuclei (Galaburda & Livingstone, 1993; Livingstone, Rosen, Drislane, & Galaburda, 1991). In additional studies of language functioning in dyslexia, Tallal and colleagues have found difficulties in auditory and somatosensory processing of linguistic and nonlinguistic speech sounds (Tallal & Piercy, 1973; Tallal, 1980; Tallal & Stark, 1982; Tallal, Stark, & Mellits, 1985; Tallal & Katz, 1989). These findings were later supported by Binder and colleagues (1994), who utilized functional magnetic resonance imaging (fMRI) of language functioning in healthy adults, and found that the superior temporal lobes are involved in decoding the acoustic signals of speech. Thus the left temporal lobe appears to be specialized for language processing, and is therefore crucial in a variety of school-related abilities including development of reading skills (Molfese & Molfese, 1986).
Functioning of the right temporal lobe is less well researched and its function is also not as clearly defined. Early studies have documented deficits in visual form discrimination following right temporal lobectomy (Meier & French, 1965) and right hemisphere missile wounds (Newcombe, 1969). The right superior temporal cortex is thought to process the ‘what’ in visual perception tasks (Ungerleider & Haxby, 1994). According to Goodale and Milner (1992, 2008), there are two streams of visual processing: the dorsal “action” stream, projecting from early visual areas and the superior colliculus (via the pulvinar) to the posterior parietal cortex; and, the ventral “perception” stream, projecting from the retina to the lateral geniculate nucleus (pars dorsalis) in the thalamus, to primary visual cortex, and then to regions in the occipito-temporal cortex. The ventral stream is described to provide the detailed representations of the world that are required for cognitive processes including recognition, identification, and planning. There is increasing yet inconsistent evidence supporting a relationship between visual perception and other brain regions, particularly in the parietal lobe (Himmelbach & Karnath, 2005). Much of this evidence comes from early reports of patients with spatial neglect, which is a lack of awareness of space and object parts in the hemi-space contralateral to the brain lesion. From these early studies it has been believed that spatial neglect is associated with lesions of the right inferior parietal lobe and the temporal, parietal, and occipital lobe juncture (Heilman & Valenstein, 1972). In a study by Karnath, Ferber, and Himmelbach (2001), only patients with spatial neglect without visual-field defects were included, as it was felt that previous research may have been confounded by inclusion of those with visual-field impairments. Computed tomography (CT) or MRI scans revealed the lesions of these patients were predominantly in the superior temporal gyrus. As Karnath (2001) elucidated, the rostral portion of the superior temporal sulcus and superior temporal gyrus are located at the transition between the ventral “what/perception” and dorsal “where/action” streams of visual processing, which may account for mixed findings of temporal and parietal lobe involvement in object perception. Goodale and Milner (1992, 2008) also note that the dorsal and ventral streams both process information about the structure and spatial location of objects. They further state that although this information is mediated by different pathways, the two systems are intimately connected with the response of one stream often contingent upon the complex mechanisms of the other stream.
Although previous pediatric neuroimaging research examining brain development in healthy children has been cross-sectional (Caviness, Kenney, Richelme, Rademacher, & Filipek, 1996; Courchesne et al., 2000; Giedd et al., 1996; Jernigan, Trauner, Hesselink, & Tallal, 1991; Pfefferbaum et al., 1994; Reiss, Abrams, Singer, Ross, & Denckla, 1996), Giedd and colleagues (1999) published longitudinal data in which linear increases in white matter volume with age were observed, whereas age-related changes in gray matter were nonlinear and regionally specific. Particularly, frontal lobe gray matter volumes increased during pre-adolescence, peaked around 12 years of age for boys (11 years of age for girls), and declined during post-adolescence resulting in an overall net decrease across the age span (i.e., 4 to 20 years). Results were similar for parietal lobe volumes and differed only in that the slope of the curve was steeper and volumes peaked one year earlier for each gender. In contrast, temporal lobe volumes peaked around 16 years of age for both boys and girls with a mild decline thereafter. Occipital lobe gray matter followed a linear increase over the age range with no evidence of decline or plateau. Total cerebral gray matter volume was approximately 10% larger in boys and peaked slightly earlier in girls, but the shapes of the curves were similar for both genders.
While studying patients with known brain lesions has led to advances in the study of brain-behavior relationships, fewer studies have documented an association between regional brain volumes and neurobehavioral function in healthy children. Recently, Shaw and colleagues (2006) investigated the relationship between intellectual functioning and cortical development. They found a negative correlation between IQ and cortical thickness in young children, suggesting that timing and trajectory of changes in gray matter volume are associated with efficiency of cognitive functioning. That is, in young children, higher IQ was associated with thinner cortex, particularly in the frontal and temporal lobe regions; however, this relationship reversed in late childhood, with positive correlations observed between cortical thickness and IQ. Interestingly, children with superior IQ had thinner superior prefrontal cortex at an early age, with a rapid increase in cortical thickness peaking at age 13, and attenuating into late adolescence.
In healthy individuals, studies have documented a modest relationship between total brain volume and intellectual functioning (Reiss et al., 1996; Schoenemann, Budinger, Sarich, & Wang 2000; Willerman, Schultz, Rutledge, & Bigler, 1991). In contrast, research examining correlations between specific neuropsychological tests and regional brain volumes in healthy individuals are limited (Egan et al., 1994). Studies involving individuals with temporal lobe damage have implicated the temporal lobes in auditory sensation and perception; selective attention for auditory and visual stimuli; visual perception; organization and categorization of verbal material; language comprehension; long-term memory; personality and affective behavior; and, sexual behavior (Kolb & Wishaw, 1990). Volumetric MRI studies with temporal lobe epilepsy patients have been successful in documenting a correlation between hippocampal volume and verbal memory performance (e.g., Pegna et al., 2002; Reminger et al., 2004) and amygdalar volumes have been correlated with visuospatial memory (e.g., Pegna et al., 2002).
The purpose of the present study was to determine whether regional temporal lobe volumes would predict performance on two neuropsychological tests hypothesized to measure temporal lobe functioning in a sample of healthy children. Specifically, we hypothesized that, after consideration of age, gender, and total cranial volume, right temporal lobe gray matter volume would be the best predictor of performance on a test of visual object perception and left temporal lobe gray matter volume would be the best predictor of performance on a test of receptive vocabulary.
MATERIALS AND METHODS
Participants
Forty-eight typically developing children ages 5-18 years (24 boys) were recruited from the Baltimore, MD area by advertisement. Each participant and parent signed a consent form that met the institutional review board standards of the Johns Hopkins Medical Institutions. Participants were initially screened over the telephone and excluded if there was a history of neurological disorder, mental retardation, or learning disability. Parents of those children meeting this eligibility criterion participated in a structured diagnostic interview using the Diagnostic Interview for Children and Adolescents - Fourth Edition (DICA-IV; Reich, Welner, & Herjanic, 1997), which is based on the Diagnostic and Statistical Manual of Mental Disorders - Fourth Edition (DSM-IV; American Psychiatric Association, 1994). Children meeting DSM-IV criteria for a psychiatric disorder were subsequently excluded. Socioeconomic status of each participant was estimated by a widely used four-factor index (i.e., gender, marital status, education, and occupation; Hollingshead, 1975). All study participants received MRI scans and completed a neuropsychological assessment that included measures of attention, memory, language, visual, and motor skills. A test of receptive vocabulary and a visual perception test were analyzed for the current study. Taking into consideration the larger neuropsychological test battery, these two tests were selected as the purest and most widely-accepted measures of temporal lobe functioning (further description of the measures is provided below).
Magnetic Resonance Image Acquisition and Processing
High-resolution three-dimensional MRI images of each participant's brain were acquired with a GE-Signa 1.5 Tesla LX scanner (General Electric, Milwaukee, WI) using the standard birdcage quadrature head coil. Axial images were obtained with a 3-D volumetric radiofrequency spoiled gradient echo (SPGR) series partitioned into 124, 1.5-mm contiguous slices.
Raw, GE-Signa formatted image data was transferred from the MRI scanner at Johns Hopkins Hospital to Apple Macintosh Power PC workstations at SUNY Upstate Medical Institutions via existing networks. The image data were imported into the program BrainImage (Reiss, 2002; http://spnl.stanford.edu/tools/brainimage.htm) for visualization, processing, and quantitation (Subramaniam, Naidu, & Reiss, 1997). The importation process creates a 124-slice image stack composed of spatially registered, 8-bit images that have been processed to minimize signal artifacts related to RF field inhomogeneity. To prepare the stacks for measurement, nonbrain material (i.e., skull, scalp, and vasculature) is removed from these image stacks using a semi-automated edge detection routine that involves region growing as well as stepwise morphologic operations (Subramamiam et al., 1997). These “skull-stripped” images are resliced so that the interpolated slice thickness (z-dimension) is the same as the x and y pixel dimensions thereby converting the image stacks into cubic voxel data sets. The cubic voxel data sets are opened into the multiplanar visualization mode of BrainImage so that three orthogonal representations of the data can be viewed simultaneously.
Image Measurement
Isolated brain tissue was subdivided into cerebral lobes, subcortical regions, brainstem, and cerebellar regions using the revised Talairach (Talairach & Tournouz, 1988) stereotaxic grid atlas specific for measurement in pediatric study groups (Andreasen et al., 1993; Kaplan et al., 1997; Kates, Abrams, Kaufmann, Breither, & Reiss, 1997). With this approach, high levels of sensitivity and specificity are achieved for all revised Talairach-based calculations of lobar brain regions (Kates et al., 1999). Each region was then segmented to delineate and measure lobar volumes of gray, white, and ventricular compartments using a constrained fuzzy algorithm that assigns voxels to one or more tissue categories based on intensity values and tissue boundaries (Reiss et al., 1998). The segmentation method used was determined reliable for all gray matter, white matter, and CSF volumes (Reiss et al., 1998).
Neuropsychological Measures
Beery-Buktenica Developmental Test of Visual-Motor Integration (Fifth Edition), Visual Perception Test (Beery & Beery, 2004)
On the Visual Perception test (VP), the first three items are designed for very young children and require identification of their own body parts, picture outlines, and parts of a picture. For the remaining 27 test items, the child is shown one geometric form and is asked to choose the geometric form that is exactly the same from a group of forms within a 3-minute time limit. For example, the child is shown a target stimulus of a Necker cube and is asked to select (by pointing or circling) the exact match of the cube among five choices, four of which may be smaller, missing parts, rotated, etc. Thus, the test is designed as a measure of motor-free visual-perceptual ability and has been shown to differentiate children with deficits in perception from those having problems with motor coordination and integration of perceptual and motor skills (Kulp & Sortor, 2003). Raw score total was analyzed for the present study.
Peabody Picture Vocabulary Test, Third Edition (PPVT-III; Dunn & Dunn, 1997)
The PPVT-III is a screening test of verbal ability and a measure of receptive (i.e., listening) single-word vocabulary attainment for standard English. The child is shown a page with four pictures and the examiner provides the child with a vocabulary word. The child is asked to identify which picture best describes that word either by pointing or verbalizing the number of the picture. Thus, the test requires little to no motor or expressive language output. The child continues the test until 8 of 12 items are missed in an item set. Raw score totals were used for analysis in the present study.
Data Analysis
Intercorrelations for left and right hemisphere volumes of each lobe were examined. Pearson product-moment correlation coefficients for total cranial volume and individual lobar gray matter volume (frontal, parietal, temporal, and occipital lobes) were examined for each hemisphere and to determine the relationship between lobar gray matter volumes and the two neuropsychological test scores.
General linear model was applied to examine the relationship between the neuropsychological test scores and lobar gray matter volumes. Based on findings by Lenroot et al. (2007) of nonlinear, regional, and gender-specific changes in gray matter development, gender was entered as a fixed factor in all regression analyses. Absolute lobar gray matter volumes (i.e., the left or right gray matter volume for each lobe) and relative lobar gray matter volumes (i.e., expressed as ratios of left or right frontal, parietal, temporal, or occipital gray matter volumes to total cranial volume) were used in the calculations. Since lobar gray matter volumes were not age-corrected, raw scores for the two neuropsychological tests (i.e., VP and PPVT-III) were also used. To account for the effect of age on the neuropsychological test scores and lobar gray matter volumes (both absolute and relative), residuals for all variables (after regressing on age) were used in the statistical analyses. Homoscedasticity of the data was confirmed by employing the method suggested by Glejser (as described in Zar, 1998). Fisher's r-to-z transformation (Cohen & Cohen, 1983; Preacher, 2002) was used to compare the magnitude of the correlations between left and right temporal lobe gray matter volumes and neuropsychological test scores with the other lobar hemispheric volumes and both test scores.
RESULTS
Relevant demographic characteristics of all 48 participants are summarized in Table 1. The mean age of the study sample was 12.18 years (standard deviation = 3.56). Fifty percent of the sample was male and 81% right-handed, with no mixed-handed participants. The race composition was 54% African American, 37% Caucasian, and 8% biracial.
Table 1.
Study Sample Characteristics (n = 48)
| Variable | Range | Mean | SD |
|---|---|---|---|
| Notes: Values are unadjusted mean (SD). | |||
| Demographics | |||
| Age (years) | 5 - 18 | 12.18 | 3.56 |
| SES (Hollingshead Index)* | 16 - 66 | 44.31 | 11.99 |
| Neuropsychological Test | |||
| PPVT-III Raw Score | 89 - 187 | 144.15 | 27.88 |
| PPVT-III Standard Score | 72 - 150 | 103.02 | 16.24 |
| Visual Perception Raw Score | 17 - 30 | 25.23 | 3.26 |
| Visual Perception Standard Score | 59 - 139 | 97.06 | 17.17 |
| Volume (ml) | |||
| Total Cranial | 860.42 - 1442.17 | 1105.99 | 131.35 |
| Frontal Gray Matter | 147.52 - 272.54 | 210.03 | 31.51 |
| Left Frontal GM | 74.30 - 132.84 | 105.47 | 15.46 |
| Right Frontal GM | 73.22 - 140.99 | 104.57 | 16.49 |
| Parietal Gray Matter | 100.90 - 201.59 | 141.87 | 21.81 |
| Left Parietal GM | 50.83 - 100.66 | 71.06 | 10.93 |
| Right Parietal GM | 50.07 - 100.93 | 70.82 | 11.21 |
| Temporal Gray Matter | 92.16 - 178.21 | 127.94 | 18.74 |
| Left Temporal GM | 47.10 - 88.88 | 64.86 | 9.39 |
| Right Temporal GM | 42.13 - 89.34 | 63.08 | 9.83 |
| Occipital Gray Matter | 42.82 - 103.92 | 71.38 | 12.01 |
| Left Occipital GM | 21.33 - 53.56 | 35.75 | 6.42 |
| Right Occipital GM | 21.49 - 50.37 | 35.63 | 5.84 |
n = 47
Lobar Correlations and Intercorrelations
Analysis of the correlation among gray matter lobar volumes within the right hemisphere (e.g., right frontal lobe volume correlations with right parietal, temporal, and occipital lobes; See Table 2 values above the diagonal) were statistically significant (all p < 0.001). Correlations ranged from r = 0.65 to r = 0.97. Similarly, correlations among gray matter lobar volumes within the left hemisphere (See Table 2 values below the diagonal) were also statistically significant (all p < 0.001) and ranged from r = 0.68 to r = 0.94. When examining correlations between hemispheres within each of the four lobes of the brain, right and left hemisphere volumes were very highly intercorrelated—all p < 0.001 and greater than or equal to r = 0.90 (see Table 2).
Table 2.
Correlation (Pearson r) of Right and Left Lobar Gray Matter Volumes
| Total Cranial | Frontal | Parietal | Temporal | Occipital | |
|---|---|---|---|---|---|
| Note: Values above the diagonal represent correlations (r) within right hemisphere, whereas values below are correlations within left hemisphere; correlations on the diagonal represent left vs. right hemisphere correlations of the same lobe; p < .001 for all values. | |||||
| Total Cranial | .964 | .965 | .936 | .890 | .756 |
| Frontal | .940 | .946 | .892 | .852 | .650 |
| Parietal | .915 | .852 | .943 | .764 | .733 |
| Temporal | .877 | .773 | .727 | .900 | .648 |
| Occipital | .818 | .678 | .802 | .706 | .919 |
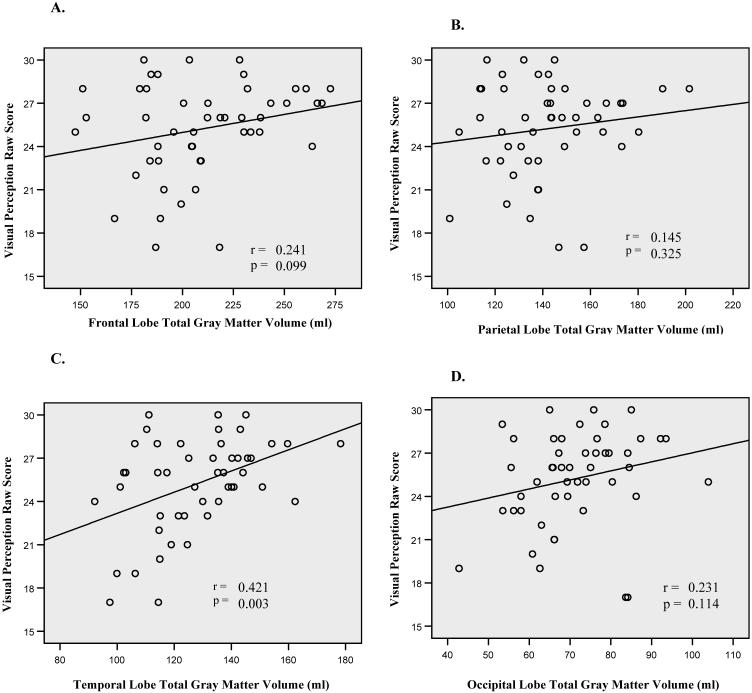
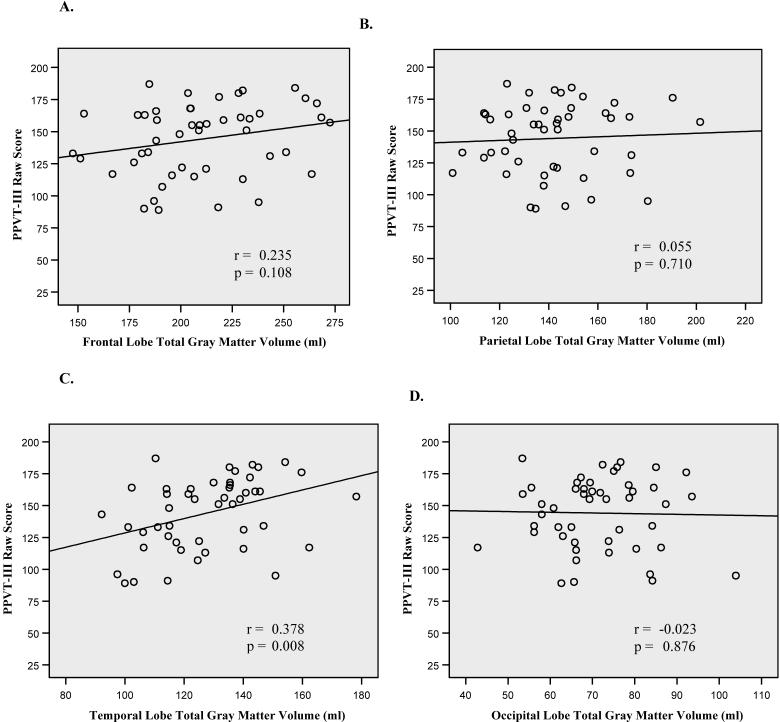
Analysis of the bivariate correlations between age, VP and PPVT-III raw scores, individual (total, left, and right) lobar gray matter volumes, and total cranial volume was performed and are presented in Table 3. Scatterplots depicting the relationship between VP scores and lobar gray matter volumes are shown in Figure 1. Among lobar volumes, temporal lobe volumes demonstrated the strongest correlation with VP scores, accounting for 18% of the total variance in the test scores. Figure 2 shows the relationship between PPVT-III and gray matter volumes for each lobe. Again, temporal lobe volumes had the strongest relationship with PPVT-III scores, accounting for 14% of the total variance in the test scores.
Table 3.
Bivariate Correlation Coefficients
| Variable (n = 48) | Age | Correlation Coefficients (r) | PPVT-III | |
|---|---|---|---|---|
| Gender | VP | |||
| Note: Values represent Pearson product-moment correlations (r). VP = Visual Perception. PPVT-III = Peabody Picture Vocabulary Test, Third Edition. | ||||
| Frontal Lobe Gray Matter Volume | -0.183 | 0.252 | 0.241 | 0.235 |
| Left Frontal GM | -0.226 | 0.256 | 0.253 | 0.207 |
| Right Frontal GM | -0.137 | 0.241 | 0.224 | 0.255 |
| Parietal Lobe Gray Matter Volume | -0.299* | 0.240 | 0.145 | 0.055 |
| Left Parietal GM | -0.318* | 0.236 | 0.146 | 0.031 |
| Right Parietal GM | -0.271 | 0.238 | 0.140 | 0.077 |
| Temporal Lobe Gray Matter Volume | 0.058 | 0.230 | 0.421** | 0.378** |
| Left Temporal GM | 0.087 | 0.258 | 0.405** | 0.377** |
| Right Temporal GM | 0.028 | 0.192 | 0.416** | 0.359* |
| Occipital Lobe Gray Matter Volume | -0.416** | 0.125 | 0.231 | -0.023 |
| Left Occipital GM | -0.386** | 0.177 | 0.240 | 0.015 |
| Right Occipital GM | -0.431** | 0.063 | 0.211 | -0.064 |
| Total Cranial Volume | -0.053 | 0.347* | 0.384** | 0.343* |
Significant at the .01 level (2-tailed)
Significant at the .05 level (2-tailed)
Figure 1. Relationship between VP Raw Scores and Lobar Gray Matter Volumes.
Scatterplots depicting the relationship between Visual Perception (VP) raw scores and (A.) frontal, (B) parietal, (C) temporal, and (D) occipital lobe gray matter volumes (ml). Temporal lobe volumes demonstrated the strongest correlation (r = 0.421), accounting for 18% of the variance.
Figure 2. Relationship between PPVT-III Raw Scores and Lobar Gray Matter Volumes.
Scatterplots depicting the relationship between PPVT-III raw scores and (A.) frontal, (B) parietal, (C) temporal, and (D) occipital lobe gray matter volumes (ml). Temporal lobe volumes demonstrated the strongest correlation (r = 0.378), accounting for 14% of the variance.
Using Fisher's r-to-z transformation, the correlation between left temporal lobe gray matter volumes and PPVT-III test scores (r = .377) was significantly greater than the correlation between left parietal (p < .05) and both left (p < .05) and right (p < .01) occipital volumes and PPVT-III scores. Similarly, the correlation between right temporal lobe gray matter volumes and PPVT-III test scores (r = .359) was significantly greater than the correlation between both left and right occipital gray matter volumes (ps < .05) and PPVT-III scores. The magnitude of the correlation between both left and right temporal lobe gray matter volumes and VP test scores was not significantly greater than the correlation between left and right frontal, parietal, and occipital volumes and VP scores. There was a trend toward a significantly larger correlation between right temporal lobe gray matter volumes and VP scores (r = .416) compared with right parietal lobe volumes and VP scores (p = .07).
Regression Analyses
A series of linear regression analyses were used to examine the relationship between absolute and relative lobar hemispheric volumes and the two neuropsychological tests of interest (i.e., VP and PPVT-III). To account for multiple comparisons, statistical significance was set to p < 0.01. In developing the regression models, all predictor variables were entered simultaneously and gender served as a covariate. Although the correlation between left and right lobar volumes was highly significant, individual hemispheric lobar volumes were used in all regression analyses to determine whether the relationship with both neuropsychological tests showed the expected higher correlation in the hypothesized hemispheric lobe (i.e., right temporal volume for VP and left temporal for PPVT-III). The results of the analyses for each neuropsychological test are summarized in Tables 4 and 5. Absolute temporal left and right and occipital left gray matter volumes, but not left or right frontal and parietal or right occipital gray matter volumes significantly predicted performance on the VP test. Absolute frontal left and right and temporal left and right gray matter volumes, but not left or right parietal or occipital gray matter volumes were significant predictors of the PPVT-III scores. None of the relative lobar volumes (corrected for total cranial volume) were significant predictors of either VP or PPVT-III test performance. In addition, none of the analyses detected a significant gender effect in predicting test performance.
Table 4.
Regression Analyses of Lobar Gray Matter Volumes and VPa Test Performance
| Predictor | R2 | F(1,47) | p |
|---|---|---|---|
| Absolute Lobar Volumes (ml) | |||
| Left Frontal Lobe Gray Matter | 0.109 | 5.425 | 0.024 |
| Right Frontal Lobe Gray Matter | 0.072 | 3.419 | 0.071 |
| Left Parietal Lobe Gray Matter | 0.062 | 2.853 | 0.098 |
| Right Parietal Lobe Gray Matter | 0.050 | 2.283 | 0.138 |
| Left Temporal Lobe Gray Matter | 0.161 | 8.498 | 0.006 |
| Right Temporal Lobe Gray Matter | 0.179 | 9.673 | 0.003 |
| Left Occipital Lobe Gray Matter | 0.150 | 8.146 | 0.006 |
| Right Occipital Lobe Gray Matter | 0.138 | 7.088 | 0.011 |
| Relative Lobar Volumes (ml)b | |||
| Left Frontal Lobe Gray Matter | 0.008 | 0.258 | 0.614 |
| Right Frontal Lobe Gray Matter | 0.024 | 1.004 | 0.322 |
| Left Parietal Lobe Gray Matter | 0.052 | 2.385 | 0.129 |
| Right Parietal Lobe Gray Matter | 0.057 | 2.605 | 0.114 |
| Left Temporal Lobe Gray Matter | 0.016 | 0.662 | 0.420 |
| Right Temporal Lobe Gray Matter | 0.040 | 1.789 | 0.188 |
| Left Occipital Lobe Gray Matter | 0.018 | 0.717 | 0.402 |
| Right Occipital Lobe Gray Matter | 0.004 | 0.108 | 0.744 |
VP = Visual Perception
Corrected for total cranial volume.
Table 5.
Regression Analyses of Lobar Gray Matter Volumes and PPVT-IIIa Test Performance
| Predictor | R2 | F(1,47) | P |
|---|---|---|---|
| Absolute Lobar Volumes (ml) | |||
| Left Frontal Lobe Gray Matter | 0.234 | 11.142 | 0.002 |
| Right Frontal Lobe Gray Matter | 0.218 | 9.995 | 0.003 |
| Left Parietal Lobe Gray Matter | 0.127 | 4.218 | 0.046 |
| Right Parietal Lobe Gray Matter | 0.136 | 4.725 | 0.035 |
| Left Temporal Lobe Gray Matter | 0.190 | 8.039 | 0.007 |
| Right Temporal Lobe Gray Matter | 0.217 | 9.911 | 0.003 |
| Left Occipital Lobe Gray Matter | 0.175 | 7.081 | 0.011 |
| Right Occipital Lobe Gray Matter | 0.140 | 5.005 | 0.030 |
| Relative Lobar Volumes (ml)b | |||
| Left Frontal Lobe Gray Matter | 0.059 | 0.702 | 0.407 |
| Right Frontal Lobe Gray Matter | 0.069 | 1.189 | 0.281 |
| Left Parietal Lobe Gray Matter | 0.060 | 0.720 | 0.401 |
| Right Parietal Lobe Gray Matter | 0.049 | 0.215 | 0.645 |
| Left Temporal Lobe Gray Matter | 0.051 | 0.270 | 0.606 |
| Right Temporal Lobe Gray Matter | 0.076 | 1.535 | 0.222 |
| Left Occipital Lobe Gray Matter | 0.058 | 0.619 | 0.435 |
| Right Occipital Lobe Gray Matter | 0.045 | 0.025 | 0.874 |
PPVT-III = Peabody Picture Vocabulary Test, Third Edition
Corrected for total cranial volume.
DISCUSSION
The present study examined the relationship between lobar gray matter volumes and two neuropsychological tests hypothesized to measure aspects of temporal lobe function (i.e., VP and PPVT-III). After controlling for age and gender, left and right temporal and left occipital lobe volumes, but not left or right frontal and parietal lobe volumes, were significant predictors of VP test scores. In contrast, left and right temporal and frontal lobe volumes were significant predictors of PPVT-III scores. For both tests, however, temporal lobe volumes emerged as the strongest correlate of raw score test performance. Thus, these results provide initial convergent and discriminant validity supporting the VP test as a measure of the temporal lobe “what” ventral perception system in healthy children and also provide evidence for the hypothesis of the PPVT-III as a complex measure of receptive language as there is evidence that it involves aspects of executive functioning.
The study findings are consistent with previous research that has emphasized temporal lobe contributions to visual-perceptual processing—especially object perception and to language functioning—especially receptive vocabulary. Research involving brain-behavior correlates of visual object perception is primarily limited to adults. The present findings support the longstanding notion from the adult literature that the visual “what” ventral perception stream is primarily processed through the temporal lobes. More specifically, our findings of bilateral temporal lobe involvement in object perception has been previously shown in several experiments. For example, Kourtzi and Kanwisher (2000) performed a series of experiments, one of which involved fMRI investigation of areas activated by line drawings. Results from this investigation revealed bilateral activation in temporal and occipital regions for identifying object structure. In another experiment, Marsolek (1995) demonstrated that abstract visual form information primarily operates in the left hemisphere, where this information is stored and then later recalled for use in distinguishing different form types. Marsolek and colleagues (1996) also provided evidence for right hemisphere involvement in holistic processing (rather than parts-based) of abstract forms for distinguishing specific details of a form within the same category. Pins, Meyer, Foucher, Humphreys, and Boucart (2004) also noted bilateral temporal lobe activation in an fMRI paradigm involving object matching. The VP task used in the present study is structured such that the participant must distinguish the exact match of a target object among a group of objects that differ only in small detail (e.g., smaller, rotated, missing line, etc.). Thus, performing the VP task involves holistic and parts-based analysis of abstract form type.
Although occipital lobe volume was not hypothesized to be a significant predictor of performance on the VP test, this result was not surprising given the visual presentation of the task. Previous research has supported the finding of primary visual cortex involvement in object perception. For example, in an fMRI study of healthy young adults, Calhoun and colleagues modified the Motor-Free Visual Perception Test - Revised (MVPT-R; a measure of visual perceptual processing involving spatial relationships, visual discrimination, figure-ground, and visual closure) to an event-related fMRI paradigm for localizing changes in blood flow/oxygenation during visual perceptual processing (Calhoun et al., 2001). They recorded activation in a large network including primary visual cortex and visual association areas. In a similar study, Moritz, Johnson, McMillan, Haughton, and Meyerand (2004) investigated the neuroanatomical correlates of the Hooper Visual Organization Test (HVOT) using fMRI. They also found a large activation network including ventral temporal-occipital cortex, posterior visual association areas, and frontal eye fields.
Numerous imaging studies have analyzed the neuroanatomical mechanisms involved in the linguistic processes underlying reading development. For example, Turkeltaub, Gareau, Flowers, Zeffro, and Eden (2003) found increased activation in the left superior temporal sulcus during an implicit word processing task using fMRI in children. Two separate studies using diffusion tensor magnetic resonance imaging (DTI) in healthy children found correlations between the left temporal lobe and word reading ability (Beaulieu et al., 2005; Nagy, Westerberg, & Klingberg, 2004). In a positron emission tomography (PET) study, Büchel, Price, and Friston (1998) investigated modes of language acquisition in blind (i.e., auditory-tactile association) and sighted adults (i.e., auditory-visual association) by presenting strings of words and non-words. They found activation of the left basal posterior temporal lobe area 37 for both groups. Recent imaging studies involving both adults and children with dyslexia have employed PET (McCrory, Mechelli, Frith, & Price, 2005), magnetic resonance spectroscopy (MRS; Rae et al., 1998), volumetric MRI (Vinckenbosch et al., 2005), and magnetoencephalography (MEG; Salmelin, Service, Kiesila, Uutela, & Salonen, 1996; Trauzettel-Klosinski, Durrwachter, Klosinski, & Braun, 2006). Across all these studies, analysis of participants' performance on behavioral measures and imaging findings revealed significant involvement of temporal lobe structures. Although the studies focus on involvement of the left temporal lobe, many also revealed involvement of the right temporal lobe in language comprehension.
Although we did not specifically predict that frontal lobe volumes would be correlated with PPVT-III performance, there is some literature to support these findings. For example, Dronkers and colleagues (2004) utilized voxel-based lesion-symptom mapping to evaluate the relationship between brain lesions and performance on a receptive language comprehension task in adult stroke patients. They found that lesions to temporal and frontal brain regions affected performance on the language task. The authors concluded that this finding likely related to the auditory rehearsal component of the language comprehension task (i.e., patients were read a sentence and then asked to select from a picture array the image most closely related to the meaning of the sentence). As previously discussed, evidence from studies of developmental dyslexia have documented decreased temporal lobe, planum temporale, lateral, and medial geniculate nuclei volumes. LGN and MGN project directly to primary visual and auditory cortex and are thought to be involved in the direction and maintenance of attention, a function that is primarily subserved by the frontal lobes. In addition, Turkletaub and colleagues (2003) found that the processes underlying reading development are associated with increased activity of left temporal and frontal structures and decreased activity of right temporal lobe areas.
Construct validity studies provide evidence of the PPVT-III as a measure of intellectual ability (e.g., Bell, Lassiter, Matthews, & Hutchinson, 2001; Campbell, Bell, & Keith, 2001; D'Amato, Gray, & Dean, 1988); however, several of these studies have also shown that the PPVT inaccurately classifies intellectual functioning (e.g., Childers, Durham, & Wilson, 1994; Tarnowski & Kelly, 1987). D'Amato, Gray, and Dean (1988) showed in their construct validity study that the PPVT was strongly associated with measures involving verbal abstract reasoning and decision-making skills. The PPVT-III task requires the participant to choose one picture from a field of four that best matches the examiner-provided word. Thus, performance of the task also involves a decision-making component, an aspect of executive functioning, which are skills primarily subserved by the frontal lobes. Results from the above mentioned imaging studies, construct validity studies of the PPVT, the brain's interconnected pathways, and the decision-making component of the task itself might provide support for the present study's finding of frontal and temporal lobe gray matter volumes as predictive of PPVT-III performance. Thus, the current results implicate the PPVT-III as a complex measure of receptive vocabulary with underlying demands for executive functioning skills.
Results of the current study are limited by comparison to large gray matter volumes and warrant follow-up with manual measurement of sub-regions of the temporal lobes. Parcellation of the temporal lobes will permit further investigation of specific structure-function relationships. In addition, the present study utilized a cross-sectional design, limiting the conclusions that can be drawn regarding the impact of brain and skill maturation. Longitudinal studies are needed to investigate factors associated with development. Finally, our finding of nonsignificant correlations between frontal, parietal, and occipital lobar gray matter volumes and both neuropsychological tests raises concern regarding the contribution of total cranial volumes to the finding of left occipital lobe volume as a predictor of VP raw score and both left and right frontal lobe volumes as predictors of PPVT-III. Thus, although the relationship between temporal lobe volume and performance on these neuropsychological tests appears robust, the relative contribution of other lobar volumes to performance on these measures warrants further investigation.
Future research employing a longitudinal design with further parcellation of the temporal lobes is warranted to investigate developmental changes as well as to address the long-standing notion of bigger volume not necessarily associated with better function. For example, recent evidence from Shaw and colleagues (2006) revealed thinner cortical thickness in frontal and temporal regions associated with higher IQ in young children, with increases in cortical thickness peaking in middle childhood and a reverse relationship (i.e., higher cortical thickness associated with higher IQ) observed by adolescence. In addition, a recent DTI study examining white matter integrity in adults diagnosed with William's Syndrome associated higher fractional anisotropy values in the right superior longitudinal fasciculus with deficits in visuospatial construction (Hoeft et al., 2007). Therefore, present findings may not directly apply to clinical populations where there is an aberrant developmental trajectory of the nervous system.
Despite the above-mentioned limitations, the present study is one of the first to demonstrate the relationship between specific brain regions and neuropsychological measures of temporal lobe functioning in, typically developing children using volumetric MRI. These findings support the continued use of volumetric MRI analysis in healthy children for examining the ecological validity of specific neuropsychological tests.
ACKNOWLEDGEMENTS
We would like to thank Dr. Christopher Ruff for his advice with statistical analyses. This study was supported by NIH grants R01 NS042851 and M01 RR 00052.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andreasen NC, Flaum M, Swayze V, II, O'Leary DS, Alliger R, Cohen G, Ehrhardt J, Yuh WT. Intelligence and brain structure in normal individuals. American Journal of Psychiatry. 1993;150:130–134. doi: 10.1176/ajp.150.1.130. [DOI] [PubMed] [Google Scholar]
- Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, Happe F, Frith C, Frith U. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–1651. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, Phillips L. Imaging brain connectivity in children with diverse reading ability. NeuroImage. 2005;25:1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Beery KE, Beery NA. The Beery-Buktenica Developmental Test of Visual Motor Integration. Fifth Edition Pearson, Inc.; Minneapolis: MN: 2004. [Google Scholar]
- Bell NL, Lassiter KS, Matthews TD, Hutchinson MB. Comparison of the Peabody Picture Vocabulary Test-Third Edition and the Wechsler Adult Intelligence Scale-Third Edition with university students. Journal of Clinical Psychology. 2001;57:417–422. doi: 10.1002/jclp.1024. [DOI] [PubMed] [Google Scholar]
- Binder JR, Rao SM, Hammeke TA, Frost JA, Bandettini BS, Hyde JS. Effects of stimulus rate on signal response during functional magnetic resonance imaging of auditory cortex. Cognitive Brain Research. 1994;2:31–38. doi: 10.1016/0926-6410(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Boatman D, Lesser RP, Gordon B. Auditory speech processing in the left temporal lobe: an electrical interference study. Brain and Language. 1995;51:269–290. doi: 10.1006/brln.1995.1061. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Barthelemy C, Mouren MC, Artiges E, Samson Y, Brunelle F, Frackowiak RS, Zilbovicius M. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. NeuroImage. 2004;23:364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Büchel C, Price C, Friston K. A multimodal language region in the ventral visual pathway. Nature. 1998;394:274–277. doi: 10.1038/28389. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, McGinty VB, Pekar JJ, Watson TD, Pearlson GD. fMRI activation in a visual-perceptual task: network areas detected using the general linear model and independent components analysis. NeuroImage. 2001;14:1080–1088. doi: 10.1006/nimg.2001.0921. [DOI] [PubMed] [Google Scholar]
- Campbell JM, Bell SK, Keith LK. Concurrent validity of the Peabody Picture Vocabulary Test-Third Edition as an intelligence and achievement screener for low SES African American children. Assessment. 2001;8:85–94. doi: 10.1177/107319110100800108. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Kenney DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7-11 years: a volumetric analysis based on magnetic resonance images. Cerebral Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Childers JS, Durham TW, Wilson S. Relation of performance on the Kaufman Brief Intelligence Test with the Peabody Picture Vocabulary Test-Revised among preschool children. Perceptual and Motor Skills. 1994;79:1195–1199. doi: 10.2466/pms.1994.79.3.1195. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Erlbaum; Hillsdale, NJ: 1983. [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- D'Amato RC, Gray JW, Dean RS. Construct validity of the PPVT with neuropsychological, intellectual, and achievement measures. Journal of Clinical Psychology. 1988;44:934–939. doi: 10.1002/1097-4679(198811)44:6<934::aid-jclp2270440614>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, J.r., Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. Third Edition American Guidance Service; Circle Pines, MN: 1997. [Google Scholar]
- Eliez S, Rumsey JM, Giedd JN, Schmitt JE, Patwardhan AJ, Reiss AL. Morphological alteration of temporal lobe gray matter in dyslexia: an MRI study. Journal of Child Psychology and Psychiatry. 2000;41:637–644. doi: 10.1111/1469-7610.00650. [DOI] [PubMed] [Google Scholar]
- Egan V, Chiswick A, Santosh C, Naidu K, Rimmington JE, Best JJK. Size isn't everything: a study of brain volume, intelligence and auditory evoked potentials. Personal and Individual Differences. 1994;17:357–367. [Google Scholar]
- Galaburda A, Livingstone M. Evidence for magnocellular defect in developmental dyslexia. Annals of the NY Academy of Sciences. 1993;682:70–82. doi: 10.1111/j.1749-6632.1993.tb22960.x. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. Human brain left-right asymmetry in temporal speech region. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cerebral Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in Neurosciences. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E. Auditory neglect in man. Archives of Neurology. 1972;26:32–35. doi: 10.1001/archneur.1972.00490070050007. [DOI] [PubMed] [Google Scholar]
- Himmelbach M, Karnath H-O. Dorsal and ventral stream interaction: contributions from optic ataxia. Journal of Cognitive Neuroscience. 2005;17:632–640. doi: 10.1162/0898929053467514. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Barnea-Goraly N, Haas BW, Golarai G, Ng D, Mills D, Korenberg J, Bellugi U, Galaburda A, Reiss AL. More is not always better: Increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in williams syndrome. The Journal of Neuroscience. 2007;44:11960–11965. doi: 10.1523/JNEUROSCI.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Hugdahl K, Heiervang E, Ersland L, Lundervold A, Steinmetz H, Smievoll AI. Significant relation between MR measures of planum temporale area and dichotic processing of syllables in dyslexic children. Neuropsychologia. 2003;41:666–675. doi: 10.1016/s0028-3932(02)00224-5. [DOI] [PubMed] [Google Scholar]
- Isquith PK, Gioia GA, Espy KA. Executive function in preschool children: examination through everyday behavior. Developmental Neuropsychology. 2004;26:403–422. doi: 10.1207/s15326942dn2601_3. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink J,R, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2949. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Kahn JV. Predicting adaptive behavior of severely and profoundly mentally retarded children with early cognitive measures. Journal of Intellectual Disability Research. 1992;36:101–114. doi: 10.1111/j.1365-2788.1992.tb00487.x. [DOI] [PubMed] [Google Scholar]
- Kaplan DM, Liu AM, Abrams MT, Warsofsky IS, Kates WR, White CD, Kaufmann WE, Reiss AL. Application of an automated parcellation method to the analysis of pediatric brain volumes. Psychiatry Research. 1997;76:15–27. doi: 10.1016/s0925-4927(97)00055-3. [DOI] [PubMed] [Google Scholar]
- Karnath H-O. New insights into the functions of the superior temporal cortex. Nature. 2001;2:568–576. doi: 10.1038/35086057. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. 2001;411:950–953. doi: 10.1038/35082075. [DOI] [PubMed] [Google Scholar]
- Kates WR, Abrams MT, Kaufmann WE, Breither SN, Reiss AL. Reliability and validity of MRI measurement of the amygdale and hippocampus in children with fragile X syndrome. Psychiatry Research. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Kates WR, Warsofsky IS, Patwardhan A, Abrams MT, Liu AM, Naisu S, Kaufmann WE, Reiss AL. Automated Talairach atlas-based parcellation and measurement of cerebral lobes in children. Psychiatry Research. 1999;91:11–30. doi: 10.1016/s0925-4927(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Kolb B, Wishaw IQ. Fundamentals of human neuropsychology. W.H. Freeman; New York: 1990. [Google Scholar]
- Kourtzi Z, Kanwisher N. Cortical regions involved in perceiving object shape. The Journal of Neuroscience. 2000;20:3310–3318. doi: 10.1523/JNEUROSCI.20-09-03310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulp MT, Sortor JM. Clinical value of the Beery Visual-Motor Integration supplemental tests of Visual Perception and Motor Coordination. Optometry and Vision Science. 2003;80:312–315. doi: 10.1097/00006324-200304000-00009. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wels EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain development trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CM, Voeller KK, Lombardino LJ, Morris MK, Hynd GW, Alexander AW, Anderson HG, Garofalakis M, Honeyman JC, Mao J, et al. Anomalous cerebral structure in dyslexia revealed with magnetic resonance imaging. Archives of Neurology. 1993;50:461–469. doi: 10.1001/archneur.1993.00540050013008. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Rosen GD, Drislane FW, Galaburda AM. Physiological and anatomical evidence for magnocellular defect in developmental dyslexia. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:7943–7947. doi: 10.1073/pnas.88.18.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolek CJ. Abstract visual-form representations in the left cerebral hemisphere. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:375–386. doi: 10.1037//0096-1523.21.2.375. [DOI] [PubMed] [Google Scholar]
- Marsolek CJ, Shachter DL, Nicholas CD. Form-specific visual priming for new associations in the right cerebral hemisphere. Memory and Cognition. 1996;24:539–556. doi: 10.3758/bf03201082. [DOI] [PubMed] [Google Scholar]
- Meresse IG, Zilbovicius M, Boddaert N, Robel L, Philippe A, Sfaello I, Laurier L, Brunelle F, Samsson Y, Mouren MC, Chabane N. Autism severity and temporal lobe functional abnormalities. Annals of Neurology. 2005;58:466–469. doi: 10.1002/ana.20597. [DOI] [PubMed] [Google Scholar]
- Meier MJ, French LA. Lateralized deficits in complex visual discrimination and bilateral transfer of reminiscence following unilateral temporal lobectomy. Neuropsychologia. 1965;3:261–272. [Google Scholar]
- Milner AD, Goodale MA. Two visual systems re-viewed. Neuropsychologia. 2008;46:774–785. doi: 10.1016/j.neuropsychologia.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Molfese DL, Molfese VJ. Psychophysiological indices of early cognitive processes and their relationship to language. In: Obrzut JE, Hynd GW, editors. Child Neuropsychology: Theory and research. Academic Press; New York: 1986. pp. 95–115. [Google Scholar]
- Moritz CH, Johnson SC, McMillan KM, Haughton VM, Meyerand ME. Functional MRI neuroanatomic correlates of the Hooper Visual Organization Test. Journal of the International Neuropsychological Society. 2004;10:939–947. doi: 10.1017/s1355617704107042. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Newcombe F. Missile wounds of the brain. Oxford University Press; London: 1969. [Google Scholar]
- Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, Sasaki M. Abnormal regional cerebral blood flow in childhood autism. Brain. 2000;123:1838–1844. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- Pegna AJ, Caldara-Schnetzer AS, Perrig SH, Lazeyras F, Khateb A, Landis T, Seeck M. Is the right amygdala involved in visuospatial memory? Evidence from MRI volumetric measures. European Neurology. 2002;47:148–155. doi: 10.1159/000047973. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pins D, Meyer ME, Foucher J, Humphreys G, Boucart M. Neural correlates of implicit object identification. Neuropsychologia. 2004;42:1247–1259. doi: 10.1016/j.neuropsychologia.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Preacher KJ. Calculation for the test of the difference between two independent correlation coefficients [computer software] 2002 May; Available from http://www.quantpsy.org. [Google Scholar]
- Price KJ, Joschko M, Kerns K. The ecological validity of pediatric neuropsychological tests of attention. The Clinical Neuropsychologist. 2003;17:170–181. doi: 10.1076/clin.17.2.170.16506. [DOI] [PubMed] [Google Scholar]
- Rae C, Lee MA, Dixon RM, Blamire AM, Thompson CH, Styles P, Talcott J, Richardson AJ, Stein JG. Metabolic abnormalities in developmental dyslexia detected by 1H magnetic resonance spectroscopy. Lancet. 1998;351:1849–1852. doi: 10.1016/S0140-6736(97)99001-2. [DOI] [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. The Diagnostic Interview for Children and Adolescents-IV. Multi-Health Systems; North Tonawanda: 1997. [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children: a volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Hennessey JG, Rubin M, Beach L, Abrams MT, Warsofsky IS, Liu AM, Links JM. Reliability and validity of an algorithm for fuzzy tissue segmentation of magnetic resonance images. Journal of Computer Assisted Tomography. 1998;22:471–479. doi: 10.1097/00004728-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Reiss AL. BrainImage [computer software] Stanford University, Department of Psychiatry; 1999. [Google Scholar]
- Reminger SL, Kaszniak AW, Labiner DM, Littrell LD, David BT, Ryan L, Herring AM, Kaemingk KL. Bilateral hippocampal volume predicts verbal memory function in temporal lobe epilepsy. Epilepsy and Behavior. 2004;5:687–695. doi: 10.1016/j.yebeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Dorwart R, Vermess M, Denckla MB, Kruesi MJ, Rapoport JL. Magnetic resonance imaging of brain anatomy in severe developmental dyslexia. Archives of Neurology. 1986;43:1045–1046. doi: 10.1001/archneur.1986.00520100053014. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Service E, Kiesila P, Uutela K, Salonen O. Impaired visual word processing in dyslexia revealed with magnetoencephalography. Annals of Neurology. 1996;40:157–162. doi: 10.1002/ana.410400206. [DOI] [PubMed] [Google Scholar]
- Schoenemann PT, Budinger TF, Sarich VW, Wang WS-Y. Brain size does not predict general cognitive ability within families. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4932–4937. doi: 10.1073/pnas.97.9.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Subramaniam B, Naidu S, Reiss AL. Neuroanatomy in Rett syndrome: cerebral cortex and posterior fossa. Neurology. 1997;48:399–407. doi: 10.1212/wnl.48.2.399. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournouz . Co-Planar Stereotaxic Atlas of the Human Brain: 3-dimensional proportional system - an approach to cerebral imaging. Stuttgart; Thieme: 1988. [Google Scholar]
- Tallal P. Auditory temporal perception, phonics, and reading disabilities in children. Brain and Language. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Tallal P, Katz W. Neuropsychological and neuroanatomical studies of developmental language/reading impaired disorders: Recent advances. In: von Euler C, Lundberg I, Lennerstrand G, editors. Brain and reading. MacMillan; London: 1989. pp. 183–196. [Google Scholar]
- Tallal P, Piercy M. Defects of non-verbal auditory perception in children with developmental aphasia. Nature. 1973;16:468. doi: 10.1038/241468a0. [DOI] [PubMed] [Google Scholar]
- Tallal P, Stark RE. Perceptual/motor profiles of reading impaired children with or without concomitant oral language deficits. Annals of Dyslexia. 1982;32:163–176. [Google Scholar]
- Tallal P, Stark RE, Mellits DE. Identification of language-impaired children on the basis of rapid perception and production skills. Brain and Language. 1985;25:314–322. doi: 10.1016/0093-934x(85)90087-2. [DOI] [PubMed] [Google Scholar]
- Tarnowski KJ, Kelly PA. Utility of the PPVT-R for pediatric intellectual screening. Journal of Pediatric Psychology. 1987;12:611–614. doi: 10.1093/jpepsy/12.4.611. [DOI] [PubMed] [Google Scholar]
- Trauzettel-Klosinski S, Durrwachter U, Klosinski G, Braun C. Cortical activation during word reading and picture naming in dyslexia and non-reading-impaired children. Clinical Neurophysiology. 2006;117:1085–1097. doi: 10.1016/j.clinph.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Current Opinion in Neurobiology. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Vinckenbosch E, Robichon F, Eliez S. Gray matter alteration in dyslexia: converging evidence from volumetric and voxel-by-voxel MRI analyses. Neuropsychologia. 2005;43:324–331. doi: 10.1016/j.neuropsychologia.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Willerman L, Schultz R, Rutledge JN, Bigler ED. In vivo brain size and intelligence. Intelligence. 1991;15:223–228. [Google Scholar]
- Zar JH. Biostatistical Analysis. 4th ed. Prentice Hall; NJ: 1998. p. 356. [Google Scholar]
- Zilbovicius M, Boddaert N, Belin P, Poline JB, Remy P, Mangin JF, Thivard L, Barthelemy C, Samson Y. Temporal lobe dysfunction in childhood autism: a PET study. Positron emission tomography. The American Journal of Psychiatry. 2000;157:1988–1993. doi: 10.1176/appi.ajp.157.12.1988. [DOI] [PubMed] [Google Scholar]