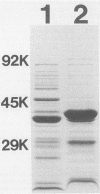
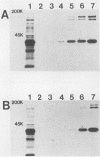
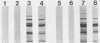
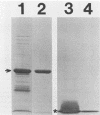
Abstract
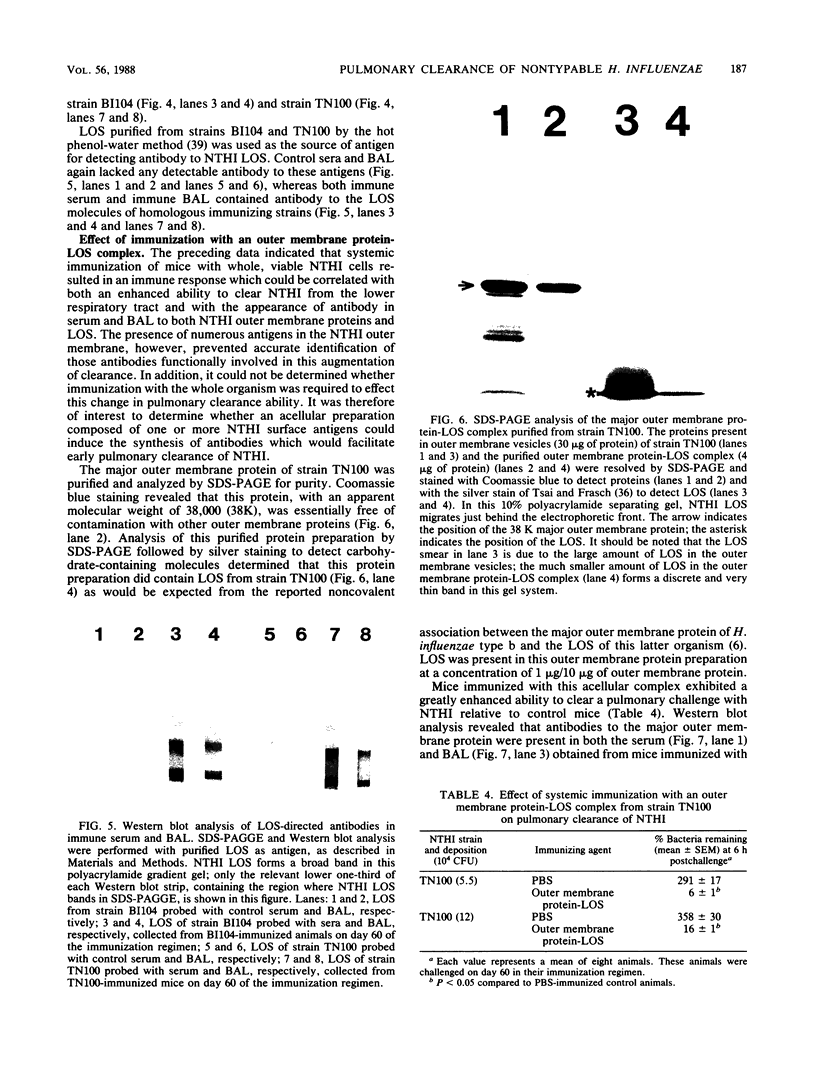
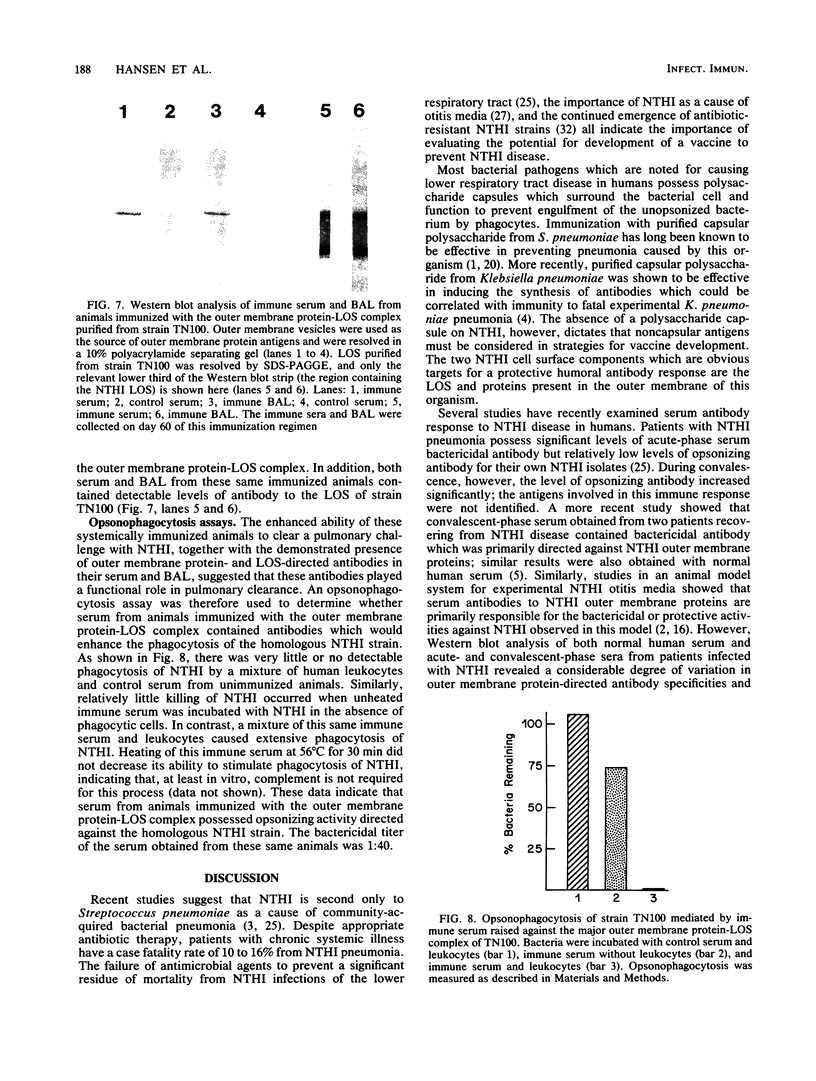
BALB/c mice systemically immunized by intraperitoneal injection with whole, viable cells of two different strains of nontypable Haemophilus influenzae (NTHI) exhibited a markedly enhanced ability to clear the homologous strain of NTHI from the lower respiratory tract. Immunization did not influence the number of phagocytic cells recovered by bronchoalveolar lavage from mice before or after intrapulmonary challenge with NTHI. Immunization also induced the synthesis of relatively large quantities of NTHI-directed antibodies which were detectable in both the bloodstream and the alveolar spaces of the lung. Radioimmunoprecipitation and Western blot (immunoblot) analyses indicated that these antibodies were directed against both the proteins and lipooligosaccharide (LOS) in the NTHI outer membrane. Bactericidal and opsonophagocytic assays determined that the NTHI-directed antibodies in the serum were functional and able to kill or opsonize the homologous NTHI strain. Mice immunized with an NTHI major outer membrane protein-LOS complex also had an increased ability to effect pulmonary clearance of NTHI. Serum and bronchoalveolar lavage fluid collected from these animals immunized with the outer membrane protein-LOS complex contained relatively high levels of antibodies to both of these antigens. The serum from these animals also possessed bactericidal and opsonic activity against the homologous NTHI strain. These results indicate that systemic immunization can enhance the ability of experimental animals to clear NTHI from the lower respiratory tract and suggest that immunoprophylaxis of NTHI pulmonary disease may be feasible.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austrian R., Douglas R. M., Schiffman G., Coetzee A. M., Koornhof H. J., Hayden-Smith S., Reid R. D. Prevention of pneumococcal pneumonia by vaccination. Trans Assoc Am Physicians. 1976;89:184–194. [PubMed] [Google Scholar]
- Barenkamp S. J. Protection by serum antibodies in experimental nontypable Haemophilus influenzae otitis media. Infect Immun. 1986 May;52(2):572–578. doi: 10.1128/iai.52.2.572-578.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk S. L., Holtsclaw S. A., Wiener S. L., Smith J. K. Nontypeable Haemophilus influenzae in the elderly. Arch Intern Med. 1982 Mar;142(3):537–539. [PubMed] [Google Scholar]
- Campagnari A. A., Gupta M. R., Dudas K. C., Murphy T. F., Apicella M. A. Antigenic diversity of lipooligosaccharides of nontypable Haemophilus influenzae. Infect Immun. 1987 Apr;55(4):882–887. doi: 10.1128/iai.55.4.882-887.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Immunization against fatal experimental Klebsiella pneumoniae pneumonia. Infect Immun. 1986 Nov;54(2):403–407. doi: 10.1128/iai.54.2.403-407.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnehm H. E., Pelton S. I., Gulati S., Rice P. A. Characterization of antigens from nontypable Haemophilus influenzae recognized by human bactericidal antibodies. Role of Haemophilus outer membrane proteins. J Clin Invest. 1985 May;75(5):1645–1658. doi: 10.1172/JCI111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Robertson S. M., Gulig P. A., Frisch C. F., Haanes E. J. Immunoprotection of rats against Haemophilus influenzae type B disease mediated by monoclonal antibody against a haemophilus outer-membrane protein. Lancet. 1982 Feb 13;1(8268):366–368. doi: 10.1016/s0140-6736(82)91394-0. [DOI] [PubMed] [Google Scholar]
- Hansen M. V., Musher D. M., Baughn R. E. Outer membrane proteins of nontypable Haemophilus influenzae and reactivity of paired sera from infected patients with their homologous isolates. Infect Immun. 1985 Mar;47(3):843–846. doi: 10.1128/iai.47.3.843-846.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooke A. M., Bellanti J. A., Oeschger M. P. Live attenuated bacterial vaccines: new approaches for safety and efficacy. Lancet. 1985 Jun 29;1(8444):1472–1474. doi: 10.1016/s0140-6736(85)92252-4. [DOI] [PubMed] [Google Scholar]
- Huxley E. J., Viroslav J., Gray W. R., Pierce A. K. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978 Apr;64(4):564–568. doi: 10.1016/0002-9343(78)90574-0. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C., Ashton F. E. Conjugation of meningococcal lipopolysaccharide R-type oligosaccharides to tetanus toxoid as route to a potential vaccine against group B Neisseria meningitidis. Infect Immun. 1984 Jan;43(1):407–412. doi: 10.1128/iai.43.1.407-412.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltreider H. B., Chan M. K. The class-specific immunoglobulin composition of fluids obtained from various levels of the canine respiratory tract. J Immunol. 1976 Feb;116(2):423–429. [PubMed] [Google Scholar]
- Karasic R. B., Trumpp C. E., Gnehm H. E., Rice P. A., Pelton S. I. Modification of otitis media in chinchillas rechallenged with nontypable Haemophilus influenzae and serological response to outer membrane antigens. J Infect Dis. 1985 Feb;151(2):273–279. doi: 10.1093/infdis/151.2.273. [DOI] [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Hansen E. J. Antigenic and phenotypic variations of Haemophilus influenzae type b lipopolysaccharide and their relationship to virulence. Infect Immun. 1986 Jan;51(1):69–79. doi: 10.1128/iai.51.1.69-79.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E. Synthesis of immunogenic oligosaccharide-protein conjugates from the lipopolysaccharide of Neisseria gonorrhoeae P9. J Immunol Methods. 1982;48(2):233–240. doi: 10.1016/0022-1759(82)90197-1. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Apicella M. A. Antigenic heterogeneity of outer membrane proteins of nontypable Haemophilus influenzae is a basis for a serotyping system. Infect Immun. 1985 Oct;50(1):15–21. doi: 10.1128/iai.50.1.15-21.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Bartos L. C., Campagnari A. A., Nelson M. B., Apicella M. A. Antigenic characterization of the P6 protein of nontypable Haemophilus influenzae. Infect Immun. 1986 Dec;54(3):774–779. doi: 10.1128/iai.54.3.774-779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Dudas K. C., Mylotte J. M., Apicella M. A. A subtyping system for nontypable Haemophilus influenzae based on outer-membrane proteins. J Infect Dis. 1983 May;147(5):838–846. doi: 10.1093/infdis/147.5.838. [DOI] [PubMed] [Google Scholar]
- Musher D. M., Kubitschek K. R., Crennan J., Baughn R. E. Pneumonia and acute febrile tracheobronchitis due to haemophilus influenzae. Ann Intern Med. 1983 Oct;99(4):444–450. doi: 10.7326/0003-4819-99-4-444. [DOI] [PubMed] [Google Scholar]
- Onofrio J. M., Toews G. B., Lipscomb M. F., Pierce A. K. Granulocyte-alveolar-macrophage interaction in the pulmonary clearance of Staphylococcus aureus. Am Rev Respir Dis. 1983 Mar;127(3):335–341. doi: 10.1164/arrd.1983.127.3.335. [DOI] [PubMed] [Google Scholar]
- Paradise J. L. Otitis media in infants and children. Pediatrics. 1980 May;65(5):917–943. [PubMed] [Google Scholar]
- Patrick C. C., Kimura A., Jackson M. A., Hermanstorfer L., Hood A., McCracken G. H., Jr, Hansen E. J. Antigenic characterization of the oligosaccharide portion of the lipooligosaccharide of nontypable Haemophilus influenzae. Infect Immun. 1987 Dec;55(12):2902–2911. doi: 10.1128/iai.55.12.2902-2911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Thompson R. E. Pulmonary host defenses. I. Analysis of protein and lipids in bronchial secretions and antibody responses after vaccination with pseudomonas aeruginosa. J Immunol. 1973 Aug;111(2):358–368. [PubMed] [Google Scholar]
- Robertson S. M., Frisch C. F., Gulig P. A., Kettman J. R., Johnston K. H., Hansen E. J. Monoclonal antibodies directed against a cell surface-exposed outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1982 Apr;36(1):80–88. doi: 10.1128/iai.36.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shann F., Gratten M., Germer S., Linnemann V., Hazlett D., Payne R. Aetiology of pneumonia in children in Goroka Hospital, Papua New Guinea. Lancet. 1984 Sep 8;2(8402):537–541. doi: 10.1016/s0140-6736(84)90764-5. [DOI] [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Barenkamp S. J., Granoff D. M. Further studies of the role of noncapsular antibody in protection against experimental Haemophilus influenzae type b bacteremia. Infect Immun. 1983 Oct;42(1):257–263. doi: 10.1128/iai.42.1.257-263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syriopoulou V., Scheifele D., Smith A. L., Perry P. M., Howie V. Increasing incidence of ampicillin resistance in Hemophilus influenzae. J Pediatr. 1978 Jun;92(6):889–892. doi: 10.1016/s0022-3476(78)80354-0. [DOI] [PubMed] [Google Scholar]
- Toews G. B., Vial W. C., Hansen E. J. Role of C5 and recruited neutrophils in early clearance of nontypable Haemophilus influenzae from murine lungs. Infect Immun. 1985 Oct;50(1):207–212. doi: 10.1128/iai.50.1.207-212.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews G. B., Viroslav S., Hart D. A., Hansen E. J. Pulmonary clearance of encapsulated and unencapsulated Haemophilus influenzae strains. Infect Immun. 1984 Aug;45(2):437–442. doi: 10.1128/iai.45.2.437-442.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Musher D. M., Septimus E. J., McGowan J. E., Jr, Quinones F. J., Wiss K., Vance P. H., Trier P. A. Haemophilus influenzae infections in adults: characterization of strains by serotypes, biotypes, and beta-lactamase production. J Infect Dis. 1981 Aug;144(2):101–106. doi: 10.1093/infdis/144.2.101. [DOI] [PubMed] [Google Scholar]