Abstract
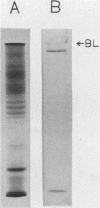
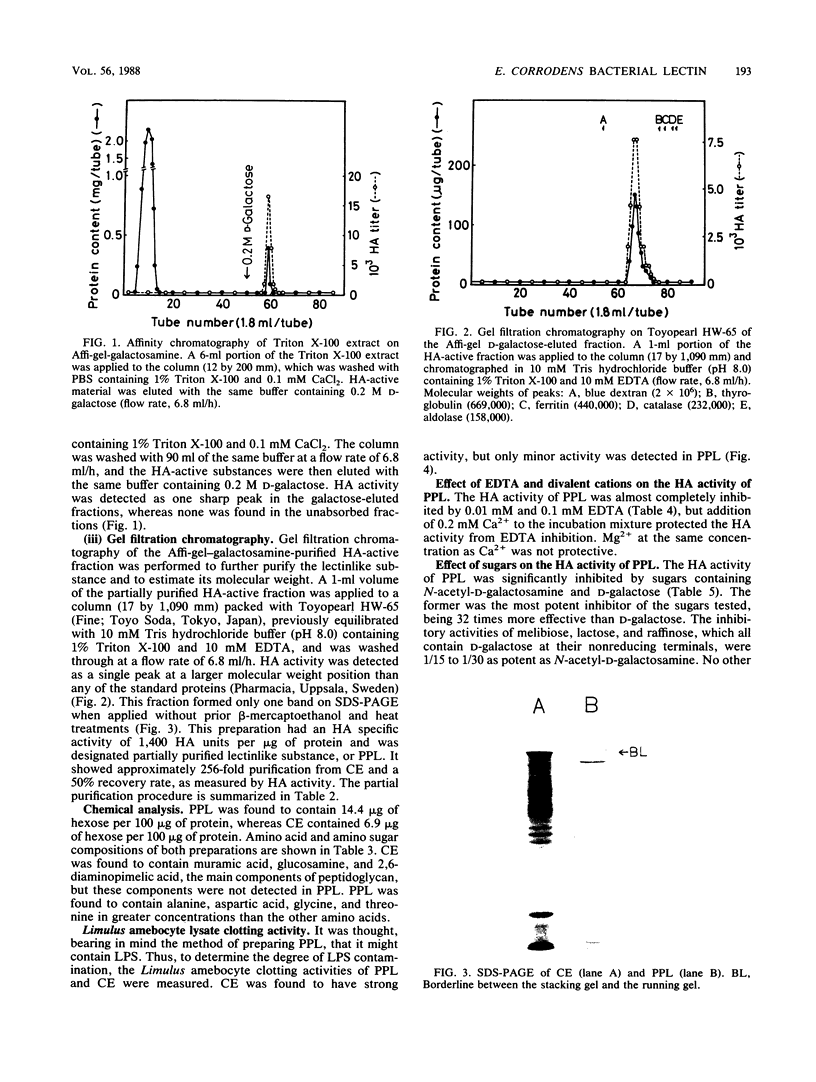
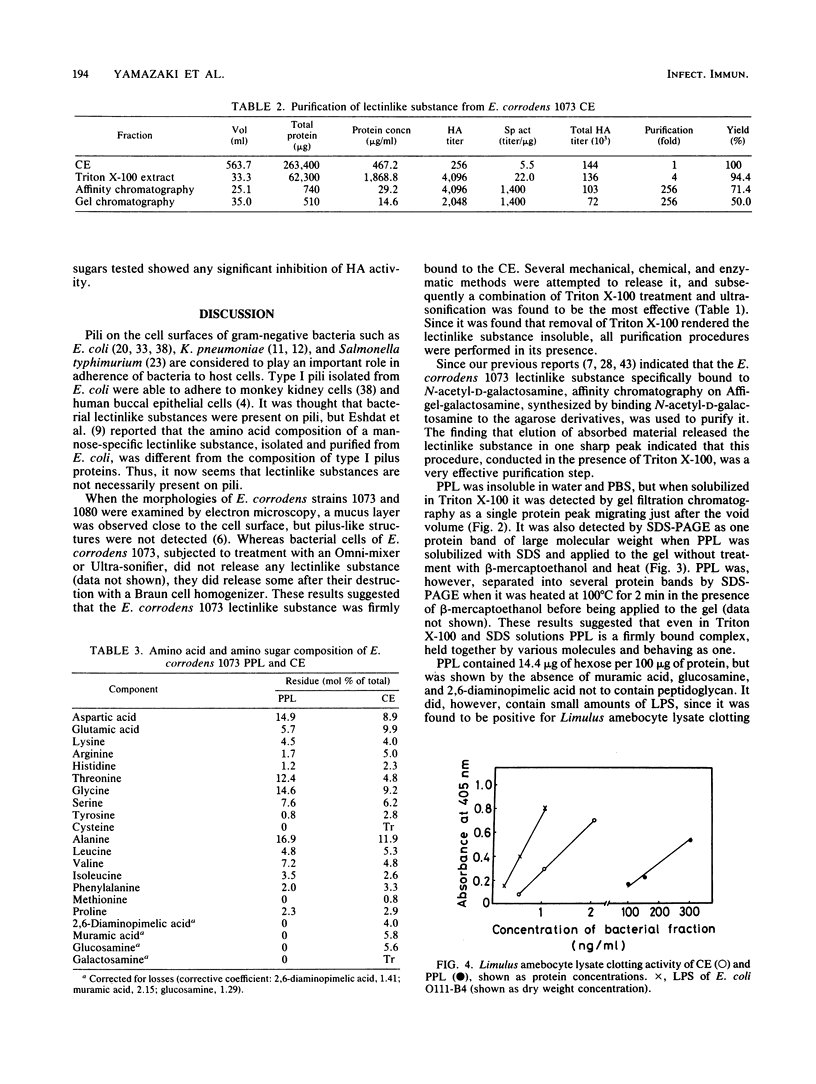
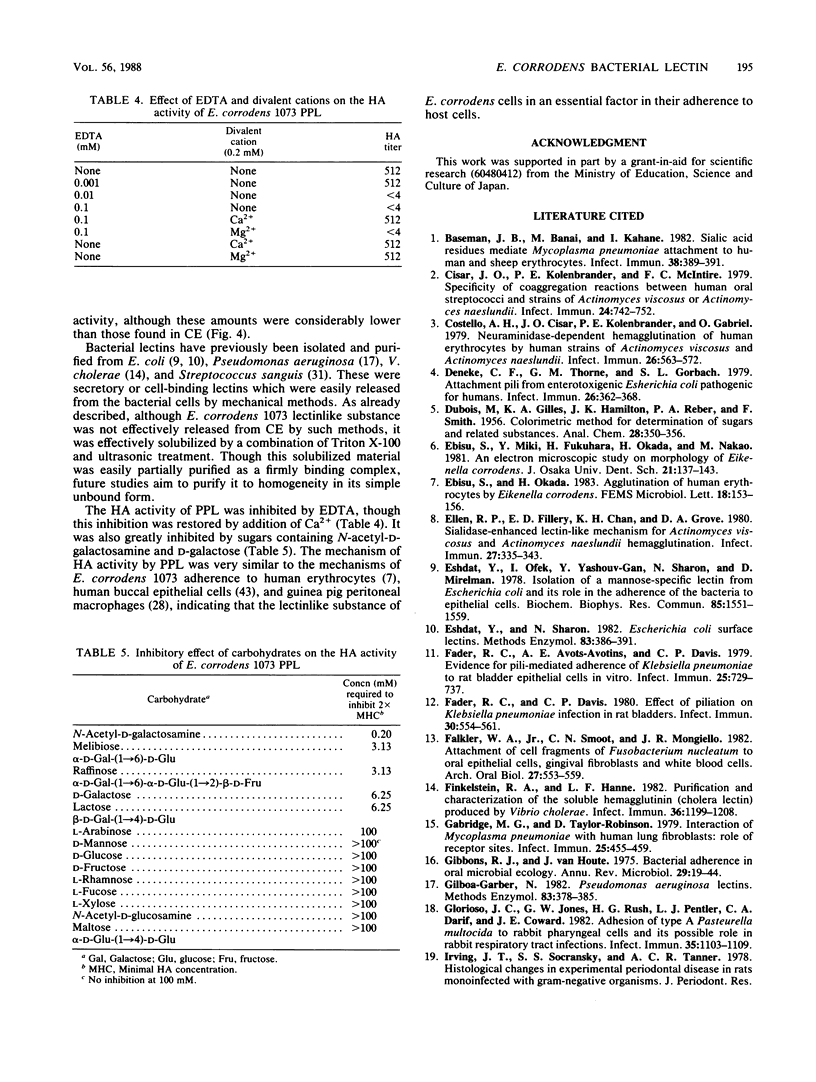
A bacterial lectinlike substance, which is considered to participate in the adherence of Eikenella corrodens to various host cells, was purified from E. corrodens cells. The substance was extracted in 1% Triton X-100 with sonication from the cell envelope of E. corrodens 1073 and partially purified by galactosamine affinity chromatography and gel filtration chromatography based on its hemagglutination (HA) activity. The lectinlike substance was purified about 256-fold as evaluated by its specific HA activity. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the partially purified lectinlike substance (PPL) produced a single protein band of large molecular weight when it was applied to the gel without the addition of beta-mercaptoethanol and heating. Chemical analysis showed that PPL contained 14.4 micrograms of hexose per 100 micrograms of protein and that it did not contain muramic acid, glucosamine, or 2,6-diaminopimelic acid, which are characteristic of peptidoglycans. The HA activity of PPL was inhibited by EDTA but restored by adding Ca2+. The HA activity was remarkably inhibited by sugars containing N-acetyl-D-galactosamine and D-galactose. These results indicate that the lectinlike substance on the E. corrodens cells is an essential factor for the adherence to host cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baseman J. B., Banai M., Kahane I. Sialic acid residues mediate Mycoplasma pneumoniae attachment to human and sheep erythrocytes. Infect Immun. 1982 Oct;38(1):389–391. doi: 10.1128/iai.38.1.389-391.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A. H., Cisar J. O., Kolenbrander P. E., Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneke C. F., Thorne G. M., Gorbach S. L. Attachment pili from enterotoxigenic Escherichia coli pathogenic for humans. Infect Immun. 1979 Oct;26(1):362–368. doi: 10.1128/iai.26.1.362-368.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisu S., Miki Y., Fukuhara H., Okada H., Nakao M. An electron microscopic study on the morphology of Eikenella corrodens. J Osaka Univ Dent Sch. 1981 Dec;21:137–143. [PubMed] [Google Scholar]
- Ellen R. P., Fillery E. D., Chan K. H., Grove D. A. Sialidase-enhanced lectin-like mechanism for Actinomyces viscosus and Actinomyces naeslundii hemagglutination. Infect Immun. 1980 Feb;27(2):335–343. doi: 10.1128/iai.27.2.335-343.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshdat Y., Ofek I., Yashouv-Gan Y., Sharon N., Mirelman D. Isolation of a mannose-specific lectin from Escherichia coli and its role in the adherence of the bacteria to epithelial cells. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1551–1559. doi: 10.1016/0006-291x(78)91179-8. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., Sharon N. Escherichia coli surface lectins. Methods Enzymol. 1982;83:386–391. doi: 10.1016/0076-6879(82)83035-8. [DOI] [PubMed] [Google Scholar]
- Fader R. C., Avots-Avotins A. E., Davis C. P. Evidence for pili-mediated adherence of Klebsiella pneumoniae to rat bladder epithelial cells in vitro. Infect Immun. 1979 Aug;25(2):729–737. doi: 10.1128/iai.25.2.729-737.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader R. C., Davis C. P. Effect of piliation on Klebsiella pneumoniae infection in rat bladders. Infect Immun. 1980 Nov;30(2):554–561. doi: 10.1128/iai.30.2.554-561.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkler W. A., Jr, Smoot C. N., Mongiello J. R. Attachment of cell fragments of Fusobacterium nucleatum to oral epithelial cells, gingival fibroblasts and white blood cells. Arch Oral Biol. 1982;27(7):553–559. doi: 10.1016/0003-9969(82)90069-3. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Hanne L. F. Purification and characterization of the soluble hemagglutinin (cholera lectin)( produced by Vibrio cholerae. Infect Immun. 1982 Jun;36(3):1199–1208. doi: 10.1128/iai.36.3.1199-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Taylor-Robinson D. Interaction of Mycoplasma pneumoniae with human lung fibroblasts: role of receptor sites. Infect Immun. 1979 Jul;25(1):455–459. doi: 10.1128/iai.25.1.455-459.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gilboa-Garber N. Pseudomonas aeruginosa lectins. Methods Enzymol. 1982;83:378–385. doi: 10.1016/0076-6879(82)83034-6. [DOI] [PubMed] [Google Scholar]
- Glorioso J. C., Jones G. W., Rush H. G., Pentler L. J., Darif C. A., Coward J. E. Adhesion of type A Pasteurella mulocida to rabbit pharyngeal cells and its possible role in rabbit respiratory tract infections. Infect Immun. 1982 Mar;35(3):1103–1109. doi: 10.1128/iai.35.3.1103-1109.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Fusco P. C., Brinton C. C., Moon H. W. In vitro adhesion of Escherichia coli to porcine small intestinal epithelial cells: pili as adhesive factors. Infect Immun. 1978 Aug;21(2):392–397. doi: 10.1128/iai.21.2.392-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Kotani S. [Method for the study of chemical structure of the bacterial cell wall. 3. Identification and determination of structural constituents of cell wall peptide glycan]. Tanpakushitsu Kakusan Koso. 1969 Sep;14(10):920–925. [PubMed] [Google Scholar]
- Korhonen T. K., Leffler H., Svanborg Edén C. Binding specificity of piliated strains of Escherichia coli and Salmonella typhimurium to epithelial cells, saccharomyces cerevisiae cells, and erythrocytes. Infect Immun. 1981 May;32(2):796–804. doi: 10.1128/iai.32.2.796-804.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A., Johnson D., Nowotny A., Tanner A. C., Socransky S. S. Histopathology of periodontal disease in gnotobiotic rats monoinfected with Eikenella corrodens. J Periodontal Res. 1978 Mar;13(2):134–148. doi: 10.1111/j.1600-0765.1978.tb00162.x. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y., Ebisu S., Okada H. The adherence of Eikenella corrodens to guinea pig macrophages in the absence and presence of anti-bacterial antibodies. J Periodontal Res. 1987 Sep;22(5):359–365. doi: 10.1111/j.1600-0765.1987.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Mongiello J. R., Falkler W. A., Jr Sugar inhibition of oral Fusobacterium nucleatum haemagglutination and cell binding. Arch Oral Biol. 1979;24(7):539–545. doi: 10.1016/0003-9969(79)90133-x. [DOI] [PubMed] [Google Scholar]
- Morita T., Harada T., Nakamura S., Iwanaga S., Niwa M. Horseshoe crab (Tachypleus tridentatus) clotting enzyme: a new sensitive assay method for bacterial endotoxin [proceedings]. Jpn J Med Sci Biol. 1978 Apr;31(2):178–181. [PubMed] [Google Scholar]
- Nagata K., Nakao M., Shibata S., Shizukuishi S., Nakamura R., Tsunemitsu A. Purification and characterization of galactosephilic component present on the cell surfaces of Streptococcus sanguis ATCC 10557. J Periodontol. 1983 Mar;54(3):163–172. doi: 10.1902/jop.1983.54.3.163. [DOI] [PubMed] [Google Scholar]
- Newman M. G. The role of Bacteroides melaninogenicus and other anaerobes in periodontal infections. Rev Infect Dis. 1979 Mar-Apr;1(2):313–324. doi: 10.1093/clinids/1.2.313. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Bacterial adherence. Adv Intern Med. 1980;25:503–532. [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Reed W. P., Williams R. C., Jr Bacterial adherence: first step in pathogenesis of certain infections. J Chronic Dis. 1978 Feb;31(2):67–72. doi: 10.1016/0021-9681(78)90091-7. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S. Microbiology of periodontal disease -- present status and future considerations. J Periodontol. 1977 Sep;48(9):497–504. doi: 10.1902/jop.1977.48.9.497. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y., Ebisu S., Okada H. Eikenella corrodens adherence to human buccal epithelial cells. Infect Immun. 1981 Jan;31(1):21–27. doi: 10.1128/iai.31.1.21-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]