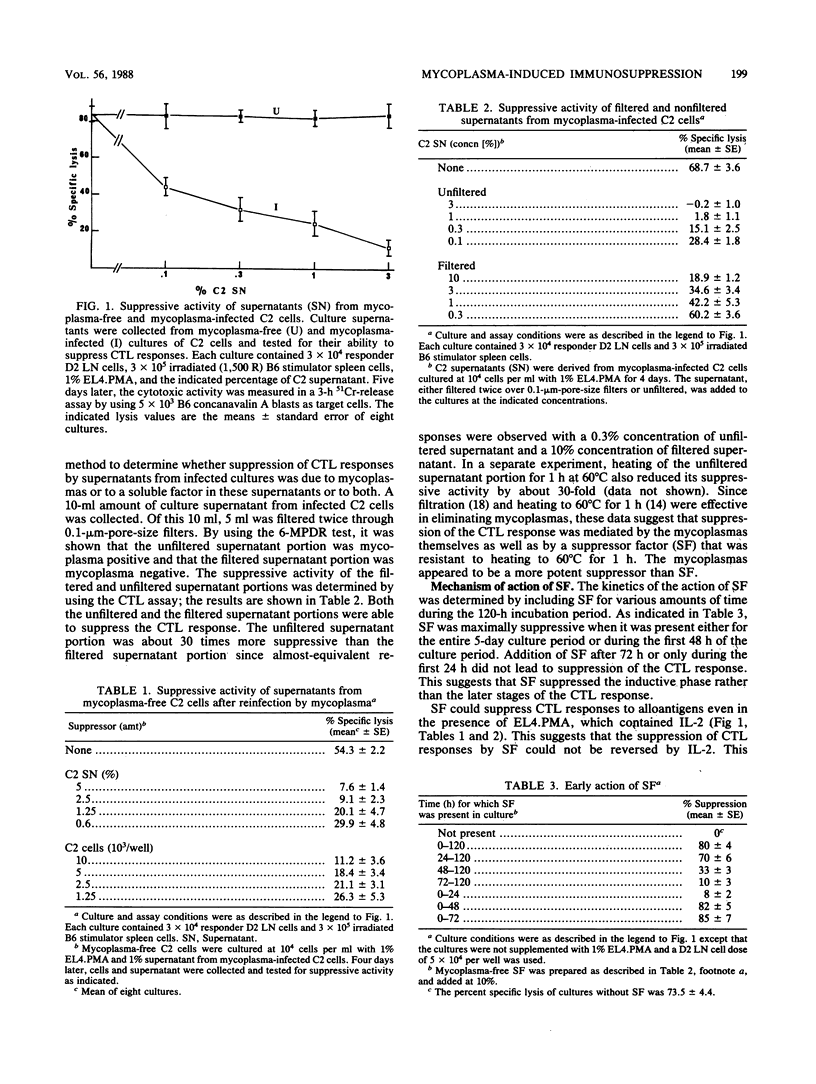
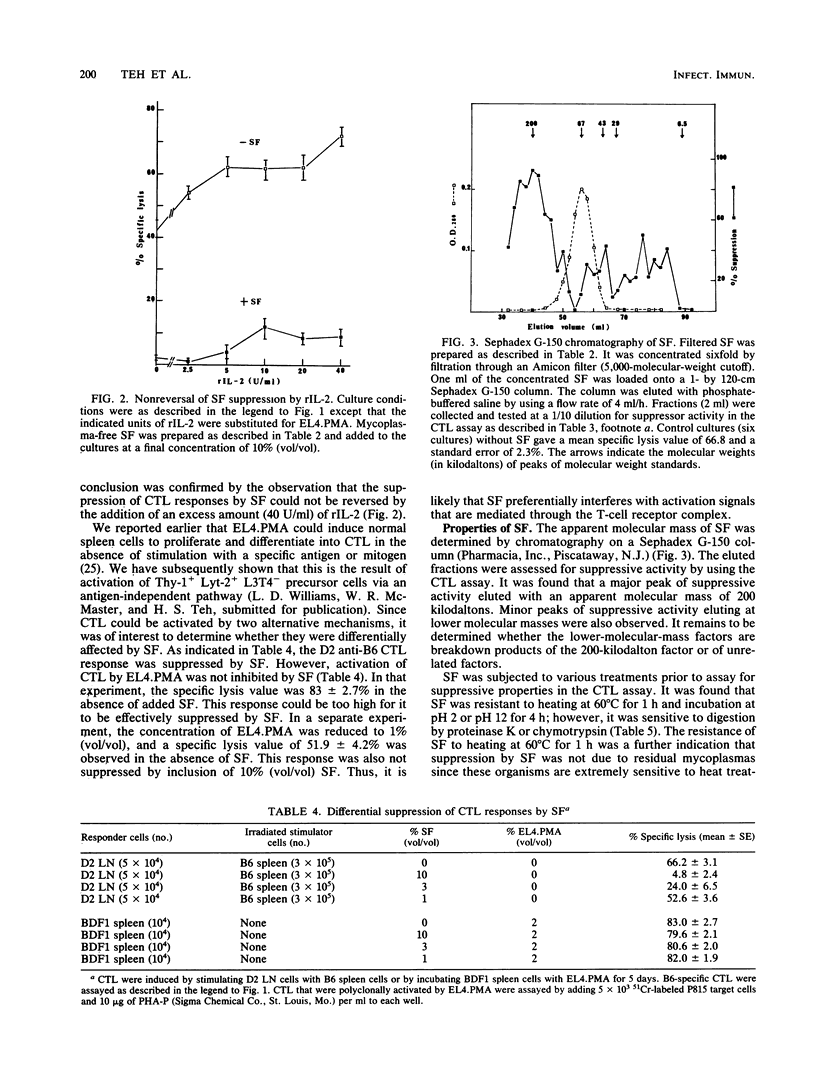
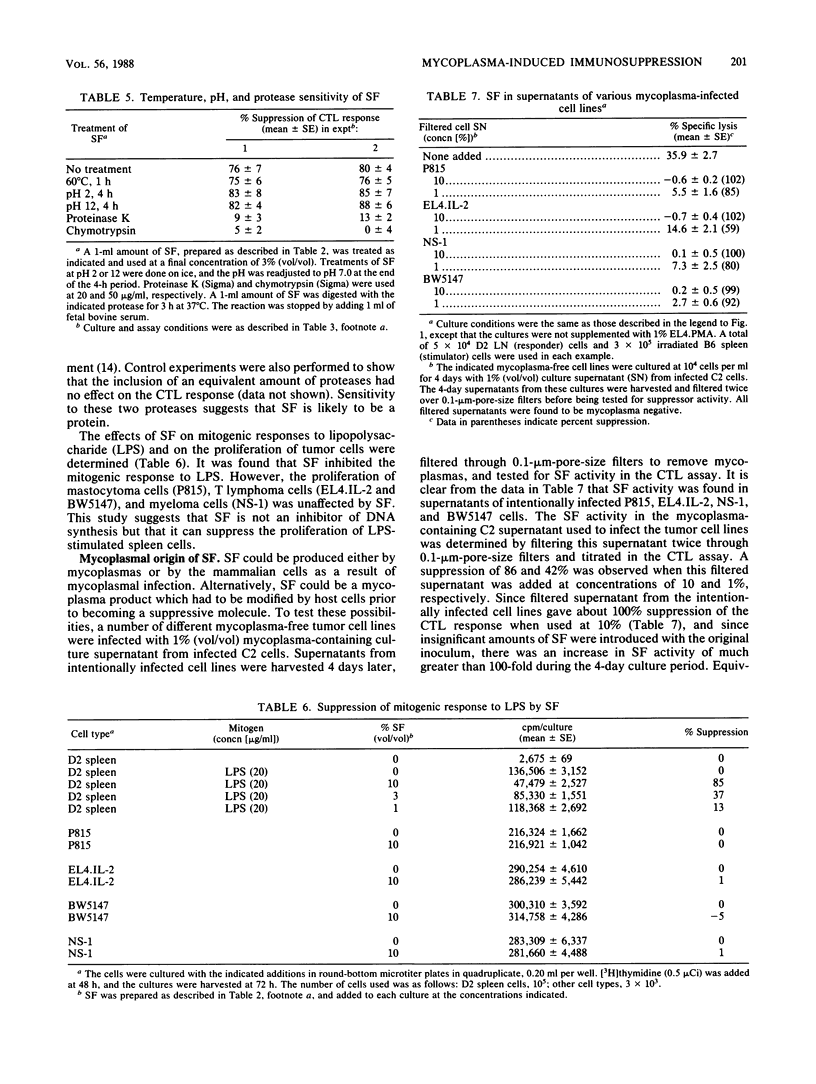
Abstract
A class of interleukin-2-dependent T-cell clones, isolated from a murine fetal thymus, was previously shown to suppress the induction of cytotoxic responses to alloantigens (H.-S. Teh, M. Ho, and W. R. McMaster, J. Immunol. 135:1582-1588, 1985). In that article, the immunosuppressive properties of these T-cell clones were shown to be a direct consequence of infection by Mycoplasma hyorhinis. Suppression of cytotoxic responses was mediated by both the mycoplasmas and a 200-kilodalton factor present in supernatants of infected cultures. This factor was sensitive to proteases but was resistant to heating to 60 degrees C for 1 h and to incubation on ice at pH 2 or pH 14 for 4 h. The production of suppressor factor in infected cultures was independent of the viability or the protein synthesis capability of the mammalian cells, suggesting that it was produced by M. hyorhinis. The factor was most suppressive when it was added during the early stages of the cytotoxic response. Its suppressive effects on cytotoxic responses were not reversed by the addition of an excess of recombinant interleukin-2. This factor also suppressed mitogenic responses to lipopolysaccharide. However, it is not a growth inhibitor since it did not affect the proliferation of tumor cell lines. A simple method for detecting M. hyorhinis in the infected T-cell clones and for eliminating it is described.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barile M. F., Leventhal B. G. Possible mechanism for Mycoplasma inhibition of lymphocyte transformation induced by phytohaemagglutinin. Nature. 1968 Aug 17;219(5155):750–752. doi: 10.1038/219751a0. [DOI] [PubMed] [Google Scholar]
- Bassett M., Coons T. A., Wallis W., Goldberg E. H., Williams R. C., Jr Suppression of stimulation in mixed leukocyte culture by newborn splenic lymphocytes in the mouse. J Immunol. 1977 Nov;119(5):1855–1857. [PubMed] [Google Scholar]
- Callewaert D. M., Kaplan J., Peterson W. D., Jr, Lightbody J. J. Suppression of lymphocyte activation by a factor produced by Mycoplasma arginini. J Immunol. 1975 Dec;115(6):1662–1664. [PubMed] [Google Scholar]
- Cole B. C., Sullivan G. J., Daynes R. A., Sayed I. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. II. Cellular requirements for T cell transformation mediated by a soluble Mycoplasma mitogen. J Immunol. 1982 May;128(5):2013–2018. [PubMed] [Google Scholar]
- Farrar J. J., Fuller-Farrar J., Simon P. L., Hilfiker M. L., Stadler B. M., Farrar W. L. Thymoma production of T cell growth factor (Interleukin 2). J Immunol. 1980 Dec;125(6):2555–2558. [PubMed] [Google Scholar]
- Hatanaka M., Del Giudice R., Long C. Adenine formation from adenosine by mycoplasmas: adenosine phosphorylase activity. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1401–1405. doi: 10.1073/pnas.72.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellung-Larsen P., Frederiksen S. Influence of mycoplasma infection on the incorporation of different precursors into RNA components of tissue culture cells. Exp Cell Res. 1976 May;99(2):295–300. doi: 10.1016/0014-4827(76)90586-3. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Lau C. Y., Budz-Tymkewycz S., Wang E. Y., Ishaque A. A mutant human T-cell line producing immunosuppressive factor(s). Cell Immunol. 1984 Aug;87(1):35–52. doi: 10.1016/0008-8749(84)90128-x. [DOI] [PubMed] [Google Scholar]
- Lau C. Y., Wang E. Y., Li D., Budz-Tymkewycz S., Visconti V., Ishaque A. Mechanism of action of a suppressor-activating factor (SAF) produced by a human T cell line. J Immunol. 1985 May;134(5):3155–3162. [PubMed] [Google Scholar]
- Levine E. M. Mycoplasma contamination of animal cell cultures: a simple, rapid detection method. Exp Cell Res. 1972 Sep;74(1):99–109. doi: 10.1016/0014-4827(72)90484-3. [DOI] [PubMed] [Google Scholar]
- McGarrity G. J., Carson D. A. Adenosine phosphorylase-mediated nucleoside toxicity. Application towards the detection of mycoplasmal infection in mammalian cell cultures. Exp Cell Res. 1982 May;139(1):199–205. doi: 10.1016/0014-4827(82)90333-0. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Mizel D. Purification to apparent homogeneity of murine interleukin 1. J Immunol. 1981 Mar;126(3):834–837. [PubMed] [Google Scholar]
- Mosier D. E., Mathieson B. J., Campbell P. S. Ly phenotype and mechanism of action of mouse neonatal suppressor T cells. J Exp Med. 1977 Jul 1;146(1):59–73. doi: 10.1084/jem.146.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez A. G., Kim J. H., Gelbard A. S., Djordjevic B. Altered incorporation of nucleic acid precursors by mycoplasma-infected mammalian cells in culture. Exp Cell Res. 1972 Feb;70(2):301–310. doi: 10.1016/0014-4827(72)90140-1. [DOI] [PubMed] [Google Scholar]
- Proust J. J., Buchholz M. A., Nordin A. A. A "lymphokine-like" soluble product that induces proliferation and maturation of B cells appears in the serum-free supernatant of a T cell hybridoma as a consequence of mycoplasmal contamination. J Immunol. 1985 Jan;134(1):390–396. [PubMed] [Google Scholar]
- Riendeau D., Harnish D. G., Bleackley R. C., Paetkau V. Purification of mouse interleukin 2 to apparent homogeneity. J Biol Chem. 1983 Oct 25;258(20):12114–12117. [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J. Comparison of methods for the detection of Mycoplasmal contamination of cell cultures: a review. In Vitro. 1975 Jan-Feb;11(1):20–34. doi: 10.1007/BF02615318. [DOI] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Simberkoff M. S., Thorbecke G. J., Thomas L. Studies of PPLO infection. V. Inhibition of lymphocyte mitosis and antibody formation by mycoplasmal extracts. J Exp Med. 1969 Jun 1;129(6):1163–1181. doi: 10.1084/jem.129.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh H. S., Ho M., McMaster W. R. Isolation and characterization of novel suppressor T cell clones from murine fetal thymus. J Immunol. 1985 Sep;135(3):1582–1588. [PubMed] [Google Scholar]
- Teh H. S., Yu M. Activation of nonspecific killer cells by interleukin 2-containing supernatants. J Immunol. 1983 Oct;131(4):1827–1833. [PubMed] [Google Scholar]
- Vennegoor C., Polak-Vogelzang A. A., Hekman A. Monoclonal antibodies against Mycoplasma hyorhinis. A secondary effect of immunization with cultured cells. Exp Cell Res. 1982 Jan;137(1):89–94. doi: 10.1016/0014-4827(82)90011-8. [DOI] [PubMed] [Google Scholar]
- Wang A., Lu S. D., Mark D. F. Site-specific mutagenesis of the human interleukin-2 gene: structure-function analysis of the cysteine residues. Science. 1984 Jun 29;224(4656):1431–1433. doi: 10.1126/science.6427925. [DOI] [PubMed] [Google Scholar]
- Yowell R. L., Cole B. C., Daynes R. A. Utilization of T cell hybridomas to establish that a soluble factor derived from Mycoplasma arthritidis is truly a genetically restricted polyclonal T cell activator. J Immunol. 1983 Aug;131(2):543–545. [PubMed] [Google Scholar]
- van Diggelen O. P., Phillips D. M., Shin S. I. Endogenous HPRT activity in a cryptic strain of mycoplasma and its effect on cellular resistance to selective media in infected cell lines. Exp Cell Res. 1977 Apr;106(1):191–203. doi: 10.1016/0014-4827(77)90256-7. [DOI] [PubMed] [Google Scholar]



