Abstract
This longitudinal study was designed to provide data on sex differences in the course of illness in schizophrenia and other psychotic disorders. Ninety-seven participants (43 women and 54 men) were assessed during index hospitalization when they were in the acute phase of illness, and then re-assessed prospectively at 6 consecutive followups over a 20 year period. Patients were evaluated by a series of standardized measures on many aspects of illness including the presence of psychosis, global outcome, and rate of recovery. When women were compared to men in this sample, the data demonstrated a lower percentage of psychotic activity for women over the course of illness (significant at the 7.5 and 20 year followups), and a significant improvement in psychotic activity over 20 years for women (p<.05), but not for men. Additionally, women showed significantly better global functioning (p<.05) at 3 of the 6 followups (the 2, 7.5, and 10-year followups). Significantly higher percentages (p<.05) of women were in recovery at 2 of the 6 followup years (the 2 and 10-year followups). Cumulatively, 61% of the women with schizophrenia showed a period of recovery at some point during the 20 year period, compared to 41% of the men. The sex difference patterns were similar for patients with schizophrenia and for those with other types of psychotic disorders. Sex differences in this sample were specifically not attributable to differences in age of onset or premorbid developmental achievements.
Keywords: Sex Differences, Schizophrenia, Psychosis, Recovery, Outcome
The current research provides 20-year multi-followup longitudinal data comparing sex difference in psychosis, outcome, and recovery in patients with schizophrenia and other types of psychotic disorders. Previous research has suggested that women with schizophrenia have a milder course of illness than men (1–3). Various factors have been proposed to account for this sex difference hypothesis including psychosocial differences, older age of onset for women, and differences in premorbid developmental achievement (4–6).
We previously conducted a prospective followup study of sex differences in outcome, based on 5 followups over a 15-year period (7). Our previous study, which to our knowledge was the only longitudinal study to examine sex differences in patients with other psychotic disorders compared to those with schizophrenia, found that sex differences in outcome were found for both patients with schizophrenia and those with other psychotic disorders, with women consistently showing better functioning over time, more frequent periods of good functioning and periods of recovery, less likelihood of uniformly poor outcome, and fewer and shorter rehospitalizations. Unlike both groups of patients who were psychotic, the patients with nonpsychotic disorders showed no significant sex differences in outcome. The current study builds on the previous research by studying different outcome variables and by studying patients who were followed up over a longer period (6 followups over 20 years), providing information on the followup sample as patients aged.
It is generally believed that women with schizophrenia have better courses of illness and global outcomes than men. For example, women with schizophrenia have been reported to have fewer negative symptoms (8–11), better responses to neuroleptics (12), better social functioning (13–16), and less time in the hospital (1). However, in other studies, no sex differences have been found in negative, affective, and psychotic symptoms (17–19), neurocognitive functioning, MRI findings (20), and number and duration of hospitalizations (21). Some studies suggest that the outcome of women with schizophrenia declines over time, and eventually approximates that of men with schizophrenia (22–24).
The current research builds upon previous literature and addresses the following specific questions regarding sex differences:
Do schizophrenia patients show sex differences in frequency and severity of psychotic activity over a 20 year period?
Do patients show sex differences in long-term global outcome and rate of recovery?
If there are sex differences, are they limited to schizophrenia, or are they also characteristic of other types of psychotic disorders?
Method
Patient Characteristics
The current prospectively designed longitudinal research assessed 97 patients, 6 times over 20 years (43 women and 54 men). The patients were participants in the Harrow Chicago Followup Study, a prospective longitudinal research program studying major dimensions of psychopathology, including symptoms, longitudinal course of illness, and global outcome (7,25–31). This study was approved by the Human Subjects Review Committee, and subjects participated with informed, voluntary, written consent. Research diagnoses were made at index hospitalization based on structured diagnostic research interviews, including the Schedule for Affective Disorders and Schizophrenia or SADS (32) and/or the Schizophrenia State Inventory (33), plus admission clinical diagnostic interviews and detailed inpatient observations. Inter-rater reliability for the diagnosis of schizophrenia was K=.88.
Diagnostic groups, based on the Research Diagnostic Criteria (34), included 55 patients with schizophrenia (23 women and 32 men) and 42 with other psychotic disorders (20 women and 22 men). The group of patients with other psychotic disorders included 24 with bipolar disorder, 8 with unipolar depression, and 10 with unspecified functional psychosis. At index hospitalization, all patients were administered a standardized battery of semi-structured interviews, questionnaires, and psychological tests. Patients were re-assessed prospectively at 6 followups, at a mean of 2 years, 4.5, 7.5, 10, 15, and 20 years following index hospitalization. Raters were not informed of the results of previous assessments.
All 97 patients were assessed at the 20-year followup. Sixty-five patients (67%) were studied at all 6 followups over 20 years. Another 21 patients were studied at 5 of the 6 followups, including the 20-year followup. Overall, 86 patients (89%) of the sample were studied at 5 or 6 of the 6 followups.
In some previous sex difference research, older women with schizophrenia are often compared to younger men with schizophrenia, since women’s onset of schizophrenia is typically later (i.e., at an older age) than that of men. In the present study, women with schizophrenia and men with schizophrenia of the same age were compared. This technique offsets differences in age of onset and reduces the potential effects of long-term treatment. Thus this sample was relatively young when first assessed: the women’s mean age at index was 23.5 years (s.d.= 4.9) and the men’s was 22.6 (s.d.=3.5). Thus they entered the study as a young, nonchronic group: 44% of the women and 48% of the men were experiencing their first hospitalization at index. Seventy-six percent had either one or no previous hospitalizations. At the 20-year followup, 15% of the schizophrenia patients were 39 years old or younger, 46% were 40–44, and 39% were 45–54.
The sample included 67 white and 30 African American patients. The mean parental socioeconomic status (35) was 3.24_(SD=1.4, range=1–5). The education level ranged from 9–19 years of school, with a mean of 12.9 school years (SD=2.3 years). There were no significant sex differences for the demographic variables. At the 20 year followup, among the patients with schizophrenia, 63% of the women were taking antipsychotic medication and 36% were not taking such medication, compared with 45% and 55% of the men respectively. Among the patients with other psychotic disorders, 10% of the women were taking antipsychotic medication and 90% were not taking such medication, compared with 41% and 59% of the men respectively.
Followup Assessment
At each followup, ratings of psychosis reflected the presence of delusions and/or hallucinations during the previous month, based on data from the SADS (32), and determined by a system of assessment used in previous research (36). Scores for psychosis ranged from 1 to 3, (1=absent, 2=weak or equivocal, and 3= definitely present). At the 20-year followup, data on psychotic activity were obtained for 52 schizophrenia patients and 41 patients with other psychotic disorders (42 women and 51 men). Negative symptoms were rated from the Behavior Rating Scale of the Psychiatric Assessment Interview (37) which was completed at the end of each interview. The measure of negative symptoms is designed to assess flat affect, poverty of speech, and psychomotor retardation/poverty. Individual items were combined into three subscales reflecting poverty of speech, flat affect, and psychomotor retardation (38,39). Intra-class correlations between raters were 0.96 for the poverty of speech scale, 0.86 for the flat affect scale, and 0.85 for the psychomotor retardation/poverty scale.
Additionally, patients were administered the Harrow Functioning interview (40,41), a semi-structured interview providing information regarding global functioning and adjustment, symptoms, work and social functioning, family adjustment, and rehospitalization. Two examples of questions on work functioning from this interview are: “Are you employed at present? What jobs have you held since the last followup?” Outcome ratings were based on the 8-point scale developed by Levenstein, Klein, and Pollack, the LKP scale (42), which has been used previously to assess global functioning and adjustment (7,25–31). LKP ratings are based on work and social adjustment, level of self-support, life disruptions, symptoms, relapse, and rehospitalization. These functional domains are combined providing a comprehensive score that reflects patient’s global functioning. We obtained a correlation of r=.85 (p<.0001) between the 8-point LKP scale and scores on the Global Assessment Scale (GAS, 43) which is almost identical to the Global Assessment of Functioning (GAF, 44). Inter-rater reliability was r=.92 for the LKP scale. The LKP provides a means of separating the sample into 3 groups showing: 1) good global outcome, remission, or recovery during the followup year based on scores of 1 or 2, indicating adequate or near-adequate functioning in all areas, 2) moderate impairment, based on scores of 3–6, indicating difficulties in some but not all areas of adjustment during the followup year, and 3) poor outcome during the last year, based on scores of 7–8, indicating uniformly poor functioning, or poor functioning in almost all areas, including poor psychosocial functioning and severe symptoms. To measure psychosocial and instrumental work functioning, we used the Strauss-Carpenter Scale (45).
To investigate any potential heterogeneity among the patients in the group of patients with psychotic disorders other than schizophrenia, we conducted a repeated measures analyses of variance (ANOVA) on overall outcome (LKP score), with main effects for sex, diagnosis (bipolar psychotic disorder vs. the combined group of unipolar psychotic disorder and unspecified psychosis), and time (6 followup assessments). There was no significant sex-by-diagnosis-by time interaction. Therefore, we combined the patients with psychotic disorders other than schizophrenia into the group called “other psychotic disorders.”
1.3 Operational Measure of Recovery
We used the LKP, Strauss-Carpenter scale, and the Behavior Rating Scale of the Psychiatric Assessment Interview (32,37,45) scales to derive an operational measure of recovery: 1) the absence of major symptoms throughout the followup year (i.e., psychotic and negative symptoms), 2) adequate psychosocial functioning including instrumental work half-time or more during the followup year, 3) the absence of a very poor social activity level, and 4) no psychiatric rehospitalizations during the year. To rate whether patients were in recovery, we applied the algorithm that recovery was determined by a score of 1–2 on the LKP scale and a score of “2” or higher on both the work and psychosocial functioning subscales of the Strauss-Carpenter Scale (45). If patients met all of these criteria at any given followup, they were considered to have been in recovery at that time. The recovery index we used provides data both on the percentage of patients in recovery at any followup year and the cumulative percentage of patients who, over the 20-year period, ever show a one-year or longer recovery period. Previous research (37) has shown the Strauss-Carpenter scale to have adequate reliability for social functioning (r=.96, p<.001), and for work functioning (r=.87, <.001).
Measure of Premorbid Developmental Achievement
To assess premorbid developmental achievements (which includes both premorbid developmental achievements and premorbid adjustment), we analyzed data from a widely-used measure collected prospectively, at index hospitalization using the Zigler-Phillips Scale (46, 47). The Zigler-Phillips Scale is based on patients’ work history, education, marital status, age at first break, and IQ. This scale has been linked to developmental formulations and theories concerning premorbid competence and adjustment. It has been used in studies applying developmental theory to adult psychopathology and outcome, to self image, and to mental retardation (46–49). Scoring is reliable and the many studies using the scale provide support for its construct validity (46).
Results
Sex Differences in Psychotic Activity over 20 Years: Analysis of 6 Consecutive Followups
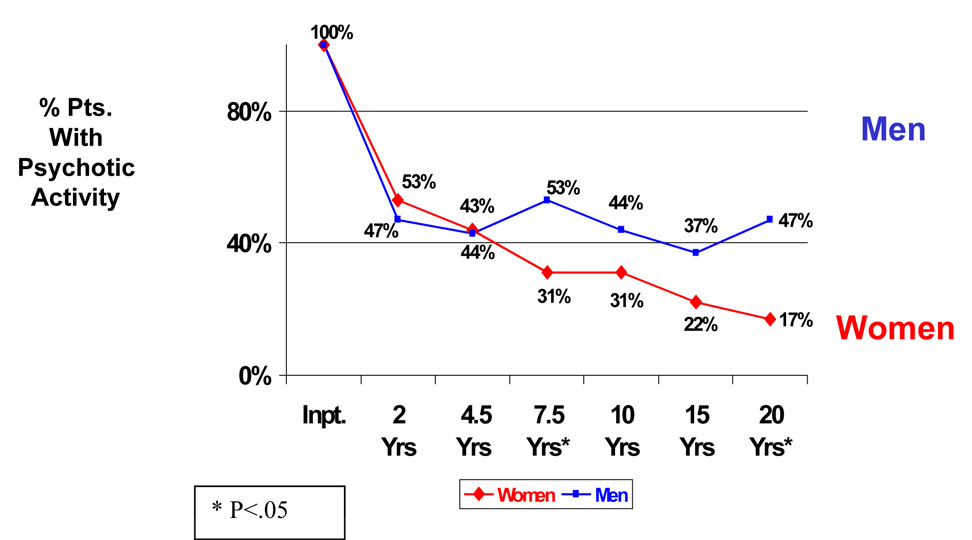
Figure 1 presents the percentage of patients with schizophrenia and other psychotic disorders showing evidence of psychotic activity at each of the 6 followups over 20 years (Insert Figure 1). At index hospitalization, all patients were psychotic. At the 2-year followup when most of the patients had improved from the period of acute hospitalization, there was no sex difference in psychosis. However, over time, after the 4.5-year followup, women showed a consistent pattern of less psychotic activity than men. At each followup after the 4.5-year followup, a lower percentage of women than men showed psychotic activity. Chi-square analyses indicated that this difference was significant at the 7.5-year followup (χ2=4.23, df=1, p<.05) and the 20-year followup (χ2=9.57, df=1, p=.002). In general, the male patients with schizophrenia showed a more oscillating pattern in psychosis in contrast to the more steady decline shown by the women with schizophrenia. The percentages of women showing psychosis ranged from 17% to 33%, while the percentages of men ranged from 41% to 50%. The same pattern emerged when the diagnostic groups were analyzed separately, but the chi-square differences were only significant at the 20 year followup for schizophrenia patients (χ2=4.45, df=1, p<.05) and the patients with other psychotic disorders (χ2=5.23, df=1, p<.05).
Figure 1. Sex Differences of % of Patients with Schizophrenia and Other Psychotic Disorders Showing Psychotic Activity: 6 Followups over 20 Years.
As a second step, we studied potential changes over time, analyzing whether patients had psychotic activity at the 2-year followup compared to the 20-year followup. We chose this interval because it represented the longest possible interval in the data set. The data indicated a significant improvement for women (t=2.41, df=21; p=.025), but no analogous improvement for men. When patients were analyzed according to diagnostic groups, the same pattern occurred for the schizophrenia patients, but the women with schizophrenia only showed a trend toward a significant improvement (t=1.74, df=14, p=.10), while the women with other psychotic disorders showed significant improvement (t=3.22, df=15, p<.01).
Sex Differences in Global Outcome over the 20 Years: Analysis of 6 Consecutive Followups
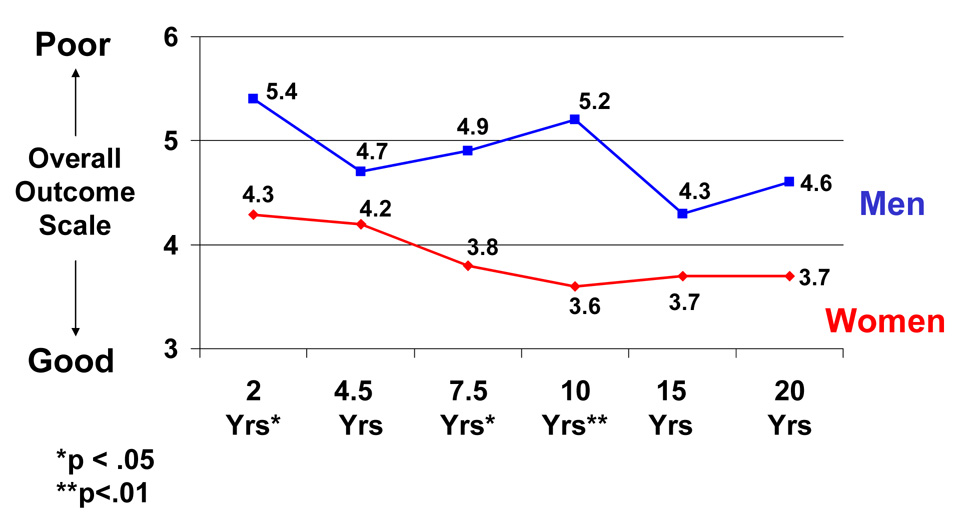
To study sex differences in global outcome, we analyzed the data from the LKP scale over the 20-year period (Insert Figure 2). Individual t-tests were conducted at each followup for women vs. men. Figure 2 presents the data on sex differences in global functioning at all 6 followups for the combined group of patients with schizophrenia and other types of psychotic disorders (see Figure 2). Women showed better global functioning at all 6 followups. These sex differences were significant for the 2-year (t=2.03, df=78, p<.05), 7.5-year (t=2.4, df=87, p<.05), and 10-year followups (t=3.21, df=84, p<01). To determine whether sex differences may have been associated with differences in premorbid developmental achievement, we conducted individual t-tests for women and men separately, comparing patients with good premorbid developmental achievement with patients with poor premorbid developmental achievement, based on the Zigler-Phillips scale (46). This analysis indicated no sex differences, suggesting that sex differences in outcome which emerged were not attributable to differences in premorbid developmental achievement. Among patients with schizophrenia, women showed significantly better global functioning at the 2-year followup (t=2.32, df=41, p<.05) and the 15-year followup (t=3.30, df=46, p<.03). For patients with other psychotic disorders, the sex difference was significant at the 10-year followup (t=2.00, df=36, p=.05).
Figure 2. Sex differences in global functioning in schizophrenia and other psychotic disorders: 6 followups over 20 years1.
1Higher scores represent poorer global outcome
Sex Differences in Rate of Recovery
We studied the patterns of sex differences in patients’ rate of recovery by assessing the cumulative percentage of women and men who were in recovery at any year (Figure 3) and using the cumulative data, the percentage who were ever in recovery during the 20-year period (Figure 4). (Insert Figure 3 and Figure 4.)
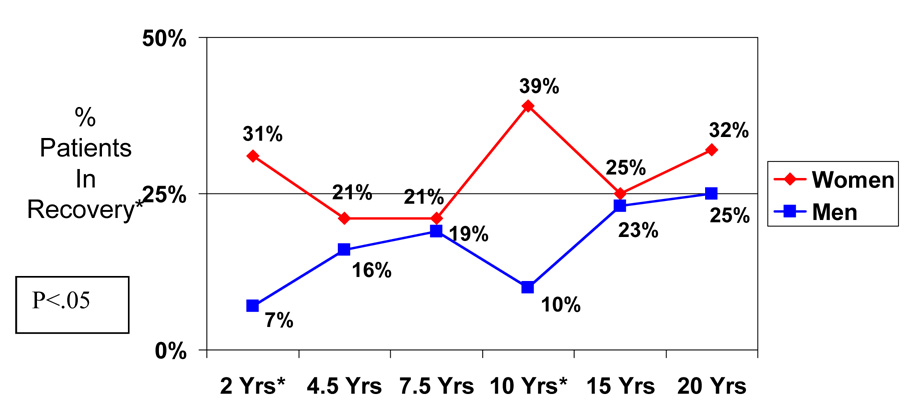
Figure 3. Sex Differences in Percentage of Schizophrenia Patients Experiencing Recovery Over 20 Years.
*Recovery = Period of one or more years with no major symptoms, no rehospitalizations, and at least partially adequate instrumental work and social functioning
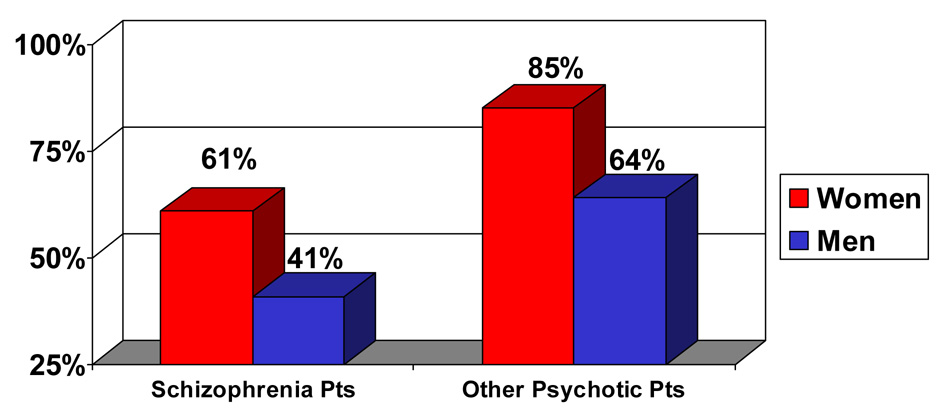
Figure 4. Cumulative % of women and men with schizophrenia and other psychotic disorders who ever experienced a period of recovery* (6 followups over 20 years).
*Recovery = Period of one or more years with no major symptoms, no rehospitalizations, and at least partially adequate instrumental work and social functioning
Figure 3 presents a trajectory of recovery based on the percentage of women and men with schizophrenia showing recovery at each followup. In all cases, a larger percentage of women were in recovery. These sex differences were significant at the 2-year (χ2 = 4.19, df=1, p<.05) and the 10-year followups (χ2= 5.69, df=1, p<.05). Despite the number of points in time when a sex difference was significant, for most of the time periods (4 of 6 assessment points), they were similar. The differences that occurred generally were defined by a marked improvement for women and decline for men between the 2nd and 5th years. Over time, the pattern was irregular and did not fit a neat pattern. For patients with other psychotic disorders, significant sex differences did not emerge.
Figure 4 presents the cumulative percentage of patients who ever experienced a period of recovery (of one year or longer) over the 20-year study period (see Figure 4). The data indicated that 61% of the women with schizophrenia showed a period of recovery, compared to 41% of the men. Sex differences in patients with other types of psychotic disorders were less robust, but still occurred in the same direction: 85% of the women with other types of psychotic disorders compared with 64% of the men experienced a period of recovery. While these analyses did not reach statistical significance, they suggest a potential for more women to have a period of recovery than men.
Discussion
This research examined whether there are sex differences in psychosis, global outcome, and rate of recovery in individuals with schizophrenia and other types of psychotic disorders. Twenty-year longitudinal prospective multi-followup data on sex differences in psychosis and cumulative 20-year data on recovery have not previously been available to the field. The general area has become more important because of the greater emphasis on recovery in schizophrenia and other psychotic disorders, and because previous research on sex differences has produced mixed results. Previous studies have not usually focused on whether this pattern of sex differences is unique to schizophrenia.
Furthermore, many sex difference studies do not examine samples of patients whose age of onset occurs at such a young age as it did for the patients in the current sample, and there is evidence that early onset of schizophrenia is a negative prognostic indicator (46,49,50–52). Our group and others have proposed that for schizophrenia, a first break at an older age is a positive prognostic factor, or a protective factor, because: 1) older age prior to a first psychotic break allows for development of greater knowledge, better social skills, and experience in the world prior to the illness, and 2) a first break at a later age may suggest greater internal resiliency, or greater resistance to psychopathology. There were no significant differences between groups in age of onset. We addressed this issue by assessing a sample who were young when first evaluated (23 years old), and who were most often (76%) undergoing their first or second hospitalization. Thus the women with schizophrenia included many whose first break occurred early for women, since many women with schizophrenia experience their first break in their mid-to-late 20s (10,50–52). The current study involves seriously ill women with schizophrenia, and compares these women with men whose first break occurred at an age more typical for men.
Sex Differences in Patients with Psychotic Disorders other than Schizophrenia
The finding that sex differences are not unique to schizophrenia, but also occur in patients with other psychotic disorders has not previously been studied in detail. A recent study of ours involving sex differences over a 15-year period showed that while women appear to have the advantage in coping with schizophrenia other psychotic disorders, this does not appear to extend to nonpsychotic disorders (7). Why women with psychotic disorders have better outcomes than their male counterparts is not definitively known. Possible reasons could include genetic factors (2, 53), difference in brain structure (54, 55), higher rates of marriage among women (56, 57), lower rates of substance abuse (57), greater social expectations and pressure concerning vocational expectations for men, and differences in social support systems. Hormonal differences may also play a role (58–62).
Global Outcome and Recovery
The current data show a significant tendency for women with schizophrenia and other psychotic disorders to have better global outcomes than their male counterparts. Although the analyses did not produce uniformly significant results at each followup, many of the sex differences were significant. Nearly every analysis showed men’s outcomes as poorer than women’s. More women than men showed a period of recovery at some point during the 20-year period, i.e., one or more years with no major symptoms, no rehospitalizations, and partially adequate work and social functioning. Additionally, there were no significant sex differences in premorbid developmental achievements at onset of illness. This may be partly attributable to the fact that the women in this sample had an early age of onset. However, even when age of onset is addressed by assessing a uniformly young sample, and when there is no sex difference in patients’ level of premorbid developmental achievements, women show a better course of illness. Our 20-year followup data do not support the hypothesis advanced by some that outcome of women with schizophrenia declines throughout the course, and eventually approximates that of men with schizophrenia (22–24), although further research, using even longer followup periods, would be desirable.
Psychosis and the Estrogen Hypothesis
Several researchers have suggested that hormonal differences, specifically in estrogen, may be important in the development and frequency of psychotic symptoms. Seeman has proposed that estrogen serves as a "natural" antipsychotic agent by inhibiting postsynaptic dopamine transmission (58–59). Psychopathology has been shown to improve when estradiol levels rise, and vice-versa (60–62). If the estrogen hypothesis is correct, and estrogen truly exerts a natural antipsychotic property, it would follow that during child-bearing years, women with schizophrenia would show reduced psychotic activity compared to men over time, at least until menopause. However, as the women grow older and approach the menopausal phase of life, the estrogen hypothesis would predict that psychosis should become more prevalent as the women grow older, and that sex differences in psychosis should become less pronounced as the sample ages.
This pattern was not obtained in the current longitudinal study: As the women became older (perhaps with slightly less estrogen production at the 20-year followup when they were approaching and reaching age 45), their psychotic symptoms tended to continue to improve. Women, but not men, showed significant improvement in psychotic activity between the 2-year and the 20-year followup. These data would indicate that if estrogen is a factor, other factors may be even more important in regard to their psychosis. Further research in this area with regular measures of estrogen levels would seem to be the next step to produce more definitive answers about this.
Acknowledgments
Supported, in part, by USPHS Grants MH-26341 and MH-068688 from the National Institute of Mental Health, USA (Dr. Harrow).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Linda S. Grossman, Department of Psychiatry, University of Illinois College of Medicine, Chicago
Martin Harrow, Department of Psychiatry, University of Illinois College of Medicine, Chicago
Cherise Rosen, Department of Psychiatry, University of Illinois College of Medicine, Chicago
Robert Faull, Department of Psychiatry, University of Illinois College of Medicine, Chicago
Gregory P. Strauss, Department of Psychiatry, University of Illinois College of Medicine, Chicago
References
- 1.Angermeyer MC, Goldstein JM, Kuehn L. Gender differences in schizophrenia: rehospitalization and community survival. Psychol Med. 1989;19:365–382. doi: 10.1017/s0033291700012411. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein JM. Gender differences in the course of schizophrenia. Am J Psychiatry. 1988;145:684–689. doi: 10.1176/ajp.145.6.684. [DOI] [PubMed] [Google Scholar]
- 3.Seeman MV. Current outcome in schizophrenia: Women versus men. Acta Psychiatr Scand. 1986;73:609–617. doi: 10.1111/j.1600-0447.1986.tb02732.x. [DOI] [PubMed] [Google Scholar]
- 4.Foerster A, Lewis S, Owen MJ, Murray R. Pre-morbid adjustment and personality in psychosis: effects of sex and diagnosis. Brit J Psychiatry. 1991;158:171–176. doi: 10.1192/bjp.158.2.171. [DOI] [PubMed] [Google Scholar]
- 5.Lewine R. Gender and schizophrenia. In: Tsuang M, Simpson J, editors. Handbook of Schizophrenia. Amsterdam: Elsevier; 1988. pp. 131–158. [Google Scholar]
- 6.Mueser K, Bellack A, Morrison R, Wixted J. Social competence in schizophrenia: premorbid adjustment, social skill, and domains of functioning. J Psychiatric Res. 1990;24:51–53. doi: 10.1016/0022-3956(90)90024-k. [DOI] [PubMed] [Google Scholar]
- 7.Grossman LS, Harrow M, Rosen C, Faull R. Sex differences in outcome and recovery for schizophrenia and other psychotic and nonpsychotic disorders. Psychiatr Serv. 2006;57:844–850. doi: 10.1176/ps.2006.57.6.844. [DOI] [PubMed] [Google Scholar]
- 8.Gur R, Petty R, Turetsky B, Gur R. Schizophrenia throughout life; sex differences in severity and profile of symptoms. Schizophr Res. 1996;21:1–12. doi: 10.1016/0920-9964(96)00023-0. [DOI] [PubMed] [Google Scholar]
- 9.Schultz SK, Miller DD, Oliver SE, Arndt S, Flaum M, Andreasen NC. The life course of schizophrenia: age and symptom dimensions. Schizophr Res. 1997;23:15–23. doi: 10.1016/S0920-9964(96)00087-4. [DOI] [PubMed] [Google Scholar]
- 10.Shtasel DL, Gur R, Gallacher F, Heimberg C, Gur RC. Gender differences in the clinical expression of schizophrenia. Schizophr Res. 1992;7:225–231. doi: 10.1016/0920-9964(92)90016-x. [DOI] [PubMed] [Google Scholar]
- 11.Wieselgren IM, Lindstrom E, Lindstrom LH. Symptoms at index admission as predictor for 1–5 year outcome in schizophrenia. Acta Psychiatr Scand. 1996;94:311–319. doi: 10.1111/j.1600-0447.1996.tb09866.x. [DOI] [PubMed] [Google Scholar]
- 12.Lewine R. Reflections on Saugstad's "Social Class, Marriage, and Fertility in Schizophrenia". Schizophr Bull. 1990;16:171–174. doi: 10.1093/schbul/16.2.171. [DOI] [PubMed] [Google Scholar]
- 13.McGlashan TH, Bardenstein KK. Gender differences in affective, schizoaffective, and schizophrenic disorders. Schizophr Bull. 1990;16:319–329. doi: 10.1093/schbul/16.2.319. [DOI] [PubMed] [Google Scholar]
- 14.Palacios-Araus L, Herran A, Sandoya M, Gonzalez de la Huebra E, Vazquez-Barquero JL, Diez-Manrique JF. Analysis of positive and negative symptoms in schizophrenia. A study from a population of long-term outpatients. Acta Psychiatr Scand. 1995;92:178–182. doi: 10.1111/j.1600-0447.1995.tb09564.x. [DOI] [PubMed] [Google Scholar]
- 15.Perry W, Moore D, Braff D. Gender differences on thought disturbance measures among schizophrenic patients. Am J Psychiatry. 1995;152:1298–1301. doi: 10.1176/ajp.152.9.1298. [DOI] [PubMed] [Google Scholar]
- 16.Tamminga CA. Gender and schizophrenia. J Clin Psychiatry. 1997;58(Suppl 15):33–37. [PubMed] [Google Scholar]
- 17.Addington D, Addington J, Patten S. Gender and affect in schizophrenia. Can J Psychiatry. 1996;41:265–268. [PubMed] [Google Scholar]
- 18.Huber G, Gross G, Schuttler R, Linz M. Longitudinal studies of schizophrenic patients. Schizophr Bull. 1980;6:592–605. doi: 10.1093/schbul/6.4.592. [DOI] [PubMed] [Google Scholar]
- 19.Klinkenberg WD, Calsyn RJ. Gender differeneces in the receipt of aftercare and psychiatric hospitalization among adults with severe mental illness. Comprehen Psychiatry. 1998;39:137–142. doi: 10.1016/s0010-440x(98)90072-4. [DOI] [PubMed] [Google Scholar]
- 20.Andia AM, Zisook S, Heaton RK, Hesselink J, Jernigan T, Kuck J, Morganville J, et al. Gender differences in schizophrenia. J Nerv Ment Dis. 1995;183:522–528. doi: 10.1097/00005053-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Cernovsky ZZ, Landmark JA, O'Reilly RL. Symptom patterns in schizophrenia for men and women. Psychol Rep. 1997;80:1267–1271. doi: 10.2466/pr0.1997.80.3c.1267. [DOI] [PubMed] [Google Scholar]
- 22.Jonsson H, Nyman K. Predicting long-term outcome in schizophrenia. Acta Psychiatr Scand. 1991;83:342–346. doi: 10.1111/j.1600-0447.1991.tb05553.x. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd D, Simpson J, Tsuang M. Are there sex differences in the long-term outcome of schizophrenia? J Nerv Ment Dis. 1985;73:643–649. doi: 10.1097/00005053-198511000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Opjordsmoen S. Long-term clinical outcome of schizophrenia with special references to gender differences. Acta Psychiatr Scand. 1991;3:307–313. doi: 10.1111/j.1600-0447.1991.tb05545.x. [DOI] [PubMed] [Google Scholar]
- 25.Fichtner CG, Grossman LS, Harrow M, Goldberg JF, Klein D. Cyclothymic mood swings in the course of affective disorders and schizophrenia. Am J Psychiatry. 1989;46:1149–1154. doi: 10.1176/ajp.146.9.1149. [DOI] [PubMed] [Google Scholar]
- 26.Harrow M, Grossman LS, Herbener E, Davis EW. Ten-Year Outcome: Patients with Schizoaffective Disorders, Schizophrenia, Affective Disorders, and Mood-Incongruent Psychotic Symptoms. Brit J Psychiatry. 2000;77:421–426. doi: 10.1192/bjp.177.5.421. [DOI] [PubMed] [Google Scholar]
- 27.Grossman LS, Harrow M. Interactive behavior in bipolar manic patients and its link to thought disorder. Comp Psychiatr. 1996;37:245–252. doi: 10.1016/s0010-440x(96)90003-6. [DOI] [PubMed] [Google Scholar]
- 28.Harrow M, Sands JR, Silverstein ML, Goldberg JF. Course and outcome for schizophrenia versus other psychotic patients: a longitudinal study. Schizophr Bull. 1997;23:287–303. doi: 10.1093/schbul/23.2.287. [DOI] [PubMed] [Google Scholar]
- 29.Racenstein JM, Harrow M, Reed R, Martin E, Herbener E, Penn DL. The relationship between positive symptoms and instrumental work functioning in schizophrenia: a 10 year follow-up study. Schizophr Res. 2002;56:95–103. doi: 10.1016/s0920-9964(01)00273-0. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg JF, Harrow M, Grossman LS. Course and outcome in bipolar affective disorder: a longitudinal follow- up study. Am J Psychiatry. 1995;152:379–384. doi: 10.1176/ajp.152.3.379. [DOI] [PubMed] [Google Scholar]
- 31.Grossman LS, Harrow M, Goldberg JF, Fichtner CG. Outcome of schizoaffective disorder at two long-term follow-ups: comparisons with outcome of schizophrenia and affective disorders. Am J Psychiatry. 1991;148:1359–1365. doi: 10.1176/ajp.148.10.1359. [DOI] [PubMed] [Google Scholar]
- 32.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 33.Grinker R, Sr, Harrow M. Clinical Research in Schizophrenia: A Multidimensional Approach. Springfield, Il: Charles C Thomas; 1987. [Google Scholar]
- 34.Spitzer R, Endicott J, Robins E. Research diagnostic criteria. Arch Gen Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- 35.Hollingshead AB, Redlich RC. Social class and mental illness. New York: John Wiley & Sons; 1958. [Google Scholar]
- 36.Harrow M, Herbener ES, Shanklin A, Jobe TH, Rattenbury F, Kaplan KJ. Followup of psychotic outpatients: dimensions of delusions and work functioning in schizophrenia. Schizophr Bull. 2004;30:147–161. doi: 10.1093/oxfordjournals.schbul.a007059. [DOI] [PubMed] [Google Scholar]
- 37.Carpenter WT, Sacks MH, Strauss JS, Bartko JJ, Rayner J. Evaluating signs and symptoms: comparison of structured interview and clinical approaches. Br J Psychiatry. 1976;128:136–138. doi: 10.1192/bjp.128.4.397. [DOI] [PubMed] [Google Scholar]
- 38.Pogue-Guile M, Harrow M. Negative symptoms in schizophrenia: Their longitudinal course and prognostic importance. Schizophr Bull. 1985;23:273–285. doi: 10.1093/schbul/11.3.427. [DOI] [PubMed] [Google Scholar]
- 39.Pogue-Geile MF, Harrow M. Negative and positive symptoms in schizophrenia and depression: A followup study. Schizophr Bull. 1985;10:371–387. doi: 10.1093/schbul/10.3.371. [DOI] [PubMed] [Google Scholar]
- 40.Harrow M, Grossman L, Jobe T, Herbener E. Do patients with schizophrenia ever show periods of recovery? A 15-year multi-followup study. Schizophr Bull. 2005;31:723–734. doi: 10.1093/schbul/sbi026. [DOI] [PubMed] [Google Scholar]
- 41.Harrow, Sands J, Silverstein M, Goldberg J. Course and outcome for schizophrenia vs. other psychotic patients: a longitudinal study. Schizophr. 1997;23:287–303. doi: 10.1093/schbul/23.2.287. [DOI] [PubMed] [Google Scholar]
- 42.Levenstein S, Klein D, Pollack M. Follow-up study of formerly hospitalized voluntary psychiatric patients: the first two years. Am J Psychiatry. 1966;122:1102–1109. doi: 10.1176/ajp.122.10.1102. [DOI] [PubMed] [Google Scholar]
- 43.Endicott J, Spitzer R, Fleiss J, Cohen J. The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiat. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 44.American Psychiatric Association. Diagnostical and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 45.Strauss J, Carpenter W. The prediction of outcome in schizophrenia: I. Characteristics of outcome. Arch Gen Psychiatry. 1972;27:739–746. doi: 10.1001/archpsyc.1972.01750300011002. [DOI] [PubMed] [Google Scholar]
- 46.Zigler E, Phillips L. Social competence and outcome in psychiatric disorder. J Abnormal Soc Psychol. 1964;63:264–271. [Google Scholar]
- 47.Zigler E, Glick M. The developmental approach to adult psychopathology. The Clinical Psychologist. 2001;54:2–11. [Google Scholar]
- 48.Zigler E, Levine J. Hallucinations vs. delusions: A developmental approach. J Nerv Ment Dis. 1993;171:141–146. doi: 10.1097/00005053-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Westermeyer J, Harrow M. Predicting outcome in schizophrenics and nonschizophrenics of both sexes: The Zigler-Phillips Social Competence Scale. J Abnormal Psychol. 1986;95:406–409. doi: 10.1037//0021-843x.95.4.406. [DOI] [PubMed] [Google Scholar]
- 50.Lewine R, Strauss J, Gift T. Sex differences in age at first hospital admission for schizophrenia: Fact or artifact? Am J Psychiatr. 1981;138:440–444. doi: 10.1176/ajp.138.4.440. [DOI] [PubMed] [Google Scholar]
- 51.Loranger W. Sex difference in age of onset of schizophrenia. Arch Gen Psychiatry. 1984;41:157–161. doi: 10.1001/archpsyc.1984.01790130053007. [DOI] [PubMed] [Google Scholar]
- 52.Szymanski S, Lieberman JA, Alvir JM, Mayerhoff D, Loebel A, Geisler S, et al. Gender differences in onset of illness, treatment response, course, and biologic indexes in first-episode schizophrenic patients. Am J Psychiatry. 1995;152:698–703. doi: 10.1176/ajp.152.5.698. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein JM, Faraone SV, Chen WJ, Tsuang MT. Gender and the familial risk for schizophrenia. Disentangling confounding factors. Schizophr Res. 1992;7:135–140. doi: 10.1016/0920-9964(92)90043-5. [DOI] [PubMed] [Google Scholar]
- 54.Lewine R, Seeman M. Anatomy of difference -- difference in anatomy. Gender and Psychopathology. 1995:131–158. [Google Scholar]
- 55.Lieberman J, Bogerts B, Degreef G, Ashtari M, Lantos G, Alvir J. Qualitative assessment of brain morphology in acute and chronic schizophrenia. Am J Psychiatry. 1992;149:784–794. doi: 10.1176/ajp.149.6.784. [DOI] [PubMed] [Google Scholar]
- 56.Bhatia T, Franzos M, Wood J, Nimgaonkar V, Despande S. Gender and procreation among patients with schizophrenia. Schizophr Res. 2004;68:387–394. doi: 10.1016/j.schres.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Walker E, Bettes B, Kain E, Harvey P. Relationship of gender and marital status with symptomatology in psychotic patients. J Abnormal Psychol. 1985;94:42–50. doi: 10.1037//0021-843x.94.1.42. [DOI] [PubMed] [Google Scholar]
- 58.Seeman M, Lang M. The role of estrogens in schizophrenia gender differences. Schizophr Bull. 1990;16:185–194. doi: 10.1093/schbul/16.2.185. [DOI] [PubMed] [Google Scholar]
- 59.Seeman M. Gender and the onset of schizophrenia: neurohormonal influences. Psychiatr J Univ Ottawa. 1981;6:136–138. [Google Scholar]
- 60.Reicher-Rossler A, Hafner H, Dutsch-Strobel A, Oster M, Stumbaum M, van Gulick-Bailer M, et al. Further evidence for a specific role of estradiol in schizoprhenia? Biol Psychiatr. 1994;36:492–494. doi: 10.1016/0006-3223(94)90649-1. [DOI] [PubMed] [Google Scholar]
- 61.Riecher-Rossler A, Hafner H, Strumbaum M, Maurer K, Schmidt R. Can estradiol modulate schizophrenic symptomatology? Schizophr Bull. 1994;20:203–214. doi: 10.1093/schbul/20.1.203. [DOI] [PubMed] [Google Scholar]
- 62.Akhondzadeh S, Nejatisafa A, Amini H, Mohammadi M, Larijani B, Kashani L, et al. Adjunctive estrogen treatment in women with chronic schizophrenia: a double-blind, randomized, and placebo-controlled trial. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27:1007–1012. doi: 10.1016/S0278-5846(03)00161-1. [DOI] [PubMed] [Google Scholar]